Abstract
Transplantation of mesenchymal stem cells (MSCs) prepared from adult bone marrow (BMSCs) has been reported to ameliorate functional deficits in several CNS diseases in experimental animal models. Bone marrow was enriched in MSCs by selecting for plastic-adherent cells that were grown to confluency in appropriate culture conditions as flattened fibroblast-like cells. Despite the fact that the stem/precursor cells in peripheral blood are widely used for reconstruction in the hematopoietic system, it is not fully understood whether peripheral blood-derived plastic-adherent precursor/stem cells (PMSCs) can differentiate into a neural lineage. To compare the potential of PMSCs and BMSCs for neural differentiation in vitro, BMSCs and PMSCs were prepared from the adult rat and expanded in culture. Although the growth rate of PMSCs was less than BMSCs, immunocytochemical and RT-PCR analyses indicated that both MSC types were successfully induced to nestin-positive neurospheres in the presence of EGF and bFGF. After withdrawal of the mitogens, these cells could differentiate into neurofilament-positive neurons or GFAP-positive glia. Thus, our findings suggest the potential use of PMSCs for a cell therapy in CNS diseases.
Keywords: Blood, Bone marrow, Mesenchymal stem cell, Transplantation
1. Introduction
Mesenchymal stem cells (MSCs) are thought to represent a very small proportion of cells in the mononuclear population of bone marrow. As originally described by Freidenstein (1976), these cells will grow to confluency in appropriate culture conditions as flattened fibroblast-like cells (Majumdar et al., 1998), and have been suggested to differentiate into bone, cartilage (Kobune et al., 2003), cardiac myocytes (Toma et al., 2002) and neurons and glia (Prockop, 1997; Woodbury et al., 2000; Kobune et al., 2003; Iihoshi et al., 2004; Honma et al., 2006; Nomura et al., 2005) both in vitro and in vivo. MSCs prepared from human bone marrow (BMSCs) have been used in clinical studies for metachromatic leukodystrophy (Koc et al., 2002), Hurler syndrome (Koc et al., 2002), myeloablative therapy for breast cancer (Koc et al., 2000), graft-versus-host disease (Aggarwal and Pittenger, 2005), and stroke (Bang et al., 2005).
Human mesenchymal precursor cells found in the blood of normal subjects proliferated in culture with an adherent-spread morphology, and displayed cytoskeletal, cytoplasmic and surface markers (CD34-, CD45-, and CD105+) of mesenchymal precursors (Zvaifler et al., 2000). These cells had a capacity for differentiation into fibroblast, osteoblast, and adipocyte lineages. A canine CD34- fibroblast-like cell in the peripheral blood showed mesenchymal stem cell characteristics (Huss et al., 2000). Because peripheral blood is readily accessible, stem cells isolated from blood may be a good candidate for a cell therapy. For example, the hematopoietic stem/precursor cells in peripheral blood are widely used for reconstruction in the hematopoietic system (Brown et al., 1997; Auner et al., 2005).
Thus, although the potential of mesenchymal precursor cells in peripheral blood (PMSCs) has received much attention, it is not known whether peripheral blood-derived plastic-adherent precursor cells (PMSCs) can differentiate into a neural lineage. Here, we compared the growth properties and differentiating potential to neural lineages of rat PMSCs with those of rat BMSCs.
2. Results
2.1. Characteristics of BMSCs and PMSCs
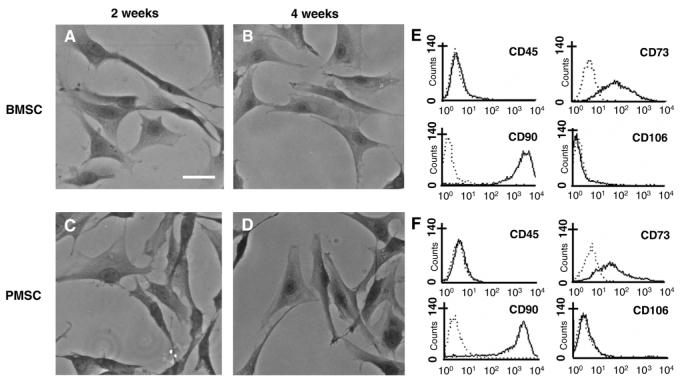
After removing nonadherent cells by replacing the medium (day 2 in culture), a small portion of attached nucleated cells was visualized in the BMSC culture dish. By day 14 in culture, the attached BMSCs had developed into an adherent layer containing abundant dispersed fibroblast-like cells, and each colony was predominantly formed by several fibroblast-like cells (Fig. 1A). By day 28 in culture, the BMSCs had proliferated and tended to form a near continuous layer comprising mainly fibroblast-like cells (Fig. 1B).
Fig. 1.
Phase-contrast photomicrograph of May-Giemsa stained BMSCs (A) and PMSCs (B) at 2 and 4 weeks in culture, respectively. Flow cytometric analysis of cultured BMSCs (E) and PMSCs (F) with CD45, CD73, CD90, and CD106 antibodies. Dotted lines in each panel indicate isotype-matched mouse IgG antibody control staining. Scale bar=10 μm.
In the cultures of PMSCs derived from peripheral blood, fibroblast-like cells with thin elongated processes around a central nucleus made their appearance at 2 weeks after culture initiation (Fig. 1C). By day 28 in culture, the cells also continued proliferating and formed a layer of flattened cells (Fig. 1D), with morphological features resembling those of BMSCs.
Figs. 1E and F are flow cytometric data of the expression of surface antigens on BMSCs and PMSCs, respectively. These results show that both BMSCs and PMSCs express a similar pattern of surface antigens: CD45-, CD73+, CD90+, and CD106-.
2.2. Growth rate
The number of BMSCs and PMSCs was counted at weekly intervals in order to characterize the proliferation rate (Fig. 2). BMSCs slowly proliferated in the initial 2 weeks, and entered a rapid growth phase for the next 4 weeks. Proliferation of BMSCs became slower after 6 weeks, but cell number was maintained for the next 2 weeks. The number of BMSCs increased more than 4 logs for cultures maintained for 8 weeks. In contrast, PMSCs displayed slow but constant growth over 8 weeks in culture, and expanded over 6-fold.
Fig. 2.

Culture expansion of BMSCs (black) and PMSCs (open square). The cell numbers of both MSCs were counted at each week. Error bars represent one SD from the mean. *p<0.05 (n=16).
2.3. Transformation of MCSs to neurospheres
BMSCs transformed to nestin-positive neurospheres using an induction protocol (Fig. 3A) described in Experimental procedures. BMSCs began forming floating cell masses and nestin positivity when they were inhibited from adhering to the culture dishes (non-treated dishes) and maintained in the appropriate medium and growth factors (see Experimental procedures). More than 90% of MSCs transformed to neurospheres. RT-PCR analysis for nestin mRNA expression in cDNA samples of cultured adherent BMSCs (Fig. 3E-a) and floating spheric cells (neurospheres) (Fig. 3E-b) are shown in Fig. 3E. The floating spheric cells displayed an amplification of a PCR fragment of the expected size for nestin (420–430 bp), but the cultured non-transformed BMSCs did not (Fig. 3F-a). PMSCs also showed similar transformation to nestin-positive neurospheres after induction (Fig. 3B), which was confirmed by RT-PCR (Fig. 3F-b).
Fig. 3.

Transformation from MSCs to nestin-positive neurospheres. When BMSCs (A) and PMSCs (B) were placed in NPBM with growth factors and were inhibited to adhere on the culture dish, the cells formed neurospheres (scale bar=20 μm). RT-PCR analysis demonstrated that neurospheres transformed from BMSCs showed nestin positivity (E-b), which was negative before transformation (E-a). Nestin also became positive following transformation of PMSCs (F-b), which was negative in the primary PMSCs (E-a). Panels C and D showed control mRNA expression of β-actin of BMSCs and PMSCs, respectively.
2.4. Differentiation from MSC-derived neurosphere to neural cells
MSC-derived neurospheres differentiated into neuron- and glia-like cells in the appropriate culture condition. BMSC-derived neurospheres differentiated into adherent neural cells when they were mechanically dissociated, plated on plastic culture dish, and maintained in NPBM without growth factors. Adherent single layers contained abundant neuron- and glial-like cells. Immunocytochemical analysis indicated that the neuronal cells showed NF-M positivity (Fig. 4A), which was confirmed by RT-PCR. A sample of adherent cells displayed an amplification of a PCR fragment of the expected size for NF-M (330–340 bp) (Fig. 4E-b). In addition, GFAP-positive cell differentiation was also demonstrated with immunostaining (Fig. 5A) and RT-PCR analysis (Fig. 5E-b). The expected size for an amplified PCR fragment of GFAP is 430–440 bp. PMSC-derived neurosphere showed similar differentiating potential to NF-M-positive neurons (Fig. 4B) and GFAP-positive glia (Fig. 5B), which were confirmed by RT-PCR in Figs. 4F-b and 5F-b, respectively.
Fig. 4.
Neurofilament expression in differentiated neurosphere cells. Cells differentiated from neurospheres which had been transformed from BMSCs (A) or PMSCs (B) showed NF-M positivity in culture. RT-PCR analysis demonstrated that BMSCs (A) and PMSCs (B) differentiated from neurospheres showed NF-M positivity (E-b; F-b), which was negative in neurospheres (E-a; F-a). Panels C and D showed control mRNA expression of β-actin of BMSCs and PMSCs, respectively. Scale bar=10 μm.
Fig. 5.
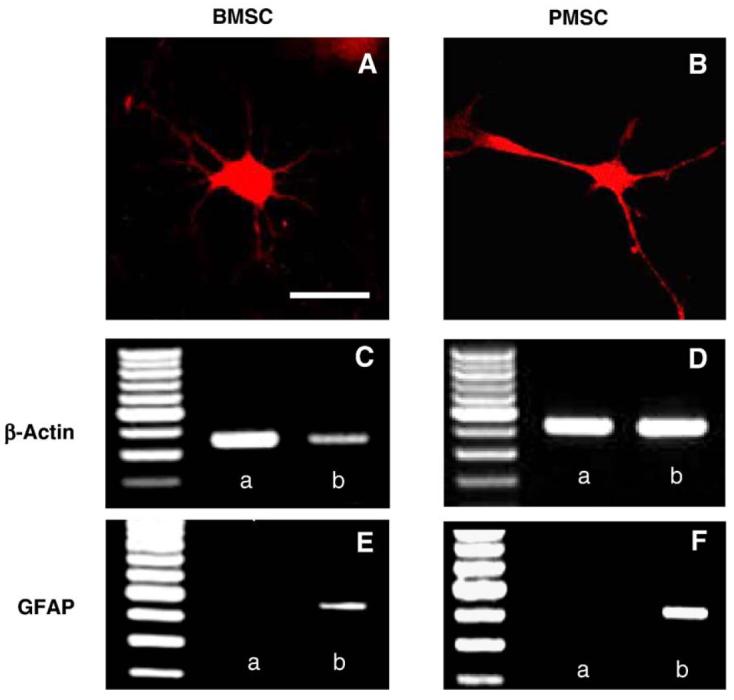
GFAP expression in differentiated neurosphere cells. Immunocytochemical analysis indicated that BMSCs (A) and PMSCs (B) differentiated from neurospheres showed GFAP positivity in culture. RT-PCR analysis demonstrated that cells differentiated from neurospheres which had been transformed from BMSCs showed the GFAP positivity (E-b), which was negative in neurospheres (E-a). GFAP also became positive following differentiation in the PMSCs group (F-b), which was negative before induction (F-a). Panels C and D demonstrated the mRNA expression of β-actin of BMSCs and PMSCs for control, respectively. Scale bar=10 μm.
3. Discussion
The present study demonstrates that fibroblast-like adherent cells with phenotypic characteristics resembling those of mesenchymal stem cells prepared from the bone marrow can be cultured from rat peripheral blood. These cells showed proliferation and differentiation into neural lineages in vitro, confirmed by immunocytochemistry and RT-PCR.
Mesenchymal precursor cell populations obtained from rat peripheral blood and bone marrow of the rat metaphysis easily expanded in vitro and exhibited a fibroblast-like morphology. Flow cytometry analysis to study the surface protein expression on undifferentiated BMSCs and PMSCs indicated that the myeloid progenitor antigen CD45 was not expressed by these cells. On the other hand, PMSCs and BMSCs expressed CD73 (SH3), which has been used to characterize mesenchymal stem cells (Majumdar et al., 1998; Kobune et al., 2003; Honma et al., 2006). In addition, nestin expression by PMSCs and BMSCs and their ability to grow in suspension in defined culture conditions brought them nearer to a neurosphere phenotype. When nestin-positive neurospheres were dissociated and plated onto an adherent surface without growth factors, neuronal and glial differentiation was observed. We are not certain from our results whether strictly regulated neuronal and glial cells were present in the PMSC population, or culture conditions determined cell fate into neurons or glia.
A distinct difference between PMSCs and BMSCs in the present study is their growth rate in culture. The BMSCs showed a sigmoidal growth curve and extensive expansion over 8 weeks. In contrast, the PMSCs had a more linear growth curve and were much less proliferative. A possible explanation is that samples of BMSCs contain more primitive progenitor cells than samples of PMSCs. Although the CD34 antigen has been used to identify hematopoietic precursor cells, CD34+ cells were present in the circulation in only one-tenth the concentration of bone marrow and had a different spectrum of antigen expression. Indeed, CD33 (myeloid antigen) and CD45 (leukocyte common antigen) were highly expressed in CD34+ cells in the peripheral circulation, but not in bone marrow (Bender et al., 1991).
Another hypothesis is that PMSCs require specific culture medium or growth factors which are different from those of BMSCs. To maintain viability and retain the ability to proliferate, stem cells need cell-to-cell contact and a microenvironment which provides necessary growth factors in adequate quantity, which may be supplied in an autocrine or paracrine fashion (Huss et al., 2000). The PMSCs in mobilized peripheral blood required BMSC conditioned medium which showed high proliferative potential with high express of Oct4, a transcriptional binding factor (Tondreau et al., 2005). Moreover, mesenchymal precursor cells in the peripheral blood of normal individuals proliferated logarithmically in 20% fetal calf serum without growth factor (Zvaifler et al., 2000). Thus, PMSCs may require a novel culture medium. The difference between cultures of BMSCs and PMSCs will be important in future considerations of PMSCs for clinical use, where a large number of cells will be necessary.
The morphological and antigenic characteristics of MSCs are different among species (Kobune et al., 2003; Honma et al., 2006; Rochefort et al., 2005), which may indicate that the appropriate culture medium or growth factors of human PMSCs is different from rat PMSCs. Nevertheless, in the present study, we showed that the rat PMSCs proliferated, highly transformed to nestin-positive neural stem cells (neurospheres), and differentiated into neuronal or glial cells in vitro.
Thus, autologous peripheral blood could be an important source of cells for a cell therapy, since they are easy to isolate and expand for autotransplantation with little risk of rejection. However, further investigations are needed to better characterize PMSCs and as well to further define their transplantability and differentiation potential in animal models.
4. Experimental procedures
4.1. Cell preparation
The use of animals in this study was approved by the animal care and use committee of Sapporo Medical University and all procedures were carried out in accordance with institutional guidelines. Bone marrow was obtained from femoral bone in adult female Sprague-Dawley rats weighing 200–250 g. Rats were anesthetized with ketamine (50 mg/kg) and xylazine (10 mg/kg) i.p. A small hole (2×3 mm) in the femoral bone was made with an air drill following skin incision (1 cm). Bone marrow (0.5 ml) was aspirated, diluted to 25 ml with Dulbecco’s modified Eagle’s medium (DMEM) (SIGMA, St. Louis, MO) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (GibcoBRL, Grand Island, NY), 2 mM L-glutamine (Gibco BRL), 100 U/ml penicillin, 0.1 mg/ml streptomycin (Gibco BRL), was plated on 50-cm2 Tissue Culture Dish (IWAKI, Tokyo, Japan), and incubated in a humidified atmosphere of 5% CO2 at 37 °C for 3 days. BMSCs, when selected by plastic adhesion, require the elimination of nonadherent cells by replacing the medium 48 h after cell seeding. When cultures almost reached confluence, the adherent cells were detached with trypsin—EDTA solution (SIGMA) and subcultured at 1×104 cells/ml.
Peripheral blood was obtained from adult Sprague—Dawley rats weighting 200–250 g. Rats were deeply anesthetized with ketamine (50 mg/kg) and xylazine (10 mg/kg) i.p. Peripheral blood (about 8 ml) was aspirated from vena cava superior with a 18-gauge needle. Peripheral blood was diluted 1:3 in Puregene RBC Lysis Solution (Gentra systems, Minneapolis, MN) and was incubated in a 50-ml conical centrifuge tube for 5 min at room temperature. The tube was centrifuged at 3500 rpm for 2 min and the supernatant was discarded. The cell pellet was suspended in DMEM supplemented with 10% FBS, 2 mM L-glutamine, 100 U/ml penicillin, 0.1 mg/ml streptomycin and was plated on 50-cm2 plastic tissue culture dishes and incubated in a humidified atmosphere of 5% CO2 at 37 °C. PMSCs, when selected by plastic adhesion, require the elimination of nonadherent cells by replacing the medium 48 h after cell seeding. When cultures almost reached confluence, the adherent cells were detached with trypsin—EDTA solution and subcultured at 1×104 cells/ml. The cell numbers of both BMSC and PMSC were counted in a cytometer every a week.
Some of cultured cells were rinsed in PBS for three times and fixed for 10 min with a fixative solution containing 4% paraformaldehyde in 0.14 M Sorensen’s phosphate buffer, pH 7.4, at room temperature. The cells were counterstained with May-Giemsa (Honma et al., 2006), and phase-contrast microphotographs were obtained using a Zeiss microscope.
4.2. Phenotypic characterization
Flow cytometric analysis of BMSCs and PMSCs was performed as previously described (Honma et al., 2006). Briefly, cell suspensions were washed twice with phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin (BSA). For direct assays fifty thousand cells were incubated with FITC-conjugated CD45 (Leukocyte Common Antigen) (BD Bioscience Pharmingen, San Jose, CA), PE-conjugated CD 73 (Ecto-5′-nucleotidase) (BD Bioscience Pharmingen), PE-conjugated CD 90 (Thy-1) (eBioscience, San Diego, CA) and PE-conjugated CD106 (VCAM-1) (BD Bioscience Pharmingen) at 4 °C for 30 min, and then washed twice with PBS containing 0.1% BSA. The cells were analyzed by cytometric analysis using a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA) with the use of CellQuest software.
4.3. Induction of MSCs to floating spheric cells
When inducing MSCs to floating spheric cells like neurospheres, MSCs were detached with trypsin—EDTA solution and were collected in a 50-ml tube in DMEM+10% FBS. After rinsing with DMEM, cells (1×106 cells/ml) were suspended in 20 ml Neural Progenitor basal medium (NPBM) (Cambrex, One Meadowlands Plaza, NJ) supplemented with 2 mM L-glutamine, 10 ng/ml epidermal growth factor (EGF), 10 ng/ml basic fibroblast growth factor (bFGF), 100 U/ml penicillin, 0.1 mg/ml streptomycin, were plated on 100 mm non-treated dish (IWAKI), and were incubated for 3 days. Growth factors (EGF and bFGF) were added every day.
4.4. Differentiation of neurospheres to neural cells
When inducing the floating spheric cells (neurospheres) to neural cells, floating spheric cells were collected by centrifuging at 1500 rpm for 5 min, suspended in NPBM supplemented with 2 mM L-glutamine, 100 U/ml penicillin, and 0.1 mg/ml streptomycin, mechanically dissociated, and were plated on plastic tissue culture dish for 1 week.
4.5. RT-PCR
Total RNA was extracted from each cell culture using RNeasy Mini Kit (QIAGEN, Hilden, Germany). We performed the reverse transcription with 100 ng RNA using the SuperScript II RNase H- reverse transcriptase (Invitrogen, Carlsbad, CA). Briefly, we used a final volume of 20 μl containing 100 ng RNA, 4 μl First Strand Buffer, 10 mM dNTPs, 100 mM DTT, 0.5 μg Oligo(dt)12–18 and 100 U of SuperScript II RNase H- reverse transcriptase. We then carried out a PCR reaction using the Hot Star Taq Master Mix Kit (QIAGEN) in a final volume of 50 μl containing 25 μl Hot Star Taq Master Mix, and 10 mM upstream sense and downstream sense primers. Cyclical parameters were denatured at 94 °C for 30 s, annealed at 60 °C for 30 s, and finally elongated at 72 °C for 30 s. Thirty five cycles were performed for each primer set. We resolved the PCR products on 2% gel agarose. Primer sequence of amplified products was: mouse β-actin sense (5′-TGGAATCCTGTGGCATCCATGAAAC-3′), mouse β-actin antisense (5′-TAAAACGCAGCTCAGTAACAGTCCG-3′), rat nestin sense (5′-CTTAGTCTGGAGGTGGCTACATACA-3′), rat nestin antisense (5′-GAGGATAGCAGAAGAACTAGGCACT-3′), rat neurofilament M (NF-M) sense (5′-GGTCACTTCACATGCCATAGTCAA-3′), rat NF-M antisense (5′-GGCTCAGTTGGTACTTTGCGTAA-3′), rat glial fibrillary acid protein (GFAP) sense (5′-ATTCCGCGCCTCTCCCTGTCTC-3′), and rat GFAP antisense (5′-GCTTCATCCGCCTCCTGTCTGT-3′).
4.6. Immunocytochemical analysis
To identify the cell type derived from the BMSCs and PMSCs, immunocytochemical studies were performed with the use of antibodies to neurons (1:400 monoclonal mouse NF-M, SIGMA), and astrocytes (1:400 monoclonal mouse anti-GFAP, SIGMA). Cultured cells were rinsed in PBS for three times and fixed for 10 min with a fixative solution containing 4% paraformaldehyde in 0.14 M Sorensen’s phosphate buffer, pH 7.4, at room temperature. After washing twice in PBS and incubating in PBS containing 0.1% Triton X-100 for 10 min at room temperature, fixed cells were incubated for 30 min in a blocking solution containing 0.1% Triton X-100, and 3% BSA before incubation with the primary antibody. Primary antibodies are labeled with Alexa Fluor 488 or Alexa Fluor 594 using Zenon mouse IgG Labeling Kits (Molecular Probes Inc., Eugene, OR) according to the manufacturer’s instruction. After immunostaining, cover-slips were mounted cell-side down on microscope slides using mounting medium (DAKO Corp., Carpinteria, CA). Confocal images were obtained using a Zeiss laser scanning confocal microscope with the use of Zeiss software.
4.7. Data analysis
All data are presented as mean values±SD. Differences among groups were assessed by ANOVA with Scheffe’s post hoc test to identify individual group differences. Differences were deemed statistically significant at P<0.05.
Acknowledgments
This work was supported in part by grants from the Japanese Ministry of Education, Science, Sports and Culture (16390414, 16591450, 16659393), Mitsui Sumitomo Insurance Welfare Foundation, the National Multiple Sclerosis Society (USA) (RG2135; CA1009A10), the National Institutes of Health (NS43432), and the Medical and Rehabilitation and Development Research Services of the Department of Veterans Affairs.
REFERENCES
- Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- Auner HW, Zebisch A, Ofner P, Sill H, Linkesch W, Krause R. Evaluation of potential risk factors for early infectious complications after autologous peripheral blood stem cell transplantation in patients with lymphoproliferative diseases. Ann. Hematol. 2005;84:532–537. doi: 10.1007/s00277-005-1025-5. [DOI] [PubMed] [Google Scholar]
- Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann. Neurol. 2005;57:874–882. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- Bender JG, Unverzagt KL, Walker DE, Lee W, Van Epps DE, Smith DH, Stewart CC, To LB. Identification and comparison of CD34-positive cells and their subpopulations from normal peripheral blood and bone marrow using multicolor flow cytometry. Blood. 1991;77:2591–2596. [PubMed] [Google Scholar]
- Brown RA, Adkins D, Goodnough LT, Haug JS, Todd G, Wehde M, Hendricks D, Ehlenbeck C, Laub L, DiPersio J. Factors that influence the collection and engraftment of allogeneic peripheral-blood stem cells in patients with hematologic malignancies. J. Clin. Oncol. 1997;15:3067–3074. doi: 10.1200/JCO.1997.15.9.3067. [DOI] [PubMed] [Google Scholar]
- Friedenstein AJ. Precursor cells of mechanocytes. Int. Rev. Cytol. 1976;47:327–359. doi: 10.1016/s0074-7696(08)60092-3. [DOI] [PubMed] [Google Scholar]
- Honma T, Honmou O, Iihoshi S, Harada K, Houkin K, Hamada H, Kocsis JD. Intravenous infusion of immortalized human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Exp. Neurol. 2006;199:56–66. doi: 10.1016/j.expneurol.2005.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huss R, Lange C, Weissinger EM, Kolb HJ, Thalmeier K. Evidence of peripheral blood-derived, plastic-adherent CD34(-/low) hematopoietic stem cell clones with mesenchymal stem cell characteristics. Stem Cells. 2000;18:252–260. doi: 10.1634/stemcells.18-4-252. [DOI] [PubMed] [Google Scholar]
- Iihoshi S, Honmou O, Houkin K, Hashi K, Kocsis JD. A therapeutic window for intravenous administration of autologous bone marrow after cerebral ischemia in adult rats. Brain Res. 2004;1007:1–9. doi: 10.1016/j.brainres.2003.09.084. [DOI] [PubMed] [Google Scholar]
- Kobune M, Kawano Y, Ito Y, Chiba H, Nakamura K, Tsuda H, Sasaki K, Dehari H, Uchida H, Honmou O, Takahashi S, Bizen A, Takimoto R, Matsunaga T, Kato J, Kato K, Houkin K, Niitsu Y, Hamada H. Telomerized human multipotent mesenchymal cells can differentiate into hematopoietic and cobblestone area-supporting cells. Exp. Hematol. 2003;31:715–722. doi: 10.1016/s0301-472x(03)00177-2. [DOI] [PubMed] [Google Scholar]
- Koc ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE, Caplan AI, Lazarus HM. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J. Clin. Oncol. 2000;18:307–316. doi: 10.1200/JCO.2000.18.2.307. [DOI] [PubMed] [Google Scholar]
- Koc ON, Day J, Nieder M, Gerson SL, Lazarus HM, Krivit W. Allogeneic mesenchymal stem cell infusion for treatment of metachromatic leukodystrophy (MLD) and Hurler syndrome (MPS-IH) Bone Marrow Transplant. 2002;30:215–222. doi: 10.1038/sj.bmt.1703650. [DOI] [PubMed] [Google Scholar]
- Majumdar MK, Thiede MA, Mosca JD, Moorman M, Gerson SL. Phenotypic and functional comparison of cultures of marrow-derived mesenchymal stem cells (MSCs) and stromal cells. J. Cell. Physiol. 1998;176:57–66. doi: 10.1002/(SICI)1097-4652(199807)176:1<57::AID-JCP7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Nomura T, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD. I.V. infusion of brain-derived neurotrophic factor gene-modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Neuroscience. 2005;136:161–169. doi: 10.1016/j.neuroscience.2005.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- Rochefort GY, Vaudin P, Bonnet N, Pages JC, Domenech J, Charbord P, Eder V. Influence of hypoxia on the domiciliation of mesenchymal stem cells after infusion into rats: possibilities of targeting pulmonary artery remodeling via cells therapies? Respir. Res. 2005;6:125. doi: 10.1186/1465-9921-6-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- Tondreau T, Meuleman N, Delforge A, Dejeneffe M, Leroy R, Massy M, Mortier C, Bron D, Lagneaux L. Mesenchymal stem cells derived from CD133-positive cells in mobilized peripheral blood and cord blood: proliferation, Oct4 expression, and plasticity. Stem Cells. 2005;23:1105–1112. doi: 10.1634/stemcells.2004-0330. [DOI] [PubMed] [Google Scholar]
- Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J. Neurosci. Res. 2000;61:364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Zvaifler NJ, Marinova-Mutafchieva L, Adams G, Edwards CJ, Moss J, Burger JA, Maini RN. Mesenchymal precursor cells in the blood of normal individuals. Arthritis Res. 2000;2:477–488. doi: 10.1186/ar130. [DOI] [PMC free article] [PubMed] [Google Scholar]