Abstract
Human olfactory ensheathing cells (OECs) were prepared from adult human olfactory nerves, which were removed during surgery for frontal base tumors, and were transplanted into the demyelinated spinal cord of immunosuppressed adult rats. Extensive remyelination was observed in the lesion site: In situ hybridization using a human DNA probe (COT-1) indicated a similar number of COT-1-positive cells and OEC nuclei within the repaired lesion. The myelination was of a peripheral type with large nuclei and cytoplasmic regions surrounding the axons, characteristic of Schwann cell and OEC remyelination. These results provide evidence that adult human OECs are able to produce Schwann cell-like myelin sheaths around demyelinated axons in the adult mammalian CNS in vivo.
Keywords: axon, demyelination, glia, myelin, multiple sclerosis
INTRODUCTION
A number of neurologic diseases, such as multiple sclerosis and spinal cord injury, elicit demyelination of axons in the CNS (Bunge et al., 1993; McDonald, 1995). Endogenous remyelination of demyelinated axons by oligodendrocytes (Gledhill et al., 1973; Clifford-Jones et al., 1980) or Schwann cells (Blakemore et al., 1977) results in the reestablishment of relatively normal impulse conduction in animals (Smith et al., 1979; Blight and Young, 1989; Felts and Smith, 1992), whereas it is very limited in humans (Ghatak et al., 1973; Prineas and Connell, 1979; Itoyama et al., 1985). Recently, myelin-forming cell transplantation has been suggested as a potential repair strategy for demyelinated CNS axons (see, e.g., Groves et al., 1993; Honmou et al., 1996; Imaizumi et al., 1998; Brustle et al., 1999; Keirstead et al., 1999; Yandava et al., 1999). Although transplantation of exogenous glial cells cultured from fetuses or neonates as well as adults can induce substantial anatomically defined myelination (Blakemore and Crang, 1985; Duncan et al., 1988) and can restore normal conduction properties in demyelinated axons in experimental animals (Utzschneider et al., 1994; Honmou et al., 1996; Imaizumi et al., 1998), transplantation of human glial cells obtained from the adult human brain failed to achieve remyelination of demyelinated rat axons in the CNS (Targett et al., 1996).
Olfactory ensheathing cells (OECs) are specialized cells that support axons of olfactory epithelium neurons that project through the olfactory nerve into the olfactory bulb of the CNS; they are pluripotential cells that can show Schwann cell-like or astrocyte-like cell properties (Devon and Doucette, 1992). Although they do not produce myelin normally, when they are transplanted into the demyelinated spinal cord, they can remyelinate the axons with a peripheral pattern of myelination similar to Schwann cell myelination (Franklin et al., 1996; Imaizumi et al., 1998) and restore conduction of the remyelinated axons (Imaizumi et al., 1998). Additional interest has focused on OECs, because olfactory epithelial neurons are replaced continuously and regenerate axons in the normal adult (Moulton, 1974; Barber and Raisman, 1978; Graziadei et al., 1979; Zhao et al., 1994). It has been reasoned that the unique properties of these cells may allow them to guide and enhance regenerating CNS axons (Ramon-Cueto and Valverde, 1995; Li et al., 1997).
Although recent work indicates that rat OEC transplantation can enhance regeneration of transected spinal cord axons (Li et al., 1997; Imaizumi et al., 1999) and remyelinate demyelinated axons in the spinal cord (Franklin et al., 1996; Imaizumi et al., 1998), it is not known whether OECs derived from human olfactory nerves can remyelinate or enhance axonal regeneration. Li et al. (1997) suggested that human OECs may be a potential cell source for human cell therapy approaches to encourage axonal regeneration. Homologous or autologous tissue represents one possible source of OECs for transplantation in patients with demyelinating disease; however, it is necessary first to demonstrate the remyelinating potential of OECs obtained from the adult human. Indeed, although numerous rodent cell types derived from the CNS and PNS display remyelinating potential on transplantation, little is known of the potential of human cells, particularly from the adult, to induce remyelination. To determine whether dissociated adult human OECs can remyelinate adult CNS after transplantation, we transplanted cells derived from adult human olfactory nerves into a demyelinated lesion in the spinal cord white matter in immunosuppressed rats. The results indicate that extensive remyelination can be induced by transplantation of adult human OECs into the demyelinated rat spinal cord.
MATERIALS AND METHODS
Preparation of Olfactory-Ensheathing Cell Cultures
Primary human OEC cultures were prepared from adult human olfactory nerves obtained from two female patients undergoing olfactory nerve resection for frontal base tumors. Patient A was a woman age 62 years and had a nasal melanoma that invaded into the frontal base through the olfactory bulb. Patient B was a woman age 48 years and had an olfactory groove meningioma. The nerves were harvested and processed for cell culture based on the method of Chuah and Au (1993) with minor modifications. Briefly, the cell suspension resulting from enzymatically and mechanically dissociated human adult olfactory nerves was plated onto 100-mm2 poly-L-lysine-coated tissue culture plates at 8 × 105 cells per plate and cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% (volume/volume) fetal calf serum. Rat OEC cultures were obtained from olfactory nerves of 12-week-old Wistar rats under deep anesthesia of ketamine (75 mg/kg, i.p.). Tissues were dissected free of meninges and surrounding connective tissue and were processed for cell culture as described above.
Characterization of Dissociated Olfactory Glial Cells in Culture
Antigenic phenotypes of cultured human and rat OECs were examined. Immunocytochemistry of both human and rat OECs was carried out as described previously by using markers that characterize OECs (Ramon-Cueto and Valverde, 1995). Selected cultures were immunostained for the presence of antiglial fibrillary acid protein (GFAP; dilution, 1:200, polyclonal rabbit anti-GFAP; Nitirei, San Francisco, CA), antivimentin (dilution, 1:100; monoclonal mouse antivimentin; Nitirei), anti-S-100 protein (S-100; dilution 1:200; polyclonal rabbit anti-S-100; Nitirei), anti-O4 (dilution, 1:100; monoclonal mouse anti O-4; Boehringer Mannheim, Mannheim, Germany), antigalactocerebrocide (GalC; dilution, 1:200; monoclonal mouse anti-GalC; Boehringer Mannheim), and anti-A2B5 (dilution, 1:100; monoclonal mouse anti-A2B5; Boehringer Mannheim).
Cells were rinsed once with phosphate-buffered saline (PBS) and fixed for 15 min with a fixative solution containing 4% paraformaldehyde in 0.14 M Sorensen’s phosphate buffer, pH 7.4. Fixed cells were incubated for 30 min in a blocking solution containing 0.2% Triton X-100 and 5 % normal goat serum before incubation with the primary antibody. Incubation with anti-O4 or anti-A2B5 antibody was performed without Triton X-100. The primary antibody was visualized by using fluorescein isothiocyanate (FITC)-labeled goat-antirabbit immunoglobulin G (IgG) antibody (dilution, 1:100; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) and FITC-labeled goat-antimouse IgG antibody (dilution, 1:100; Jackson ImmunoResearch Laboratories). After immunostaining, coverslips were mounted cell-side-down on microscope slides using fluorescent mounting medium (Dako, Carpenteria, CA) for immunofluorescence. Photographs were taken on a Zeiss immunofluorescent microscope (Axioskop FS; Zeiss, Oberkochen, Germany).
Animal Preparation and Transplantation
Experiments were performed on 23 Wistar rats (5 unoperated controls, 8 demyelinated rats, and 10 demyelinated rats with transplants). A focal demyelinated lesion was created in the dorsal column of the spinal cord of 12-week-old rats with X-irradiation and ethidium bromide injection (EB-X) by using a method similar to that of Honmou et al. (1996). Briefly, rats were anesthetized with ketamine (75 mg/kg) and xylazine (10 mg/kg) i.p., and a 40-Gray surface dose of X-irradiation was delivered through a 2 × 4 cm opening in a lead shield (4 mm thick) to the spinal cord caudal to thoracic segment 10 (Th-10) by using a Softex M-150 WZ radiotherapy machine (100 kV, 1.15 mA, SSD 20 cm; dose rate, 200 cGy/min). Three days after irradiation, rats were anesthetized as described above, and, using sterile technique, a laminectomy was performed at Th-11. The demyelinating lesion was induced by the direct injection of ethidium bromide (EB) into the dorsal column through a drawn glass micropipette. Injections of 0.5 μl of 0.3 mg/ml EB in saline were made at depths of 0.7 mm and 0.4 mm. A suspension of OECs (1 × 104 cells/μl) in 1 μl medium was injected into the middle of the EB-X-induced lesion 3 days after the EB injection. Transplant-receiving rats were immunosuppressed with cyclosporin A (10 mg/kg/day, s.c.; a kind gift from Novartis Pharma AG, Basel, Switzerland).
Histologic Examination
The rats were deeply anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and perfused through the heart, first with PBS and then with a fixative solution containing 2% glutaraldehyde and 2% paraformaldehyde in 0.14 M Sorensen’s phosphate buffer, pH 7.4. After in situ fixation for 10 min, the spinal cord was excised carefully, cut into 1-mm segments, and placed into fresh fixative. The tissue was washed several times in Sorensen’s buffer, postfixed with 1% OsO4 for 2 hours at 25°C, dehydrated in graded ethanol solutions, passed through propylene oxide, and embedded in EPON. Semithin sections (1 μm) were cut, counter-stained with 0.5% methylene blue and 0.5% azure II in 0.5% borax, and examined with a light microscope (Axioskop FS; Zeiss). Thin sections were counterstained with uranyl and lead salts and examined with a JEOL JEM1200EX electron microscope (Peabody, MA) operating at 60 kV.
In Situ Hybridization
A detailed description of the in situ hybridization procedure has been published previously (Guitteny et al., 1988). Briefly, cells cultured on poly-L-lysine-coated tissue culture plates were exposed to fixative solution of 4% paraformaldehyde in 0.1 M PBS for 20 min at 0°C and then in 0.15 M ethanolamine for 20 min at 0°C. Subsequently, they were washed with PBS (three times for 10 min each) and then exposed to RNase (10 μg/μl; Qiagen, Hilden, Germany) in 10 mM Tris and 500 mM NaCl (pH 7.5) for 10 min at 37°C. This was followed by exposure to proteinase K (5 ng/ml; Boehringer Mannheim) in 2 × standard saline citrate (SSC) buffer (2 × SSC buffer = 3 M NaCl and 0.3 M sodium citrate; Gibco, Grand Island, NY) for 10 min at 37°C. The cells were then washed in PBS (three times for 5 min each) and placed in a fixative solution of 4% paraformaldehyde in 0.1 M PBS for 1 min. This was followed by washing in PBS (three times for 5 min each). The cells were then dehydrated through an ascending series of ethanol, air dried, and then exposed to hybridization solution containing 0.5 μg/ml biotinylated human COT-1 DNA probe, 10% dextran sulfate, 2 × SSC, 0.1 M sodium phosphate, Denhardt’s solution, and 0.05 mg/ml sodium azide. The cells were then denatured on a heating block at 100°C for 5 min and incubated at 42°C in humid chamber overnight. The cells were then exposed to 0.2 × SSC (three times for 15 min each) and then to blocking solution containing 50 mg/ml protein in 100 mM Tris-HCl, 150 mM NaCl, and 0.2 mg/ml sodium azide for 15 min. They were next placed in streptoavidin-alkaline phosphate (4 μg/ml) in conjugate dilution buffer solution (100 mM Tris-HCl, 150 mM MgCl2, 10 mg/ml bovine serum albumin, and 0.2 mg/ml sodium azide) for 15 min. This was followed by incubation in Tris-buffered saline (two times for 15 min each) and exposure to alkaline-substrate buffer for 5 min. Next, a chromogen solution containing 0.3 ng/ml Nitro Blue tetrazolium and 0.166 ng/ml 5-bromo-4-chloro-3-indolyl-phosphate in alkaline-substrate buffer for 30 min at 37°C was applied followed by several changes of deionized water to stop the reaction. The cells were then dehydrated through an ascending series of ethanol, cleared, air dried, and coverslipped with Malinol (Muto Pure Chemicals, Tokyo, Japan). For control experiments, the cells were processed without probe.
The transplant-receiving rats were allowed to survive for a period of 21 days after transplantation, at which time they were killed by aortic perfusion with 4% paraformaldehyde in 0.1 M PBS under ketamine (75 mg/kg, i.p.) anesthesia. The spinal cords were removed and placed in cold fixative with 30% sucrose for 2 days for cryoprotection. Tissues were then placed in optimal cutting temperature (OCT) compound (Miles Inc., Elkhart, IN), frozen in liquid N2, and 8-μm sections were cut with a cryostat. Sections were dried onto silane-coated slides. In situ hybridization was processed as described above with the exception of the enzyme treatment (proteinase K, 40 μg/ml for 15 min). The specificity of the probe was confirmed by staining human tissue.
The numbers of COT-1-positive cells and myelinating OEC bodies on the same sections after OEC transplantation were counted per mm2. All variances represent standard error (±SEM). Differences among groups were assessed by using an unpaired, two-tailed t-test to identify individual group differences. Differences were deemed statistically significant at P < 0.05.
RESULTS
Morphologic and Antigenic Properties of Dissociated Olfactory Glial Cells in Culture
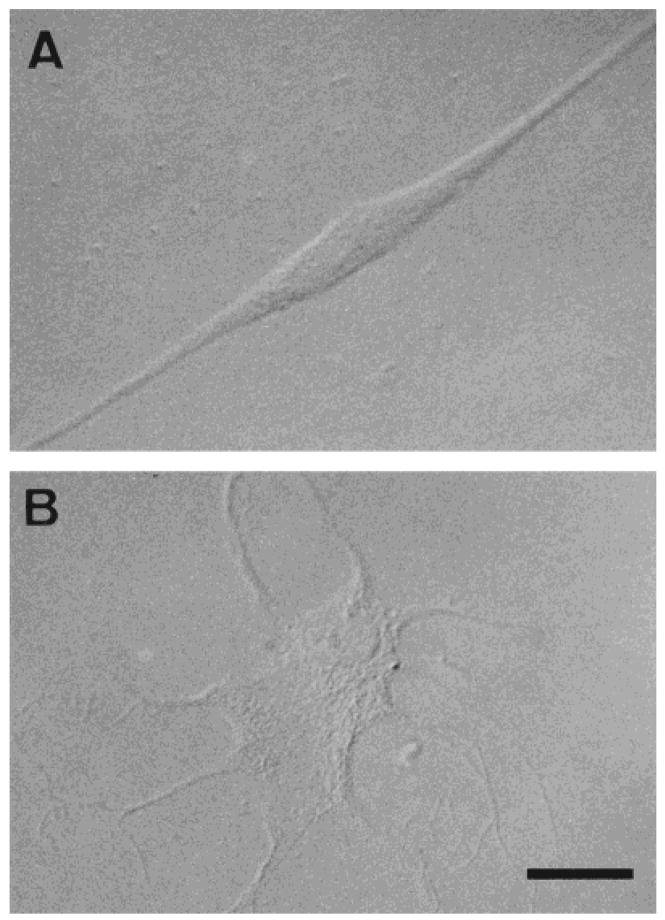
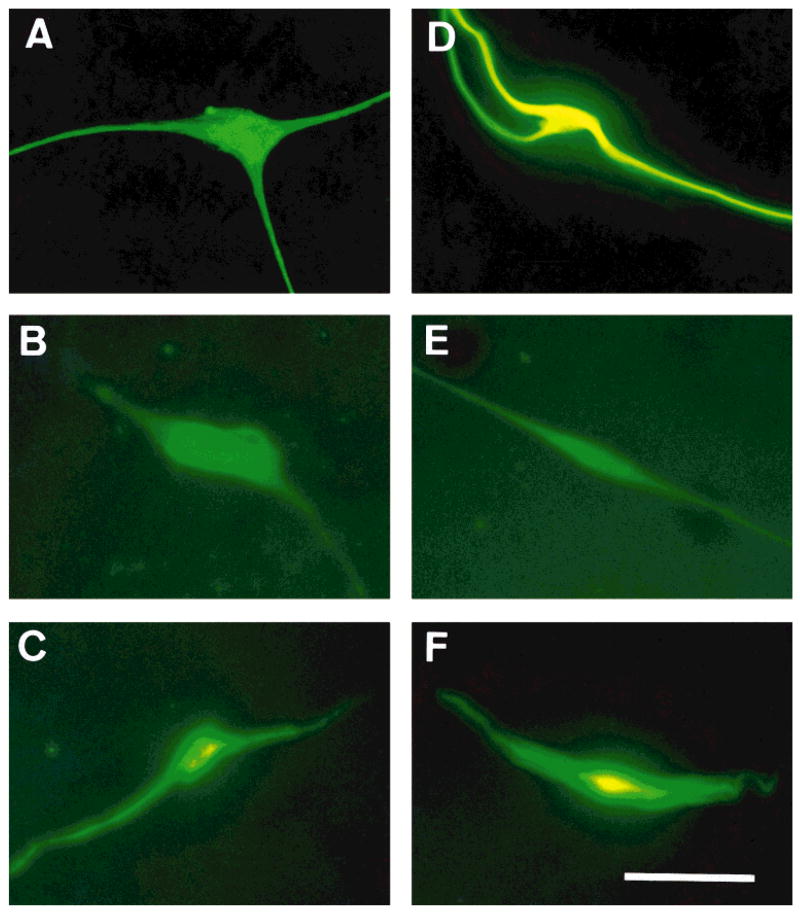
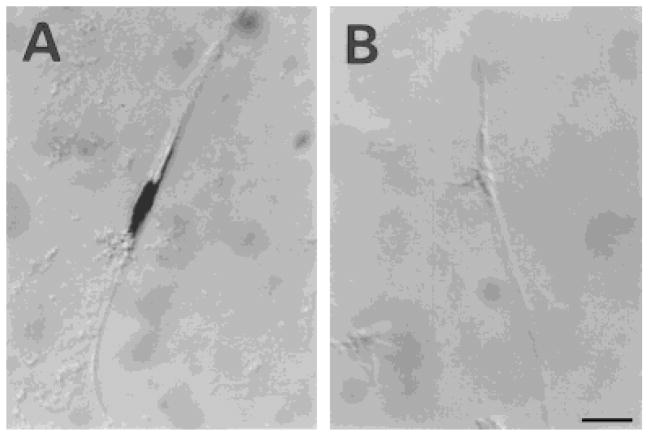
After cell dissociation of human OECs from the olfactory nerves, the cells were plated for short-term culture (2 days). The morphologic features of the human primary OEC cultures were similar to those of the rat OEC cultures that have been described in detail elsewhere (Barnett et al., 1993; Franceschini and Barnett, 1996). Briefly, these cells generally present as either spindle-shaped, Schwann-like cells (Fig. 1A) or multipolar, astrocyte-like cells (Fig. 1B). However, some display morphologies intermediate between these two types. Immunohistochemical analysis indicated that the distinct combination of antigens expressed by these cells was unlike that described previously for Schwann cells, type-1 astrocytes, O-2A progenitors, oligodendrocytes, or type-2 astrocytes but similar to that of rat OECs (Barnett et al., 1993; Franceschini and Barnett, 1996). Figure 2 shows immunolabeled cells for rat (Fig. 2A–C) and human (Fig. 2D–F) cultured OECs for GFAP (Fig. 2A,D), O4 (Fig.2B,E), and vimentin (Fig. 2C,F). Over 90% of total cells in culture were stained with anti-GFAP and antivimentin antibodies, and ≈80% of cells were O4-positive. Both rat and human OECs were not stained by antibodies for S-100, Gal-C, and A2B5. These results are in agreement with studies of rodent OECs showing a similar antigenic profile (Barnett et al., 1993; Ramon-Cueto and Valverde, 1995; Franceschini and Barnett, 1996; Franklin et al., 1996). Moreover, the negative staining for S-100, Gal-C, and A2B5 indicates that the cells are not Schwann cells (Franklin et al., 1996). This is an important consideration, because, although it is unlikely, some Schwann cells associated with blood vessels may be present in the cultures.
Fig. 1.
Human olfactory ensheathing cells (OECs) cultured from the adult human olfactory nerves. Phase-contrast photomicrographs showing Schwann cell-like (spindle) cells (A) and astrocyte-like cells (B). Scale bar = 50 μm in A, 100 μm in B.
Fig. 2.
The antigenic phenotype of rat (A–C) and human (D–F) OECs in culture. Immunolabeling of the cells with antiglial fibrillary acidic protein (anti-GFAP; A,D), monoclonal mouse anti-O-4 (B,E), and antivimentin (C,F). Scale bar = 50 μm.
Light and Electron Microscopic Observations
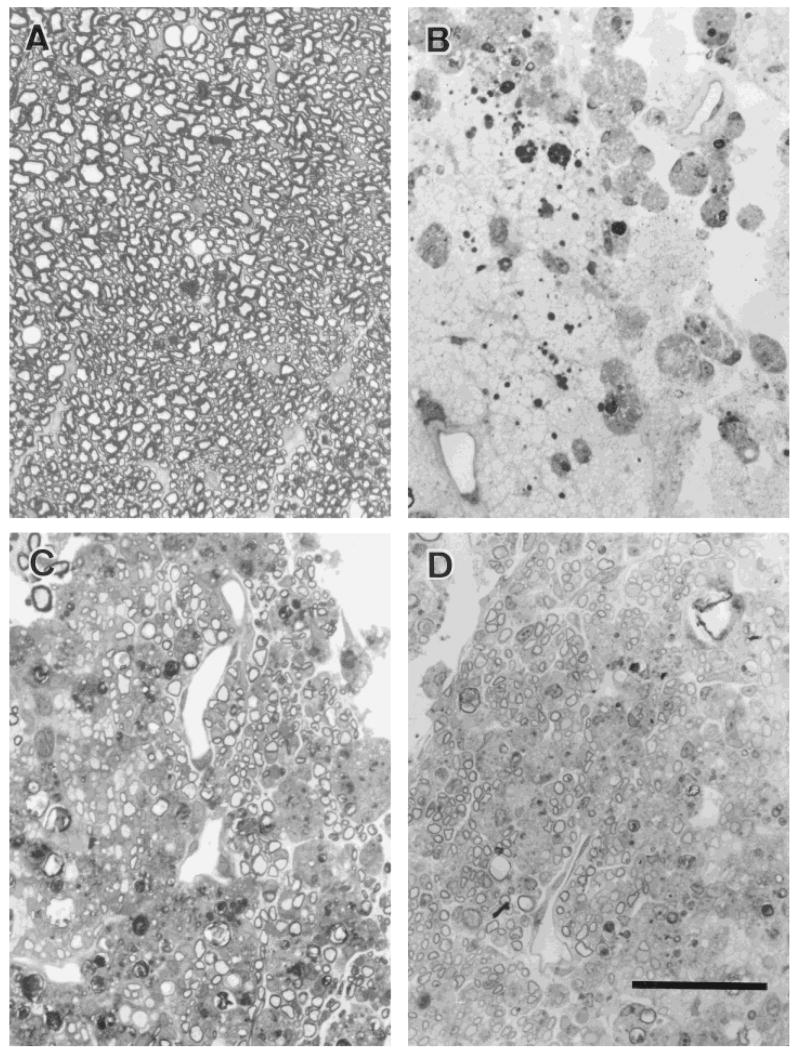
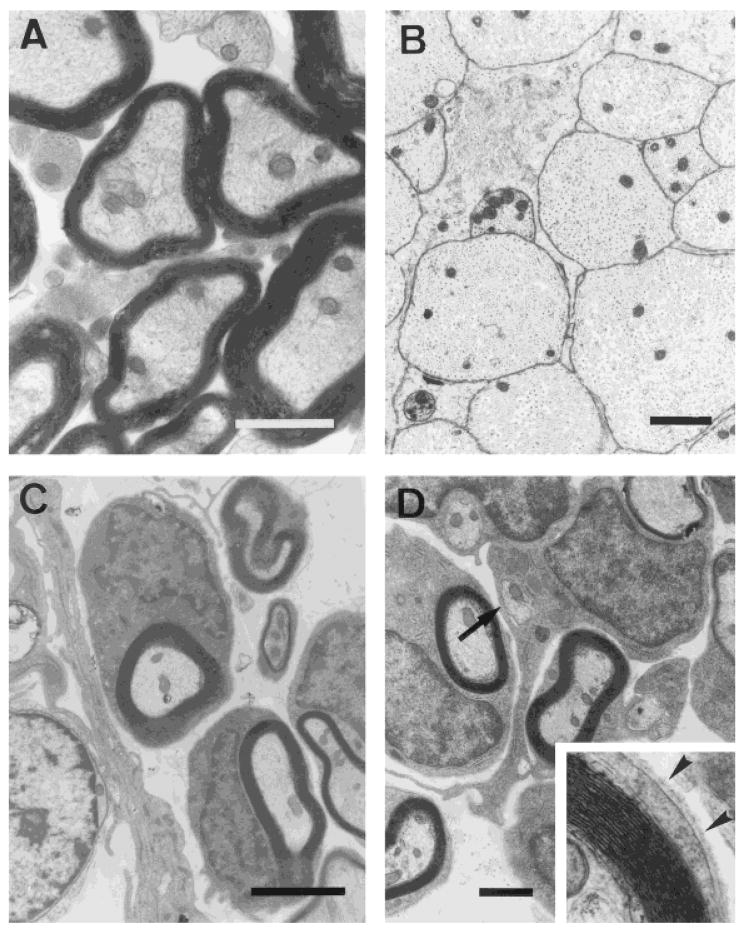
Demyelination was induced by local injection of EB, a nucleic acid-chelating agent that induces primary demyelination by killing oligodendrocytes, and was maintained by X-ray irradiation, which inhibited the endogenous remyelination (EB-X model; Blakemore and Patterson, 1978). The lesion site was largely glial cell free, well circumscribed, and encompassed 70–80% of the transverse extent of the dorsal columns: The anteroposterior extent of the lesion was 4–6 mm. Examples of the demyelinated dorsal columns are shown at the light and electron microscopic levels, respectively, in Figures 3B and 4B. Control dorsal column axons from a similar region are shown in Figures 3A and 4A for comparison. The lesion was confirmed not to be repaired by host-derived glial cells for at least for 6–8 weeks in nontransplanted rats in which some myelination around the peripheral margin of the lesion was observed (n = 5 rats).
Fig. 3.
Light photomicrographs of transverse sections through the dorsal columns 3 weeks after transplantation show that nearly the entire cross-sectional area of the lesion was repaired by OEC remyelination. Examination at higher magnification shows normal (A), demyelinated (B), and remyelinated (C) axons by rat OECs and remyelinated axons by human OECs (D) in the dorsal columns. Scale bar = 20 μm.
Fig. 4.
Electron photomicrographs showing normal (A) and demyelinated (B) axons in the dorsal columns. All demyelinated spinal cords that received human OEC injections showed clear evidence of remyelination (C) of the demyelinated axons. Some human OECs ensheathed two or more axons (D; arrow). Examination at higher magnification showed the presence of a basal lamina surrounding the fibers characteristic of peripheral myelin (inset, arrowheads). Scale bars = 1 μm in A, 3 μm in B, 2 μm in C, 1.5 μm in D.
At 3 weeks after cell transplantation of either rat OECs or human OECs, there was extensive remyelination throughout the lesion (Fig. 3C,D), with the exception of the very finest caliber axons, which normally are nonmyelinated. Closer examination of the remyelinated axons with electron microscopy indicated that virtually all demyelinated spinal cord that received cell injections showed clear evidence of remyelination, with a characteristic peripheral myelination pattern (Fig. 4C,D). The remyelinated axons had a “signet ring” appearance that was similar to the peripheral type of remyelination induced by Schwann cell or OEC transplantation in the CNS (Blakemore and Crang, 1985; Franklin et al., 1996; Honmou et al., 1996; Imaizumi et al., 1998). This was characterized by large cytoplasmic and nuclear regions surrounding the axons. Comparison of the normal oligodendrocyte (Fig. 4A) pattern of myelination with that induced by human OECs (Fig. 4D) clearly indicates morphologic differences between the two myelin-forming cell types. The normal (oligodendrocyte) myelination does not have the large nuclear and cytoplasmic domains characteristic of peripheral myelin-forming cells. Also, note the presence of a basement membrane (see Fig. 4D, inset, arrowheads) around a myelinated axon subsequent to human OEC transplantation. The large nuclear and cytoplasmic regions and the presence of a basement membrane associated with myelination is a hallmark of peripheral myelin (Berthold, 1978).
In Situ Hybridization With the Human COT-1 DNA Probe
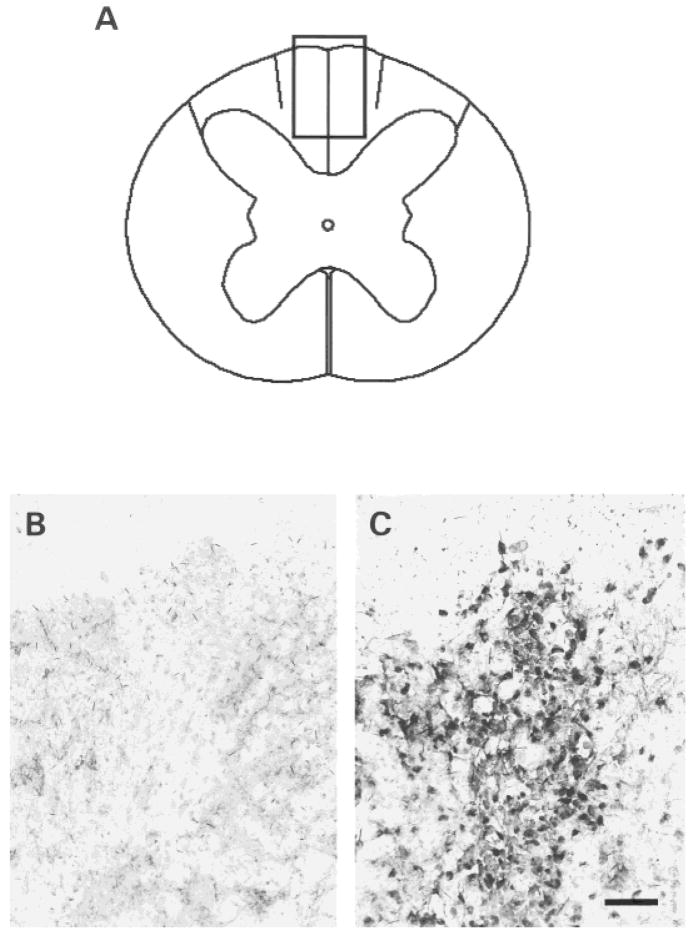
Although no endogenous remyelination occurs in this model system at the times studied (Blakemore and Patterson, 1978), we also carried out in situ hybridization studies with a probe specific to human DNA to identify the presence of donor cells in the remyelinated spinal cords. The nuclei of the human OECs in vitro was labeled by the COT-1 DNA probe, which specifically hybridizes with human DNA (Fig. 5A). Rat OECs were not labeled by the COT-1 probe (Fig. 5B). The schematic in Figure 6A shows an EB-X lesion after human OEC transplantation. The photomicrographs of Figure 6B,C are from the boxed area in Figure 6A, and show in situ hybridization for the human-specific COT-1 DNA probe after transplantation of rat OECs and human OECs, respectively. Note the abundance of labeled nuclei with the lesion after human cell transplantation (Fig. 6C) but not after rat cell transplantation (Fig. 6B). Counts of the COT-1-positive cells and the nuclei of OECs forming myelin indicated that there were 2,052.0 ± 80.7/mm2 (n = 10) COT-1-positive cells and 2,042.5 ± 72.8/mm2 (n = 10) myelinating cell bodies on the same sections (see Materials and Methods). In contrast, virtually no labeling was observed with rat OECs in the lesion (Fig. 6C), even if there was extensive remyelination throughout the lesion after rat OEC transplantation (Fig. 3C). Thus, this further indicates that the remyelination after human OEC transplantation was effectuated by the donorxenotransplanted cells.
Fig. 5.
The human and rat OECs in vitro were hybridized with the COT-1 probe, which is specific for human DNA. Note that the human OEC is labeled markedly (A), whereas the rat OEC is not labeled (B). Scale bar = 25 μm.
Fig. 6.
A–C: Schematic representation (A) of a lesion achieved by using X-irradiation and ethidium bromide injection (EB-X lesion). The boxed area is shown in (B) and (C) after in situ hybridization for the human-specific COT-1 DNA probe. The transplanted human OECs are labeled markedly in the EB-X lesion (C), whereas the transplanted rat OECs in the lesion are not labeled (B). Scale bar = 50 μm.
DISCUSSION
In this study, we demonstrated that transplanted OECs derived from adult human olfactory nerves are capable of remyelinating demyelinated axons extensively in the adult rat spinal cord in vivo. Two lines of evidence indicate that the remyelination was indeed from the transplanted human cells and not from endogenous remyelination by resident rat Schwann cells. First, our lesion model to induce demyelination consisted of X-irradiating the spinal cord to inhibit endogenous cell division followed by EB-X into the cord to kill white matter glial cells. This protocol induced a glial-free zone in the dorsal columns of the spinal cord with no endogenous myelination for at least 6–8 weeks (Blakemore and Patterson, 1978). We studied the spinal cords at 3 weeks after cell injection, a period well within the window in which no endogenous remyelination occurs. Moreover, we confirmed survival of human donor cells in the remyelination zone by in situ hybridization using a human-specific COT-1 DNA probe: Extensive labeled donor cells were present in the remyelinated zone of the dorsal columns, and the numbers of myelin-forming cell nuclei and COT-1-positive cells were similar.
The morphologic features of the myelin-forming cells and myelin in the lesion are highly reminiscent of the myelin sheaths formed by transplanted rat Schwann cells or OECs into the CNS. The myelin-forming cells had large nuclear and cytoplasmic regions and were surrounded by a basement membrane characteristic of peripheral myelin (Berthold, 1978). Indeed, observations both in vitro (Devon and Doucette, 1995) and in vivo (Franklin et al., 1996; Imaizumi et al., 1998) indicate similar morphologic features for rat OEC-induced remyelination of CNS axons.
The relative effectiveness of adult human OECs in remyelinating demyelinated axons in the adult rat is in contrast to the relative ineffectiveness of adult human oligodendrocytes in remyelinating adult rat white matter. Transplantation of human oligodendrocytes derived from the adult human brain white matter failed to form a myelin sheath around the demyelinated axons (Targett et al., 1996). Although transplanted human oligodendrocytes survived in the demyelinated lesion, no myelin sheaths were produced, and there was no evidence of cell migration or division. Targett et al. (1996) suggested that the majority of oligodendrocytes in the adult human brain white matter have little if any remyelinating potential. Several lines of evidence support this idea. In agreement with these in vivo studies, in vitro studies indicate that oligodendrocytes make contact with axons, but no myelin sheaths are formed when human white matter glia is cocultured with rodent dorsal root ganglion cells (Whittemore et al., 1993). However, human embryonic oligodendrocytes can myelinate rat axons in the immature CNS in vivo (Gumpel et al., 1987), as human Schwann cells do in the PNS (Levi and Bunge, 1994). A species mismatch could occur, because rodent axons promote human Schwann cell proliferation in vitro less effectively than similarly prepared rodent Schwann cells (Morrissey et al., 1995). However, dog and cat oligodendrocytes can myelinate rat axons, and rat oligodendrocytes can myelinate cat axons (Archer et al., 1994; Targett and Blakemore, 1994).
Astrocytes in the demyelinated lesion, where persistent glial scars often are formed, may impose significant restrictions on the use of Schwann cells to repair demyelinated CNS. Although both host-derived and transplant-derived Schwann cells are able to remyelinate demyelinated CNS axons (Blakemore and Crang, 1985) and restore conduction (Honmou et al., 1996), astrocyte-containing areas appear to exclude or impede Schwann cells from entering the lesion (Franklin and Blakemore, 1993). In this regard, OECs may be able to remyelinate demyelinated axons in the damaged CNS in a manner not shared by neural crest-derived Schwann cells, in which chronic sclerotic plaques may be formed by residual or reacting astrocytes, because OECs are able to express an astrocyte-like phenotype. Indeed, they coexist with astrocytes within the olfactory bulb and are able to invade the CNS, which contains astrocytes (Doucette, 1990).
OECs may have an important role in the support of axonal regeneration that occurs in the olfactory system, both in guiding axonal regeneration in the olfactory nerve and in supporting axon entry into the olfactory bulb (Ramon-Cueto and Nieto-Sampedro, 1994; Doucette, 1995). In addition, transplantation of OECs into ablated spinal cord tracts enhances regeneration and functional recovery (Li et al., 1997; Imaizumi et al., 1999). An important consideration in the development of a potential cell therapy approach in humans to repair the damaged CNS is the selection of an appropriate donor cell type. The demonstration of the myelinating potential of adult human OECs from surgically removed tissue indicates that transplanted human OECs may have an important role in developing repair strategies for demyelinated and transected CNS axons.
Acknowledgments
Japanese Monbusyo; Grant numbers: 09671434, 11770765; Grant sponsor: National Institutes of Health; Grant number: NS10174; Grant sponsor: National Multiple Sclerosis Society; Grant sponsor: Myelin Project (Washington, DC).
References
- Archer DR, Leven S, Duncan ID. Myelination by cryopreserved xenografts and allografts in the myelin-deficient rat. Exp Neurol. 1994;125:268–277. doi: 10.1006/exnr.1994.1029. [DOI] [PubMed] [Google Scholar]
- Barber PC, Raisman G. Cell division in the vomeronasal organ of the adult mouse. Brain Res. 1978;141:57–66. doi: 10.1016/0006-8993(78)90616-9. [DOI] [PubMed] [Google Scholar]
- Barnett SC, Hutchins AM, Noble M. Purification of olfactory nerve ensheathing cells from the olfactory bulb. Dev Biol. 1993;155:337–350. doi: 10.1006/dbio.1993.1033. [DOI] [PubMed] [Google Scholar]
- Berthold CH. Morphology of normal peripheral axons. In: Waxman SG, editor. Physiology and pathobiology of axons. New York: Raven Press; 1978. pp. 3–64. [Google Scholar]
- Blakemore WF, Crang AJ. The use of cultured autologous Schwann cells to remyelinate areas of persistent demyelination in the central nervous system. J Neurol Sci. 1985;70:207–223. doi: 10.1016/0022-510x(85)90088-7. [DOI] [PubMed] [Google Scholar]
- Blakemore WF, Eames RA, Smith KJ, McDonald WI. Remyelination in the spinal cord of the cat following intraspinal injections of lysolecithin. J Neurol Sci. 1977;33:31–43. doi: 10.1016/0022-510x(77)90179-4. [DOI] [PubMed] [Google Scholar]
- Blakemore WF, Patterson RC. Suppression of remyelination in the CNS by X-irradiation. Acta Neuropathol (Berlin) 1978;42:105–113. doi: 10.1007/BF00690975. [DOI] [PubMed] [Google Scholar]
- Blight AR, Young W. Central axons in injured cat spinal cord recover electrophysiological function following remyelination by Schwann cells. J Neurol Sci. 1989;91:15–34. doi: 10.1016/0022-510x(89)90073-7. [DOI] [PubMed] [Google Scholar]
- Brustle O, Jones KN, Learish RD, Karram K, Choudhary K, Wiestler OD, Duncan ID, McKay RDG. Embryonic stem cell-derived glial precusors: a source of myelinating transplants. Science. 1999;285:754–756. doi: 10.1126/science.285.5428.754. [DOI] [PubMed] [Google Scholar]
- Bunge RP, Puckett WR, Becerra JL, Marcillo A, Quencer RM. Observations on the pathology of human spinal cord injury. A review and classification of 22 new cases with details from a case of chronic cord compression with extensive focal demyelination. Adv Neurol. 1993;59:75–89. [PubMed] [Google Scholar]
- Chuah MI, Au C. Cultures of ensheathing cells from neonatal rat olfactory bulbs. Brain Res. 1993;601:213–220. doi: 10.1016/0006-8993(93)91713-3. [DOI] [PubMed] [Google Scholar]
- Clifford-Jones RE, Landon DN, McDonald WI. Remyelination during optic nerve compression. J Neurol Sci. 1980;46:239–243. doi: 10.1016/0022-510x(80)90082-9. [DOI] [PubMed] [Google Scholar]
- Devon R, Doucette R. Olfactory ensheathing cells myelinate dorsal root ganglion neurites. Brain Res. 1992;589:175–179. doi: 10.1016/0006-8993(92)91182-e. [DOI] [PubMed] [Google Scholar]
- Devon R, Doucette R. Olfactory ensheathing cells do not require L-ascorbic acid in vitro to assemble a basal lamina or to myelinate dorsal root ganglion neurites. Brain Res. 1995;688:223–229. doi: 10.1016/0006-8993(95)00562-5. [DOI] [PubMed] [Google Scholar]
- Doucette R. Glial influences on axonal growth in the primary olfactory system. Glia. 1990;3:433–449. doi: 10.1002/glia.440030602. [DOI] [PubMed] [Google Scholar]
- Doucette R. Olfactory ensheathing cells: potential for glial cell transplantation into areas of CNS injury. Histol Histopathol. 1995;10:503–507. [PubMed] [Google Scholar]
- Duncan ID, Hammang JP, Jackson KF, Wood PM, Bunge RP, Langford L. Transplantation of oligodendrocytes and Schwann cells into the spinal cord of the myelin-deficient rat. J Neurocytol. 1988;17:351–360. doi: 10.1007/BF01187857. [DOI] [PubMed] [Google Scholar]
- Felts PA, Smith KJ. Conduction properties of central nerve fibers remyelinated by Schwann cells. Brain Res. 1992;574:178–192. doi: 10.1016/0006-8993(92)90815-q. [DOI] [PubMed] [Google Scholar]
- Franceschini IA, Barnett SC. Low-affinity NGF-receptor and E-N-CAM expression define two types of olfactory nerve ensheathing cells that share a common lineage. Dev Biol. 1996;173:327–343. doi: 10.1006/dbio.1996.0027. [DOI] [PubMed] [Google Scholar]
- Franklin RJ, Blakemore WF. Requirements for Schwann cell migration within CNS environments: a viewpoint. Int J Dev Neurosci. 1993;11:641–649. doi: 10.1016/0736-5748(93)90052-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin RJ, Gilson JM, Franceschini IA, Barnett SC. Schwann cell-like myelination following transplantation of an olfactory bulb-ensheathing cell line into areas of demyelination in the adult CNS. Glia. 1996;17:217–224. doi: 10.1002/(SICI)1098-1136(199607)17:3<217::AID-GLIA4>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Ghatak NR, Hirano A, Doron Y, Zimmerman HM. Remyelination in multiple sclerosis with peripheral type myelin. Arch Neurol. 1973;29:262–267. doi: 10.1001/archneur.1973.00490280074011. [DOI] [PubMed] [Google Scholar]
- Gledhill RF, Harrison BM, McDonald WI. Pattern of remyelination in the CNS. Nature. 1973;244:443–444. doi: 10.1038/244443a0. [DOI] [PubMed] [Google Scholar]
- Graziadei PP, Levine RR, Monti Graziadei GA. Plasticity of connections of the olfactory sensory neuron: regeneration into the forebrain following bulbectomy in the neonatal mouse. Neuroscience. 1979;4:713–727. doi: 10.1016/0306-4522(79)90002-2. [DOI] [PubMed] [Google Scholar]
- Groves AK, Barnett SC, Franklin RJ, Crang AJ, Mayer M, Blakemore WF, Noble M. Repair of demyelinated lesions by transplantation of purified O-2A progenitor cells. Nature. 1993;362:453–455. doi: 10.1038/362453a0. [DOI] [PubMed] [Google Scholar]
- Guitteny AF, Fouque B, Mougin C, Teoule R, Bloch B. Histological detection of messenger RNAs with biotinylated synthetic oligonucleotide probes. J Histochem Cytochem. 1988;36:563–571. doi: 10.1177/36.6.3259249. [DOI] [PubMed] [Google Scholar]
- Gumpel M, Lachapelle F, Gansmuller A, Baulac M, Baron van Evercooren A, Baumann N. Transplantation of human embryonic oligodendrocytes into shiverer brain. Ann NY Acad Sci. 1987;495:71–85. doi: 10.1111/j.1749-6632.1987.tb23666.x. [DOI] [PubMed] [Google Scholar]
- Honmou O, Felts PA, Waxman SG, Kocsis JD. Restoration of normal conduction properties in demyelinated spinal cord axons in the adult rat by transplantation of exogenous Schwann cells. J Neurosci. 1996;16:3199–3208. doi: 10.1523/JNEUROSCI.16-10-03199.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi T, Lankford KL, Waxman SG, Greer CA, Kocsis JD. Transplanted olfactory ensheathing cells remyelinate and enhance axonal conduction in the demyelinated dorsal columns of the rat spinal cord. J Neurosci. 1998;18:6176–6185. doi: 10.1523/JNEUROSCI.18-16-06176.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi T, Lankford KL, Kocsis JD. Transplantation of olfactory ensheathing cells or Schwann cells restores rapid and secure conduction across the transected spinal cord. Brain Res. 2000;854:70–78. doi: 10.1016/s0006-8993(99)02285-4. [DOI] [PubMed] [Google Scholar]
- Itoyama Y, Ohnishi A, Tateishi J, Kuroiwa Y, Webster HD. Spinal cord multiple sclerosis lesions in Japanese patients: Schwann cell remyelination occurs in areas that lack glial fibrillary acidic protein (GFAP) Acta Neuropathol. 1985;65:217–223. doi: 10.1007/BF00687001. [DOI] [PubMed] [Google Scholar]
- Keirstead HS, Ben-Hur T, Rogister B, O’Leary MT, Dubois-Dalcq M, Blakemore WF. Polysialylated neural cell adhesion molecule-positive CNS precursors generate both oligodendrocytes and Schwann cells to remyelinate the CNS after transplantation. J Neurosci. 1999;19:7529–7536. doi: 10.1523/JNEUROSCI.19-17-07529.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi AD, Bunge RP. Studies of myelin formation after transplantation of human Schwann cells into the severe combined immunodeficient mouse. Exp Neurol. 1994;130:41–52. doi: 10.1006/exnr.1994.1183. [DOI] [PubMed] [Google Scholar]
- Li Y, Field PM, Raisman G. Repair of adult rat corticospinal tract by transplants of olfactory ensheathing cells. Science. 1997;277:2000–2002. doi: 10.1126/science.277.5334.2000. [DOI] [PubMed] [Google Scholar]
- McDonald WI. Overview of clinical aspects of multiple sclerosis, including cognitive deficit. In: Waxman SG, Kocsis JD, Stys PK, editors. The axon: structure, function and pathophysiology. New York: Oxford University Press; 1995. pp. 661–668. [Google Scholar]
- Morrissey TK, Levi AD, Nuijens A, Sliwkowski MX, Bunge RP. Axon-induced mitogenesis of human Schwann cells involves heregulin and p185erbB2. Proc Natl Acad Sci USA. 1995;92:1431–1435. doi: 10.1073/pnas.92.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton DG. Dynamics of cell populations in the olfactory epithelium. Ann NY Acad Sci. 1974;237:52–61. doi: 10.1111/j.1749-6632.1974.tb49843.x. [DOI] [PubMed] [Google Scholar]
- Prineas JW, Connell F. Remyelination in multiple sclerosis. Ann Neurol. 1979;5:22–31. doi: 10.1002/ana.410050105. [DOI] [PubMed] [Google Scholar]
- Ramon-Cueto A, Nieto-Sampedro M. Regeneration into the spinal cord of transected dorsal root axons is promoted by ensheathing glia transplants. Exp Neurol. 1994;127:232–244. doi: 10.1006/exnr.1994.1099. [DOI] [PubMed] [Google Scholar]
- Ramon-Cueto A, Valverde F. Olfactory bulb ensheathing glia: a unique cell type with axonal growth-promoting properties. Glia. 1995;14:163–173. doi: 10.1002/glia.440140302. [DOI] [PubMed] [Google Scholar]
- Smith EJ, Blakemore WF, McDonald WI. Central remyelination restores secure conduction. Nature. 1979;280:395–396. doi: 10.1038/280395a0. [DOI] [PubMed] [Google Scholar]
- Targett MP, Blakemore WF. The use of xenografting to evaluate the remyelinating potential of glial cell cultures. Eye. 1994;8:238–244. doi: 10.1038/eye.1994.52. [DOI] [PubMed] [Google Scholar]
- Targett MP, Sussman J, Scolding N, O’Leary MT, Compston DA, Blakemore WF. Failure to achieve remyelination of demyelinated rat axons following transplantation of glial cells obtained from the adult human brain. Neuropathol Appl Neurobiol. 1996;22:199–206. [PubMed] [Google Scholar]
- Utzschneider DA, Archer DR, Kocsis JD, Waxman SG, Duncan ID. Transplantation of glial cells enhances action potential conduction of amyelinated spinal cord axons in the myelin-deficient rat. Proc Natl Acad Sci USA. 1994;91:53–57. doi: 10.1073/pnas.91.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittemore SR, Sanon HR, Wood PM. Concurrent isolation and characterization of oligodendrocytes, microglia and astrocytes from adult human spinal cord. Int J Dev Neurosci. 1993;11:755–764. doi: 10.1016/0736-5748(93)90064-k. [DOI] [PubMed] [Google Scholar]
- Yandava BD, Billinghurst LL, Snyder EY. Global cell replacement is feasible via neural stem cell transplantation: evidence from the dysmyelinated shiverer mouse brain. Proc Natl Acad Sci USA. 1999;96:7029–7034. doi: 10.1073/pnas.96.12.7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Firestein S, Greer CA. NADPH-diaphorase localization in the olfactory system. Neuroreport. 1994;6:149–152. doi: 10.1097/00001756-199412300-00038. [DOI] [PubMed] [Google Scholar]