Summary
The enteric pathogen Toxoplasma gondii is controlled by a vigorous innate Th1 response in the murine model. We demonstrate that following oral infection the parasite rapidly recruits inflammatory monocytes (Gr1+ Ly6C+ Ly6G− F4/80+ CD11b+ CD11c−), which establish a vital defensive perimeter within the villi of the ileum in the small intestine. Knock out mice lacking the chemokine receptor CCR2 or the ligand MCP-1, failed to recruit Gr1+ (Ly6C+) inflammatory monocytes, while dendritic cells and resident tissue macrophages remained unaltered. The selective lack of Gr1+ (Ly6C+) inflammatory monocytes resulted in an inability to control replication of the parasite, high influx of neutrophils, extensive intestinal necrosis, and rapid death. Adoptive transfer of sorted Gr1+ (Ly6C+) inflammatory monocytes demonstrated their ability to home to the ileum in infected animals and protect CCR2 −/− mice, which were otherwise highly susceptible to oral toxoplasmosis. Collectively, these findings illustrate the critical importance of inflammatory monocytes as a first line of defense in controlling intestinal pathogens.
Introduction
The intestinal mucosa serves an important role in innate defense against pathogens, which often subvert this barrier to cause systemic infection. Mucosal immunity relies on dendritic cells (DCs) that are resident in the gut and which are able to recognize foreign invaders and rapidly mount innate and adaptive responses. The majority of conventional DCs in the lamina propria of the mouse are phenotypically CD11c+ CD11b+ CD8α−, although substantial populations of CD11c+ CD11b− CD8α+ and CD11c+ CD11b− CD8α− cells also exist there (Mowat, 2003). The majority of DCs in the lamina propria also express the receptor for fractaline, CX3CR1, and these cells extend dendrites into the lumen of the intestine to sample bacterial antigens (Niess et al., 2005). DCs traffic from the lamina propria to the mesenteric lymph nodes and Peyer’s patches, and thus participate in oral tolerance as well as response to foreign antigens (Johansson and Kelsall, 2005). Peyer’s patches also contain a diverse array of DCs, including a specialized subset in the subepithelial zone that express CCR6 and respond to the chemokine CCL20 (MIP-3α)(Johansson and Kelsall, 2005). CCR6+ DCs are important in regulating mucosal responses to antigens sampled by M cells, which overlie the Peyer’s patch (Cook et al., 2000). A number of pathogens enter across M cells, and not surprisingly, CCR6+ DCs have been implicated in the activation of T-cells responses to pathogens such as Salmonella (Salazar-Gonzalez et al., 2006).
Macrophages also colonize the submucosal tissues (Schenk and Mueller, 2006), although less is known about their potential roles in combating enteric pathogens. Recent studies have demonstrated that murine monocytes are comprised of two distinct subpopulations with different phenotypes (Geissmann et al., 2008; Geissmann et al., 2003). Gr1+ CCR2+ CX3CR1lo monocytes are proinflammatory and after emerging from the bone marrow, home to sites of inflammation. Gr1+ monocytes also are able to differentiate to DCs (CD11c+) in vitro (Geissmann et al., 2008; Geissmann et al., 2003) and populate nonlymphoid tissues such as the lung and lamina propria (Varol et al., 2007). Following infection by Listeria, a CCR2-dependent population of CD11c+ DCs is recruited to the spleen where they are important for production of TNF-α and expression of iNOS (Serbina et al., 2003). The marked deficiency of Gr1+ monocytes observed in CCR2−/− mice is due to a failure of these cells to exit the bone marrow, hence leading to increased susceptibility to Listeria (Serbina and Pamer, 2006). The other major subset of monocytes (Gr1− CCR2− CX3CR1hi) sets up residence in the tissues where they perform important functions in surveillance (Auffray et al., 2007). Gr1+ is expressed on neutrophils and monocytes and the most common antibody used to examine this receptor, known as RB6, recognizes both Ly6C and Ly6G isoforms. More recently, distinct mAbs to these separate Ly6 isoforms have been used to separate these markers and allow selective identification of neutrophils (CD11b+ F4/80− Ly6Ghi Ly6Cmed) versus inflammatory monocytes (CD11b+ F4/80+ Ly6Chi Ly6G−) (Daley et al., 2007). Similar subsets of monocytes exist in humans; CD14+CD16− vs. CD14loCD16+, which represent inflammatory and surveillance populations, respectively (Geissmann et al., 2008; Schenk and Mueller, 2006), although their roles in mucosal immunity have not been extensively examined.
Following oral challenge with T. gondii, the parasite infects enterocytes in the ileum and also penetrates the epithelial barrier to enter the submucosal tissue where it encounters a variety of resident leukocytes (Barragan and Sibley, 2003; Courret et al., 2005). Within the lamina propria, the parasite encounters DCs (CD11c+ CD11b+) and macrophages (CD11c− CD11b+) and once infected, trafficking of these cells from the intestine may disseminate the parasite to distant sites including the CNS (Courret et al., 2005). Control of acute toxoplasmosis relies on a potent Th1 response that requires IL-12 and IFN-γ production, which are generated through both innate and adaptive responses (Buzoni-Gatel and Kasper, 2007; Roberts et al., 2007). Previous studies have emphasized the importance of splenic CD8α+ DCs in responding to parasite antigens through recognition by CCR5 (Aliberti et al., 2000) and TLR11 (Yarovinsky et al., 2005). Gr1+ monocytes have also previously been shown to be important in control of systemic toxoplasmosis in the murine model, albeit following i.p. inoculation (Robben et al., 2005). Despite the fact that T. gondii naturally causes infection by the oral route, the specific roles of DC or macrophage cell subsets in the early response to mucosal infection with T. gondii have not been examined.
In the present report, we studied the role of monocytes and DCs in innate immune responses within the lamina propria in the small intestine, the site of initial contact with the pathogen following oral infection. Using knock out mice specifically ablated in chemokines required for recruitment of inflammatory monocytes or subsets of mucosal DCs, we demonstrate an unexpected yet crucial role for inflammatory monocytes in the control of infection in the gut. Moreover, adoptively transferred inflammatory monocytes were able to home to the lamina propria and protect mice from lethal ileitis that otherwise developed in CCR2−/− mice. Collectively, these studies demonstrate an important role for inflammatory monocytes in innate mucosal immunity.
Results
CCR2 and MCP-1 are Critical for Resistance to Oral Toxoplasmosis
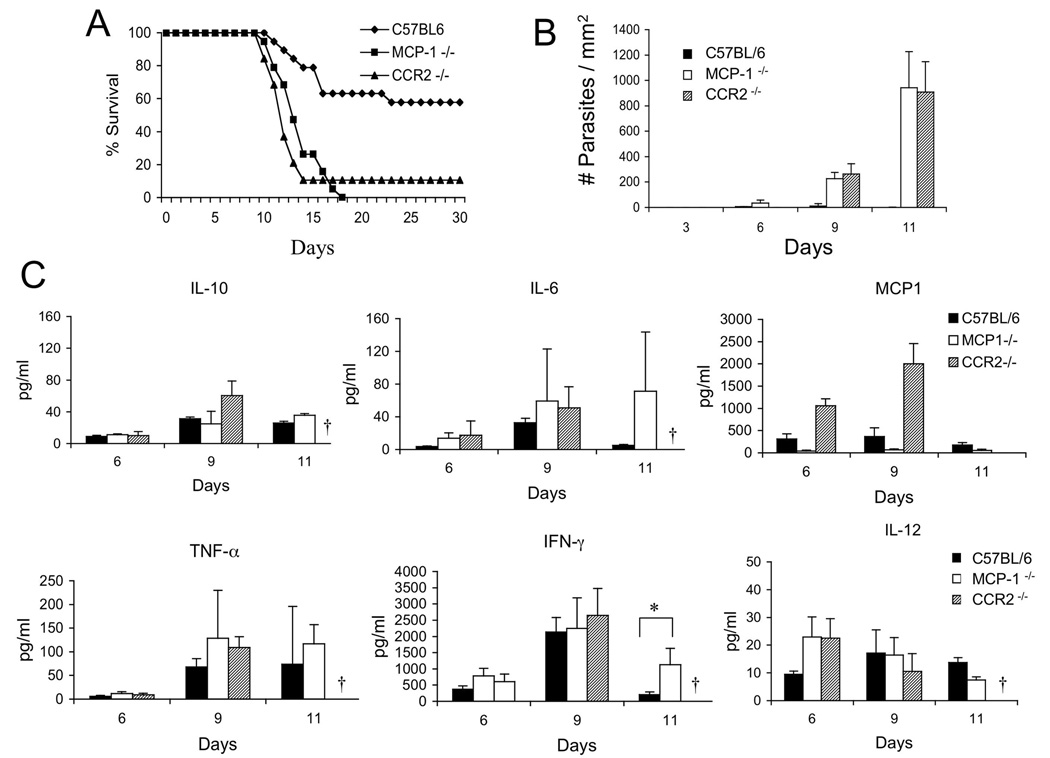
Previous studies have shown that Gr1+ monocytes, which express the chemokine receptor CCR2, are recruited by MCP-1 to the peritoneal cavity following i.p. challenge with T. gondii (Robben et al., 2005). However these studies bypassed the normal oral route of infection that begins with invasion into enterocytes, migration across the basement membrane, and entry into the lamina propria (Barragan and Sibley, 2003; Courret et al., 2005). Gr1+ monocytes have not previously been reported to participate in mucosal defense, raising the question whether they would be required following the natural route of infection by T. gondii. Oral challenge with tissue cysts of T. gondii resulted in limited mortality in wild type C57BL/C mice, whereas MCP-1−/− and CCR2−/− mice were extremely susceptible and succumbed to infection with in 12–15 days (Fig. 1A). Extremely high numbers of parasites were detected beginning on day 9 and continuing on day 11 post-infection in frozen sections of the ileum from CCR2−/− and MCP-1−/− mice when compared to control C57BL/6 mice (Fig. 1B). Following oral challenge with T. gondii, the infection is not confined to the intestine but instead quickly becomes systemic. Parasites were also detected in the spleen by day 9, however the levels of parasites were much lower than in the intestine and were not as highly elevated in CCR2−/− or MCP-1−/− mice (less than 3 fold) when compared to control C57BL/6 mice (Fig. S1).
Figure 1. Survival of mice following oral challenge with T. gondii depends on MCP-1 and CCR2.
(A) Survival of mice after peroral infection with T. gondii. Wild type C57BL/6 mice largely survived challenge while the majority of CCR2−/− and MCP-1−/− mice succumbed by day 15. Combined results of four experiments (3–6 animals per group, n=19).
(B) Tissue burdens in the ileum of infected mice. Oral challenge of CCR2−/− and MCP-1−/− mice resulted in higher numbers of T. gondii parasites compared to wild type mice. Parasites were enumerated by counting the mean number of parasites per mm2 in the ileum. Values are means ± s.d. from a representative experiment, n = 3 animals each. * P < 0.05.
(A) Cytokine production in T. gondii infected mice. Cytokines were detected in serum from C57BL/6, CCR2−/− and MCP-1−/− at intervals after peroral infection. No survivors were available from CCR2−/− at day 11 (†). Values are means ± s.d. from a representative experiment, n = 3 animals each. * P ≤ 0.05.
Despite having very high levels of intestinal parasites and failing to control infection, MCP-1−/− and CCR2−/− mice produced similar or slightly higher levels of Th1 cytokines systemically (Fig. 1C). This result is notable since IL-12 and IFN-γ are considered to be both necessary and sufficient to control acute infection (Gazzinelli et al., 1994). Significantly elevated levels of IFN-γ were observed at day 11 in MCP-1−/− mice, likely due to the higher tissue burdens of parasites. MCP-1 levels were significantly higher in CCR2−/− mice.
CCR2−/− and MCP-1−/− Mice Die from Acute Ileitis
Histological examination of intestinal tissues at day 9 post-infection revealed extensive inflammation of the ileum in MCP-1−/− and CCR2−/− mice compared to control C57BL/6 mice (Fig. 2A). Infection in C57BL/6 mice resulted in mild inflammation characterized by cellular infiltration and swelling and edema of the villus tips (Fig. 2A). In contrast, MCP-1−/− and CCR2−/− mice showed severe multifocal ulcerative and necrotic enteritis in many areas of the tissue (Fig. 2A). In the more severe areas, the villus architecture was severely disrupted due to separation of the mucosal epithelium, sloughing of necrotic cells, and extensive cellular infiltration in the underlying lamina propria (Fig. 2A). Ulcerative lesions were also seen extending through the mucosal layer and into the muscle wall (Fig. 2A). Notably, infection in C57BL/6 mice was typified by only low numbers of neutrophils (detected with mAb 1A8 to Ly6G), while extensive neutrophil influx was observed in many regions of the ileum from CCR2−/− and MCP-1−/− mice (Fig. 2B). Areas of neutrophil influx in CCR2−/− and MCP-1−/− mice were very heavily infected with parasites (Fig. 2B), and these regions corresponded to the regions of lesions observed in H&E staining. Histological examination of tissues from the spleen, liver, and lung during the first 12–15 days post-infection, showed only modest pathology consisting of small foci of infiltrating leukocytes with occasional parasites (data not shown). The extent of these changes was similar in C57BL/6 and CCR2−/− and MCP-1−/− mice. However, the few CCR2−/− animals that survived to day 30, developed 4-fold higher numbers of tissue cysts in the brain when compared to wild type C57BL/6 mice (Fig. S1B). In summary, these data indicate that MCP-1−/− and CCR2−/− mice fail to control parasite proliferation in the ileum, leading to massive neutrophil influx, tissue destruction, and resulting in death due to intestinal necrosis.
Figure 2. Absence of CCR2 or MCP-1 leads to increased parasite numbers, neutrophil recruitment and intestinal necrosis following T. gondii infection.
(A) Oral infection with T. gondii induced mild intestinal inflammation in wild type mice but extensive pathology in CCR2−/− and MCP-1−/− mice. Orally infected mice were examined at day 9 post-infection by H&E staining of paraffin sections. Normal ileum morphology was seen in noninfected C57BL/6. Infected C57BL/6 had slightly swollen villi and increased cells in the lamina propria. Lesions (asterisk) observed in the ileum of infected CCR2−/− and infected MCP-1−/− mice were characterized by disruption of the villus architecture, destruction of the inner circular layer of the tunica muscularis (ICM), and extensive replication of parasites (arrow and insets). Scale = 200 µm (15 µm in insets). Right panels are enlarged views.
(B) High numbers of neutrophils colocalized with T. gondii in the lesion areas of CCR2−/− and MCP-1−/− mice. Orally infected mice were examined at 9 days post-infection by staining with polyclonal anti-T. gondii (green) and anti-neutrophil (Ly6G, mAb 1A8) followed by Alexa-conjugated secondary antibodies. Scale bar = 20 µm.
CCR6 DCs in the Peyer’s Patch are not Essential for Control of Infection
Toxoplasma is thought to invade directly into epithelial cells in the small intestine, primarily in the ileum, where it crosses the intestinal barrier by active migration (Barragan and Sibley, 2003). Consistent with this, examination of the Peyer’s patches did not reveal high levels of parasite infection (data not shown). Nonetheless, we tested whether mice deficient in CCR6 were more susceptible to infection, since DCs in the subepithelial zone of Peyer’s patch express this chemokine receptor and have been shown to be important in mounting immune responses to some intestinal pathogens (Salazar-Gonzalez et al., 2006). CCR6−/− mice showed similar levels of survival, parasite tissue burdens, and chronic infection, when compared to heterozygote and homozygote littermate controls (Fig. S2). Collectively, these findings indicate that Peyer’s patches, and CCR6 DCs in particular, do not play a major role in the control in intestinal toxoplasmosis.
Control of Infection is Associated with Influx of Gr1+ Inflammatory Monocytes
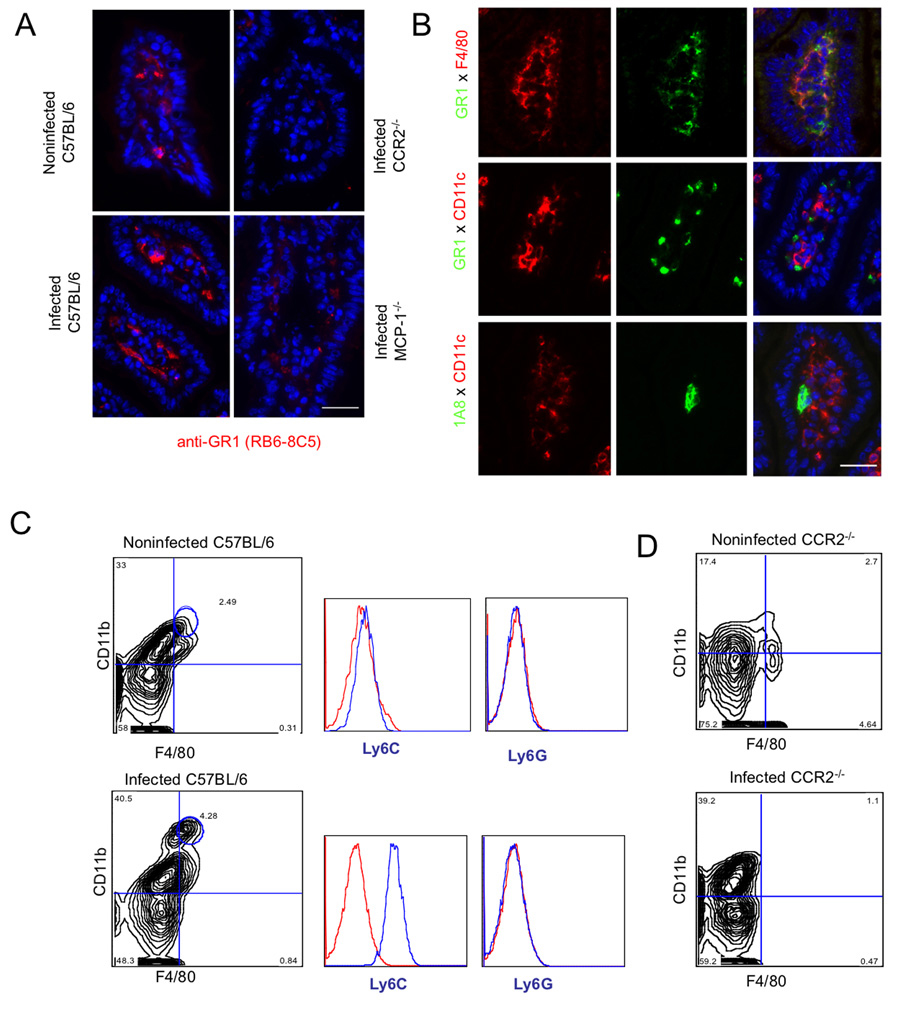
Immunofluorescence labeling of frozen sections of ileum at day 9 post-infection revealed an influx of Gr1+ cells, which localized beneath the basement membrane and surrounded cells within the lamina propria (Fig. 3A). Gr1+ cells were readily detected in noninfected C57BL/6 mice and were elevated in infected animals (Fig. 3A). To examine the distribution of Gr1+ cells in infected CCR2−/− and MCP-1−/− mice, regions of tissue that did not exhibit the high levels of neutrophil influx described in Fig. 2, where chosen for analysis. Such non-necrotic regions lacked Gr1+ cells within the lamina propria of CCR2−/− and MCP-1−/− mice, in marked contrast to the abundance of these cells in wild type mice (Fig. 3A). Necrotic regions from CCR2−/− and MCP-1−/− mice contained an abundance of neutrophils as described in Fig. 2B.
Figure 3. Recruitment of Gr1+ (Ly6C+) monocytes to the lamina propria in the ileum following T. gondii infection depends on CCR2 and MCP-1.
(A) Recruitment of Gr1+ cells in the ileum of orally infected C57BL/6 mice. Gr1+ cells increased within the lamina propria and were found beneath the basement membrane, surrounding the villus core within the ileum. Gr1+ cells were also seen in noninfected wild type (C57BL/6) but not in noninfected (data not shown) or infected CCR2−/− and MCP-1−/− mice. Frozen sections of orally infected mice were examined 9 days post-infection with anti-Gr1 (RB6-8C5) followed by Alexa-594 conjugated goat anti-rat IgG. Scale bar = 25 µm.
(B) Characterization of Gr1+ cells in the lamina propria of orally infected mice. Gr1+ cells stained with F4/80 but not CD11c or the neutrophil marker 1A8. Frozen sections of ileum from orally infected mice were examined after 9 days post-infection. Top panel: anti-Gr1 (RB6-8C5) directly conjugated to Alexa 488 and anti-F4/80 directly conjugated to Alexa 594; middle panel: anti-Gr1 (RB6-8C5) and anti-CD11c (N418) followed by Alexa-conjugated secondary antibodies; Bottom panel: anti-Ly6G (1A8) and anti-CD11c (N418), followed by Alexa-conjugated secondary antibodies Scale bar =25 µm.
(C) Oral infection with T. gondii induced recruitment of Gr1+ (Ly6C+) monocytes to the ileum. Leukocytes were isolated from the ileum of mice on day 9 after oral infection, stained for CD11b (mAb M1/70 conjugated to PE or FITC) and F4/80 (mAb A31 conjugated to APC), and quantified by FACS. Double positive macrophages (CD11b+ F4/80+) were gated based on isotype controls and circled populations were analyzed after staining with anti-Ly6C (mAb AL-21 conjugated to FITC) or Ly6G (mAb 1A8 conjugated to PE). Resident macrophages in non-infected mice (top) were negative for both Ly6C and Ly6G. In contrast, doubly positive cells from infected mice are strongly positive for Ly6C, but did not stain for Ly6G (bottom). Results shown are representative of three or more experiments; cells were pooled from three mice in each group.
(D) Analysis of peritoneal cells in noninfected (top) and infected (bottom) CCR2−/− mice. While neutrophils (CD11b+, F4/80−) increased dramatically in infected mice, the population of doubly positive CD11b+ F4/80+ cells was absent.
Gr1+ is expressed on neutrophils, inflammatory monocytes, and some populations of DCs (Tacke and Randolph, 2006). Therefore, we further explored the phenotype of cells in the small intestine of C57BL/6 mice at 9 days postinfection using a variety of antibodies to additional cell surface markers. Gr1+ cells were abundant in the lamina propria and many of these were costained with the macrophage marker F4/80, but did not express the dendritic cell marker CD11c (Fig. 3B). To explore whether these cells might be neutrophils, sections were stained with mAb 1A8 to Ly6G and CD11c to detect dendritic cells (Fig. 3B). Very few cells were positive for 1A8 (Ly6G), indicating that the abundant population of Gr1+ cells recruited to the lamina propria are not neutrophils. Instead, these studies suggest that the majority of infiltrating cells are inflammatory monocytes that do not co-express DC markers.
To further examine the phenotype of Gr1+ F4/80+ cells in the lamina propria, cells were dissociated from the ileum of C57BL/6 mice at 9 days postinfection and analyzed by flow cytometry (Fig. 3C). Cells were stained for the macrophage marker F4/80 and the leukocyte marker CD11b, which is shared between macrophages, neutrophils and some DCs. Noninfected mice showed a small proportion of macrophages (F4/80+ CD11b+) with higher numbers of singly positive CD11b+ cells (Fig. 3C). Infection resulted in an increase in the population of F4/80+ CD11b+ cells, which are presumably monocytes (Fig. 3C). To confirm the identity of these cells, they were stained with antibodies to Ly6C vs. Ly6G and analyzed by flow cytometry (Fig. 3C). These result confirm that the majority of cells found in the ileum in infected mice correspond to the phenotype of inflammatory monocytes; F4/80+ CD11b+ Ly6C Ly6G−. The doubly positive population of F4/80+ CD11b+ cells was absent in both noninfected and infected CCR2−/− mice (Fig. 3D), consistent with the idea that these cells represent inflammatory monocytes. While the numbers of singly positive F4/80+ or CD11c+ cells in the lamina propria also increased in infected C57BL/6 mice, they were similar in CCR2−/− or MCP-1−/− mice (Fig. S3). This result confirms that only inflammatory monocytes (F4/80+ CD11b+ Gr1+ Ly6C+ Ly6C−) require MCP-1 and CCR2 for trafficking to mucosal sites of infection. Collectively, these findings indicate that increased numbers of inflammatory monocytes cells are associated with protection from oral toxoplasmosis and that CCR2−/− and MCP-1−/− mice lack these protective cells in the gut and hence fail to control infection.
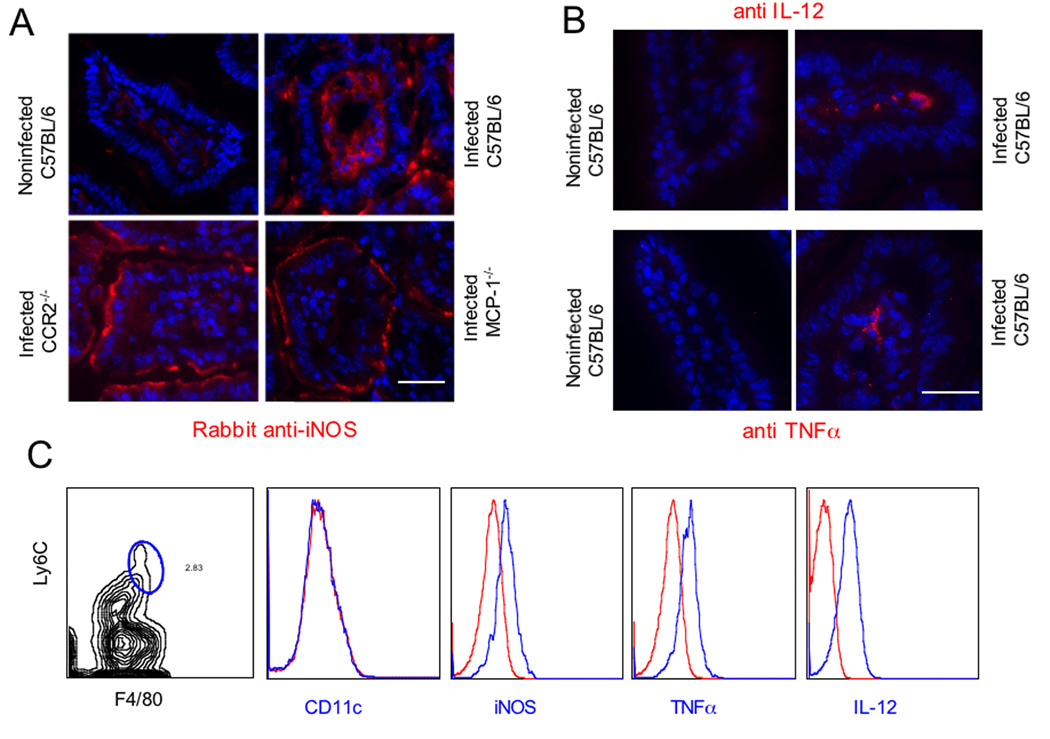
Intestinal Gr1+ (Ly6C+) Monocytes Express iNOS, IL-12, and TNF-α but not CD11c
Previous studies have shown that Gr1+ monocytes are capable of producing nitric oxide (NO) and controlling parasite replication (Mordue and Sibley, 2003). We examined the expression of inducible NO synthase (iNOS) in the ileum of infected mice by staining frozen sections with specific antibodies that were detected by immunofluorescence. iNOS expression was strongly induced within the lamina propria at the apical border of the enterocytes and within the core region of the villus of infected C57BL/6 mice (Fig. 4A). Regions of the small intestine that did not show extensive lesion formation and neutrophil influx were chosen for analysis of knockout mice. In contrast to wild type mice, iNOS staining was not detected within the core region of the villus of infected CCR2−/− or MCP-1−/− mice, but rather only the apical margin of the epithelial cells was stained (Fig. 4A). Induction of iNOS expression was only seen in infected mice and low basal levels of staining were seen in uninfected animals, regardless of genetic background (Fig. S4). Immunohistochemical staining also revealed the presence of IL-12+ and TNFα+ positive cells within the lamina propria of infected C57BL6 mice (Fig. 4B).
Figure 4. Gr1+ (Ly6C+) inflammatory monocytes in the lamina propria express iNOS, TNFα, and IL-12 but not dendritic cell markers.
(A) Induction of iNOS in the ileum of infected mice. iNOS expression was low in noninfected ileum of C57BL/6 mice, but was induced following infection in lamina propria cells. In contrast, in CCR2−/− and MCP-1−/− mice, iNOS expression was only observed at the apical region of enterocytes. Orally infected mice were examined at day 9 post-infection by staining frozen sections with rabbit anti-iNOS followed by Alex 594-conjugated secondary antibodies (red). Bar = 25 µm.\
(B) Intracellular IL-12 and TNFα expression was detected within some cell in the villus region of the Ileum in infected C57BL/6 mice. Staining for these intracellular markers was not detected in noninfected mice. Frozen sections were stained with rat anti- IL-12 (BD Pharmingen) or rat anti-TNFα (BD Pharmingen) followed by detection using Alexa-594-conjugated goat anti-rat IgG. Bar = 25 µm.
(C) Leukocytes isolated from the lamina propria at day 9 after oral infection were stained with Ly6C (mAb AL-21 conjugated to FITC) and F4/80 (mAb A3-1 conjugated to APC), gated based on isotype controls, and analyzed for expression of other surface or intracellular markers (with antibodies conjugated to PE, see methods). F4/80+ Ly6C+ monocytes did not express CD11c as shown by absence of staining with mAb to p150/90. In contrast, F4/80+ Ly6C+ monocytes stained positively for iNOS, TNFα, and IL-12. Intracellular staining was performed after incubation in brefeldin A for 4 hrs. In each case, the positive cells are plotted as the blue line vs. isotype control in the red line. Representative of three similar experiments.
To further examine the contribution of inflammatory monocytes to production of these mediators, cells were isolated from the lamina propria of C57BL6 mice at 9 days postinfection and analyzed by flow cytometry. Cells were stained for F4/80 and Ly6C to identify the population of inflammatory monocytes (Fig. 4C). Triple staining revealed that these inflammatory monocytes were positive for iNOS and yet failed to express CD11c (Fig. 4B). To examine intracellular cytokines, cells were cultivated in brefeldin A for 4 hrs prior to triple staining, which revealed that these cells were positive for TNF-α and IL-12 (Fig. 4C). Collectively these results indicate that inflammatory monocytes (F4/80+ CD11b Ly6C+) within the lamina propria express iNOS, IL-12 and TNF-α, yet these cells do not appear to differentiate into DCs.
Gr1+ (Ly6C+) Inflammatory Monocytes Protect Against Lethal Toxoplasmosis
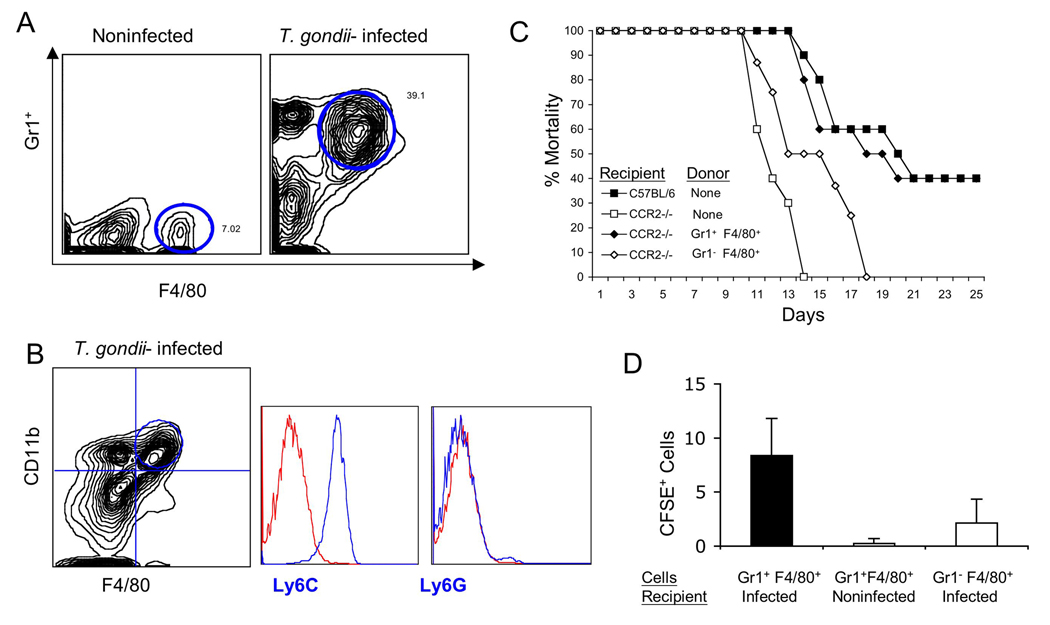
Previous studies have shown that injection of low numbers of type II strain parasites into the peritoneal cavity of mice recruits high numbers of inflammatory monocytes (Gr1+ F4/80+ CD11c−) (Mordue and Sibley, 2003; Robben et al., 2005). We compared the recruitment of inflammatory monocytes using thioglycolate, MCP-1, or different doses of live parasites. Surprisingly, only low dose challenge with parasites was effective in recruiting a large number of inflammatory monocytes, while high doses of parasites resulted in predominately neutrophils, and MCP-1 alone was ineffective in recruiting monocytes (Fig. 5A and Fig. S5). Following injection of low doses of parasites, the populations of myeloid cells found in the peritoneal cavity change dramatically: there is an increase in neutrophils (Gr1+ F4/80−) and inflammatory monocytes (Gr1+ F4/80+)(upper gate in right panel of Fig. 5A), while resident macrophages decreased (Gr1− F4/80+)(lower gate in left panel of Fig. 5A). The F4/80+ cells induced by infection also express CD11b and Ly6C but not Ly6G (Fig 5B), and express iNOS, IL-12 and TNF-α (Fig S6), indicating they are highly similar to the cell population that is upregulated in the lamina propria following oral infection. To test the ability of these cells to protect mice against lethal challenge, inflammatory monocytes (Gr1+ Ly6C+ F4/80+) were sorted from the peritoneal cavity of mice primed with low dose T. gondii infection and resident macrophages (Gr1− Ly6C− F4/80+) were isolated from uninfected mice (Fig. 5A). Adoptive transfer of inflammatory (Gr1+ Ly6C+ F4/80+) monocytes protected against otherwise lethal oral challenge with T. gondii in CCR2−/− recipients, while resident macrophages (Gr1− Ly6C− F4/80+) did not confer protection (Fig. 5C).
Figure 5. Adoptive transfer of Gr1+ (Ly6C+) inflammatory monocytes protects against death in CCR2−/− mice.
(A) High numbers of Gr1+ F4/80+ monocytes were recruited to the peritoneal cavity of mice following low dose T. gondii infection. Cell populations were characterized by flow cytometry using anti-Gr1 (mAb RB6-8C5 conjugated to Cy5) and anti-F4/80 (mAb A3-1 conjugated to PE). Double positive cells (Gr1+ F4/80+) were sorted for adoptive transfer (upper circle, right plot) Single positive F4/80+ cells were used for control transfers (lower circle, left plot). Results are representative of four experiments performed.
(B) Characterization of peritoneal monocytes elicited in T. gondii infected C57BL6 mice by staining for CD11b (mAb M1/70 conjugated to PE or FITC) and F4/80 (mAb A3-1 conjugated to APC). Doubly positive cells were gated based on isotype controls and the circled population of cells analyzed for expression of Ly6C (mAb AL-21 conjugated to FITC) and Ly6G (mAb 1A8 conjugated to PE). Inflammatory monocytes (CD11b, F4/80) strongly expressed Ly6C but did not stain for Ly6G. Representative results from three or more similar experiments. In each case, the positive cells are plotted as the blue line vs. isotype control in the red line.
(C) Adoptive transfer of Gr1+ F4/80+ inflammatory monocytes protect infected CCR2−/− mice from death. Three days after oral infection of mice with T. gondii, mice received sorted cells (see A) by i.v. injection. All infected CCR2−/− mice died on day 14 after infection, while mice that received the F4/80+ single positive cell population (negative control) died on day 18 after infection. Approximately half of C57BL/6 wild type mice and the CCR2−/− mice that received Gr1+ F4/80+cells survived the infection. Results are mean values of three separate experiments with three to four mice per group.
(D) Adoptive transfer of inflammatory monocytes into noninfected or infected C57BL/6 mice. Monocyte populations were isolated from the peritoneal cavity of T. gondii infected mice and separated by FACS after staining for Gr1 (mAb RB6-8C5 conjugated to Cy5) and F4/80 (mAb A3-1 conjugated to PE) (similar to A). Cell populations were labeled with CFSE in vitro and administered by i.v. inoculation (106 cells per animals) into wild type mice that were either noninfected, or animals that were infected 3 days previously by oral challenge. Inflammatory monocytes (Gr1+ Ly6C+ F4/80+) were recruited to the ileum in infected C57BL/6 mice (black bars) but not in noninfected mice (white bar). Gr1− Ly6C− F4/80+ macrophages were also recruited to the intestine of infected mice, albeit at much lower levels. CFSE+ cells were counted by examining frozen sections by fluorescence microscopy. Values represent the average number of cells per 20 microscope fields (40X) and were obtained from 2 separate experiments with 2 animals each group. Mean ± S.D.
Gr1+ (Ly6C+) Inflammatory Monocytes Home to the Intestine and Prevent Pathology
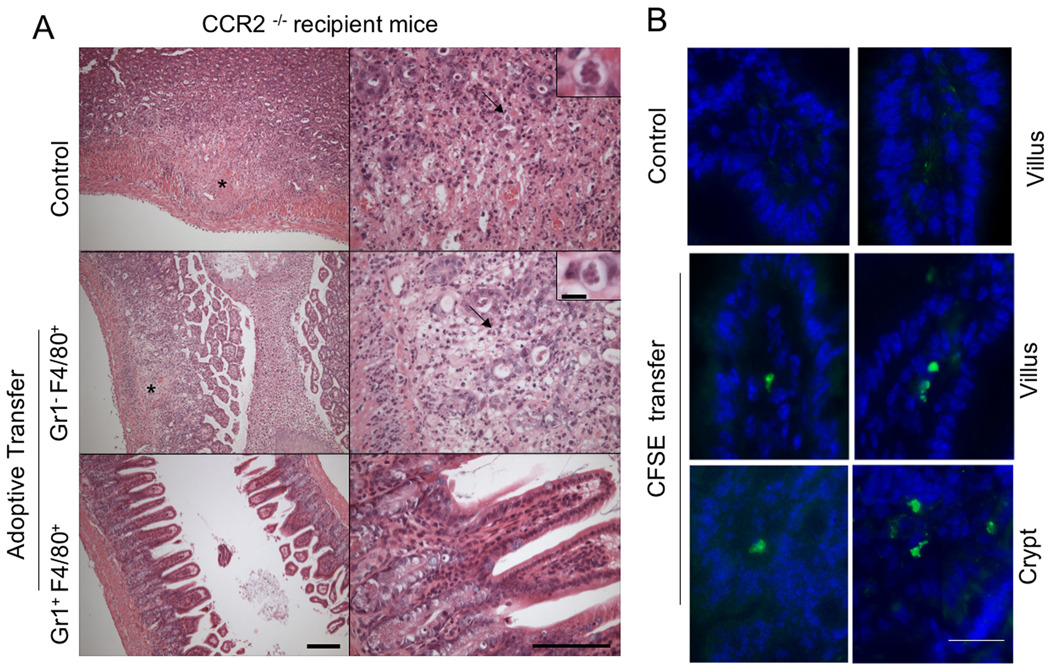
To determine the basis for the protective effects of adoptively transferred inflammatory monocytes, animals that received adoptive transfers at day 3, were sacrificed at day 9 after infection. Control CCR2−/− animals that did not receive adoptive transfer, or animals that received resident macrophages (Gr1− Ly6C− F4/80+), showed high levels of intestinal necrosis and large numbers of parasite within focal lesions (Fig. 6A). In contrast, CCR2−/− animals that received inflammatory monocytes (Gr1+ Ly6C+ F4/80+) were protected against development of intestinal pathology and showed only mild histological changes in the ileum similar to infected C57BL/6 mice (Fig. 6A). These results demonstrate that adoptively transferred inflammatory monocytes were able to control the local pathology in the gut, suggesting they are able to home to the intestine.
Figure 6. Adoptively transferred Gr1+ (Ly6C+) monocytes home to the intestine and prevent extensive pathology in CCR2−/− mice.
(A) Sorted cells were adoptively transferred to infected CCR2−/− mice on day 3 post-infection and the ilea were examined at day 9 by H&E staining of paraffin sections. Typical lesions (asterisk) were observed in the ileum of infected CCR2−/− mice that did not receive cells (Control) or received F4/80+ singly positive cells. CCR2−/− mice that received Gr1+ (Ly6C+) F4/80+ cells show well-preserved architecture in the ileum, similar to wild type mice. Representative of two similar experiments containing 2 animals per group. Scale bar = 200 µm (15 µm inset). Right panels are enlarged views.
(B) Adoptively transferred Gr1+ (Ly6C+) monocytes home to the ileum of infected mice. Sorted, CFSE labeled Gr1+ (Ly6C+) F4/80+ monocytes i.v. inoculated into C57BL/6 mice on day 3 after oral infection. One day following adoptive transfer (day 4 after oral infection), ilea were collected and frozen sections were examined by fluorescent microscopy. Recruitment of CFSE labeled Gr1+ (Ly6C+) monocytes was observed in the lamina propria region in the villi and in the crypt region, which contained lymphocytic aggregations. Control represents C57BL/6 mouse that was infected but did not receive CFSE labeled cells. Scale bar = 20 µm.
To confirm that adoptively transferred cells were able to home to the site of infection, inflammatory monocytes (Gr1+ Ly6C+ F4/80+) were isolated from the peritoneal cavity, labeled in vitro with CFSE, and injected into mice that had been orally infected 3 days earlier. Examination of frozen sections from the ileum at 24 hr after transfer, confirmed that inflammatory monocytes were able to home to the site of infection in the gut (Fig. 6B). Adoptively transferred, CFSE-positive cells were detected within lamina propria of the villus, in the crypt regions of the ileum, and in Peyer’s patches (Fig. 6B and data not shown). Comparison of the efficiency of homing indicated that Gr1+ Ly6C+ F4/80+ cells were more efficient than Gr1− Ly6C− F4/80+ cells in reaching the intestine (Fig. 5D). Migration also required active infection as minimal numbers of CSFE labeled cells were detect in frozen sections of the small intestine of noninfected mice that received inflammatory monocytes by adoptive transfer (Fig. 5D). Collectively these results indicate that Gr1+ Ly6C+ F4/80 inflammatory monocytes home to the ileum where they prevent lethal toxoplasmosis and that this pathway critically depends on MCP-1 and CCR2.
Trafficking of Inflammatory Monocytes in Wild Type and CCR2−/− Mice
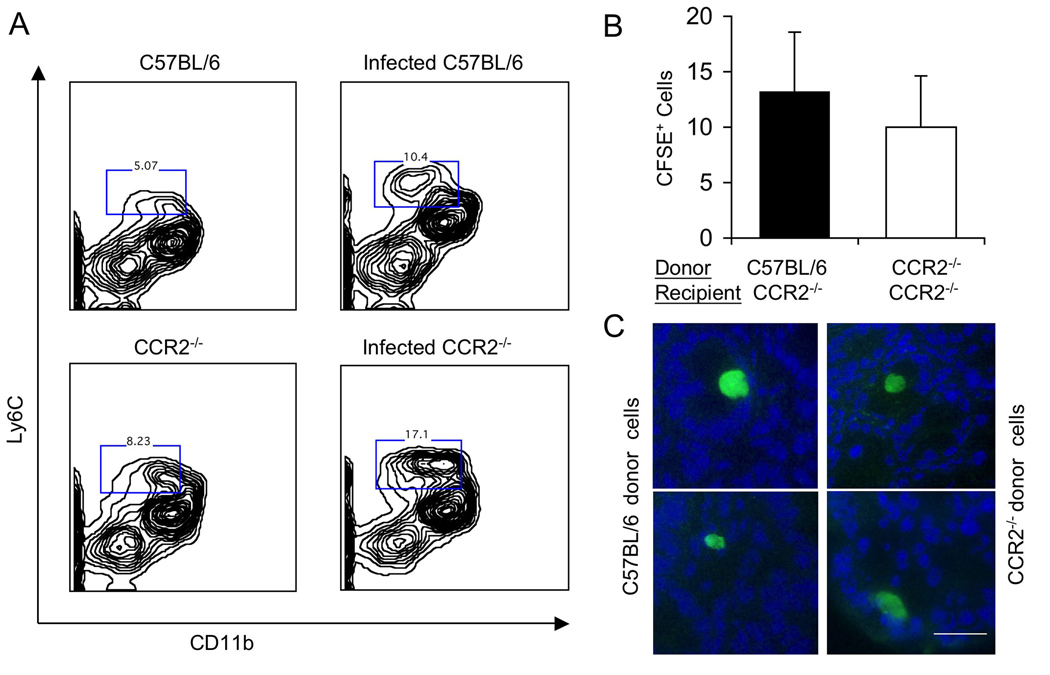
Previous studies have shown that CCR2−/− cells are defective in exiting the bone marrow and yet they are capable of migrating to the spleen in Listeria infected mice (Serbina and Pamer, 2006). To determine if a similar defect was responsible for the susceptibility of CCR2−/− mice to T. gondii, we examined the populations of myeloid cells in bone marrow of wild type and knock out mice by flow cytometry. Following T. gondii infection, the number of Ly6C+ CD11b+ monocytes increased by 1.5–2 fold in the bone marrow of C57BL/6 and in CCR2−/− mice (Fig. 7A). Consistent with earlier reports that these cells fail to exit the bone marrow in CCR2−/− mice (Serbina and Pamer, 2006), elevated levels of Gr1+ (Ly6C+) inflammatory monocytes were only seen in the blood of C57BL/6 mice (data not shown). Adoptively transferred Gr1+ (Ly6C+) CD11b+ cells were able to home to the lamina propria in the small intestine in CCR2−/− mice that were previously infected with T. gondii (Fig. 7B,C). This homing ability was similar using donor cells from wild type (CCR2+/+) and knockout (CCR2−/−) animals (Fig. 7C). Collectively, these studies indicate that in the absence of CCR2, inflammatory monocytes fail to exit the bone marrow, yet once in the periphery, they are capable of homing to sites of inflammation, including the gut.
Figure 7. Recruitment from the bone marrow and trafficking of inflammatory monocytes in wild type and CCR2−/− mice.
(A) Characterization of bone marrow cells stained with Ly6C (mAb AL-21 conjugated to FITC) and CD11b (mAb M1/70 conjugated to Cy5). In wild type mice (top), infection with T. gondii increased the number of doubly positive cells by 2 fold (boxed numbers represent the gates and are given in %). The number of doubly positive cells was elevated in the absence of infection and increased by ~2 fold in CCR2−/− mice following infection with T. gondii (lower). Representative of five similar experiments.
(B) Trafficking of Ly6C+ CD11b+ inflammatory monocytes following i.v. transfer into CCR2−/− mice. Adoptively transferred inflammatory monocytes from the bone marrow of wild type (C57BL/6) or CCR2−/− mice were able to home with comparable efficiency to the small intestine of CCR2−/− recipient mice that were infected with T. gondii. Bone marrow cells were stained as in A, sorted by FACS, and labeled in vitro using CFSE. Approximately 106 labeled cells were injected i.v. into mice that had been infected 3 days previously by oral inoculation with T. gondii. CFSE+ cells were counted by examining frozen sections by fluorescence microscopy. Values represent the average number of cells per 20 microscope fields (40X) and were obtained from 2 separate experiments with 2 animals each group. Mean ± S.D.
(C) Representative images of CFSE+ Ly6C+ CD11b+ monocytes in frozen sections of the small intestines of mice that had received donor cells from wild type (CCR2+/+) vs. CCR2 deficient (CCR2−/−) animals. Bar = 25 microns.
Discussion
Systemic infection with T. gondii results when the parasite invades across the intestinal epithelial layer in the small intestine and migrates through the basement membrane (Barragan and Sibley, 2003). Within the lamina propria, the parasite encounters leukocytes that mount the initial response to infection. We demonstrate that Gr1+ (Ly6C+) F4/80+ inflammatory monocytes increase dramatically in the lamina propria of the small intestine in response to T. gondii infection. Inflammatory monocytes form a protective barrier beneath basement membrane where they are positioned to encounter parasites as they first cross the epithelial barrier. Inflammatory monocytes upregulate iNOS, produce IL-12, and secrete TNF-α in response to infection, and these factors likely contribute to control of parasite replication. Absence of the chemokine receptor (CCR2), or major chemokine ligand (MCP-1) for this receptor, resulted in failure to populate the lamina propria with inflammatory monoctyes. The resulting inability to control parasite numbers within the intestine led to an influx of neutrophils, tissue destruction, and ultimately death due to intestinal necrosis. These findings reveal a previously unrecognized role for inflammatory monocytes as sentinels to invading enteric pathogens, thus providing an essential component of mucosal immunity.
Toxoplasma actively invades host cell using a unique form of actin-based motility (Sibley, 2004). This active penetration allows the parasite to invade directly into the intestinal epithelium and to cross the basement membrane and migrate within the lamina propria (Barragan and Sibley, 2003). Given these attributes, it is perhaps not surprising that parasites were not detected in large numbers in M cells and that CCR6+ DCs, which are important in control of pathogens that invade Peyer’s patches (Salazar-Gonzalez et al., 2006), do not appear to be essential in control of toxoplasmosis. Instead, the upregulation of inflammatory monocytes within the lamina propria was critical to control of infection.
Monocyte subsets are notoriously pleomorphic and able to change surface markers and phenotypic characteristics due to local conditions (Geissmann et al., 2008). However, our observations are consistent with the appearance of Gr1+ (Ly6C+) F4/80+ inflammatory monocytes in the lamina propria as a result of recruitment of cells from the periphery, rather than local differentiation. First, the numbers of these cells increases dramatically at the site of infection, without an obvious loss of other cell populations. Second, their appearance is dependent on the chemokine MCP-1, which is the major chemokine for recruiting circulating monocytes to sites of inflammation (Kuziel et al., 1997). MCP-1 continues to be abundantly made in the absence of peripheral monocytes bearing the major receptor for this chemokine (i.e. CCR2−/− mice). Consistent with this, the defect in CCR2−/− mice was complemented by adoptive transfer of inflammatory monocytes from wild type donors primed by infection with T. gondii. Inflammatory monocytes recovered from the peritoneum or the bone marrow of infected wild type mice were equally effective in homing to the gut of infected mice following adoptive transfer. Third, the number of inflammatory monocyte precursors increased dramatically in the bone marrow of infected mice. However, in the absence of CCR2, these inflammatory monocytes failed to exit from the bone marrow, similar to previous reports in Listeria-infected mice (Serbina and Pamer, 2006). Trafficking of inflammatory monocytes in the periphery did not require CCR2 as cells isolated from the bone marrow of infected CCR2−/− mice were also capable of homing to the intestine following adoptive transfer into infected mice. Collectively, our studies indicate that Gr1+ (Ly6C+) F4/80+ inflammatory monocytes home to the ileum in the small intestine where they are both necessary and sufficient as a first line of defense against T. gondii infection.
Our studies indicate that despite the importance of CCR2 and MCP-1 in homing of inflammatory monocytes, these cells must rely on different ligand-receptor pairs in the periphery. It has been reported that CCR6–CCL20 is essential for homing of Gr1+ monocytes to the inflamed dermis (LeBorgne et al., 2006). However, this pathway cannot explain the results observed here, since CCR6−/− mice show no deficiency in recruitment of cells to the gut (data not shown) or in increased susceptibility to T. gondii (Fig. S2). Gr1+ inflammatory monocytes have also been described to carry Listeria to the brain (Drevets et al., 2004), suggesting they may play both beneficial and detrimental roles in infections of the CNS. Myeloid cells, including both CD11b+ and CD11c+, cells may also contribute to systemic spread of T. gondii from the gut to the CNS (Courret et al., 2005), a pathway that is upregulated by enhanced migration following infection (Lambert et al., 2006). CCR2−/− mice that survived the acute infection developed much higher cysts burdens in the CNS, despite only modestly higher tissue burdens during acute infection, suggesting that inflammatory monocytes may also contribute to defense within other tissues including sites of immune privilege.
Several observations support our conclusion that Gr1+ (Ly6C+) F4/80+ inflammatory monocytes are essential for control of oral toxoplasmosis. First, inflammatory monocytes are upregulated following infection by almost 5-fold at the site of infection, within the lamina propria of the ileum. Second, in the absence of signals that allow trafficking of inflammatory monocytes out of the bone marrow (i.e. CCR2−/− mice) or to the site of infection (MCP1−/− mice), T. gondii infected animals die of overwhelming necrosis in the ileum that is associated with uncontrolled parasite numbers. In contrast, resident macrophage numbers and DCs cells (CD11c+) increase to a similar extent during infection of wild type C57BL/6, CCR2−/−, and MCP-1−/− mice (Fig. S3). While our findings do not rule out important roles for DCs during immune responses to T. gondii in the intestine, these cells are not capable of controlling infection in the absence of inflammatory monocytes. In contrast, the absence of resident macrophages (CX3CR1+ Gr1−) does not affect susceptibility to oral toxoplasmosis (Jung et al., 2000). Finally, since the major site of pathology observed in the present study is the ileum, we conclude that recruitment of inflammatory monocytes to this site provides a necessary first line of defense.
The precise function of Gr1+ (Ly6C+) inflammatory monocytes in protecting against intestinal pathology is uncertain. Initial contact between T. gondii parasites and DCs (Aliberti et al., 2000; Sousa et al., 1997) and macrophages (Robben et al., 2004) induces proinflammatory cytokines including IL-12 (Khan et al., 1994), which drives production of IFN-γ (Gazzinelli et al., 1994). IFN-γ is critical for control of parasite replication (Suzuki et al., 1988) by both hematopoetic and nonhematopoetic cells (Yap and Sher, 1999). Previous studies reported that inflammatory monocytes recruited to the peritoneal cavity following T. gondii infection express iNOS, produce IL-12, and are capable of killing T. gondii in vitro (Mordue and Sibley, 2003). Our studies indicate that a very similar subset of inflammatory monocytes are recruited to the lamina propria following oral infection. These cells express iNOS, generate IL-12, and secrete TNF-α, all three of which are key mediators in the defense against intracellular T. gondii. Hence, it is likely that inflammatory monocytes are responsible for control of infection in the gut by exerting direct anti-toxoplasmicidal activity and priming Th1 responses. A similar role has been attributed to a population of Tip-DC cells, derived from CCR2+ monocytes, in Listeria infected mice (Serbina and Pamer, 2006; Serbina et al., 2003). Whether inflammatory monocytes also contribute to antigen processing and presentation and hence T-cell activation, is presently under investigation.
Previous studies have emphasized a role for neutrophils in the primary response to T. gondii. Mouse (Bliss et al., 1999) and human (Bliss et al., 1999) neutrophils make IL-12 in vitro in response to parasite antigens, suggesting a role in early cytokine production. However, our findings indicate that very few neutrophils are recruited to the lamina propria in infected C57BL/6 mice, which are nonetheless able to control infection. Only in CCR2−/− and MCP-1−/− mice, which failed to control parasite numbers, did the numbers of neutrophils significantly increase. Neutrophils may respond to increased parasite numbers (antigen load), yet they are unable to control infection and may actually contribute to inflammation and intestinal damage. Given our current findings on the importance of Gr1+ (Ly6C+) inflammatory monocytes, it is likely that the previous studies demonstrating that depletion with anti-Gr1 (mAb RB6–8C5) results in increased susceptibility to T. gondii (Bliss et al., 2001), was due to depletion of inflammatory monocytes, rather than neutrophils. Consistent with this CXCR2−/− mice, which are deficient in neutrophil recruitment, show increased levels of chronic infection but no overt changes in acute susceptibility when challenged orally with T. gondii (Del Rio et al., 2001). While neutrophils may participate in inflammation and pathology, they are neither necessary nor sufficient to control acute toxoplasmosis in the gut.
Gr1+ (Ly6C+) inflammatory monocytes were detected in low numbers in normal ileum of uninfected C57BL/6, consistent with previous reports that these cells can populate the intestine and serve as precursors for DCs (Varol et al., 2007). Curiously, the Gr1+ (Ly6C+) inflammatory monocytes detected here did not express CD11c, suggesting they may be blocked in differentiation. Previous studies demonstrating that Gr1+ monocytes are recruited to the peritoneal cavity after i.p. challenge with T. gondii also found that these cells fail to express DC markers (Mordue and Sibley, 2003; Robben et al., 2005). This is in marked contrast to infection with Listeria, which elicits TipDC cells from CCR2+ precursors that readily differentiate to express DC markers (Serbina and Pamer, 2006; Serbina et al., 2003). Other studies also have shown that Gr1+ monocytes are able to differentiate into DCs cells when cultured in vitro (Geissmann et al., 2003). Infection of conventional DCs by T. gondii has been reported to inhibit their maturation (McKee et al., 2004), suggesting a similar phenomenon may occur in Gr1+ (Ly6C+) monocytes.
Oral infection with T. gondii has been used extensively to examine the regulation of immune responses in the gut (Buzoni-Gatel and Kasper, 2007). During the adaptive response, CD8+ αβ intraepithelial cells proliferate and these cells are able to protect against lethal infection through a process that relies on cytotoxicity and production of IFN-γ (Buzoni-Gatel et al., 1999; Buzoni-Gatel et al., 1997; Chardes et al., 1994). Previous studies have shown that CCR2 is also expressed on activated and memory T-cells and CCR2−/− mice have reduced production of IFN-γ and increased resistance to Th1 mediated disease models (reviewed in (Luther and Cyster, 2001)). These effects are unlikely to explain the results observed here for several reasons. First, the rapid death of CCR2−/− mice suggests an involvement of innate responses rather than adaptive. Second, the development of acute ileitis following oral challenge with T. gondii infection is driven by strong Th1 responses (Liesenfeld, 2002), and decreased T-cell involvement in this pathway would be expected to be protective rather than increasing susceptibility. Finally, Th1 responses were not systemically impaired in CCR2−/− mice challenged with T. gondii.
The number of macrophages in the gut increases in Inflammatory Bowel Disease (IBD) and these cells express CD14, CCR2, and co-stimulatory markers as well as TREM, a receptor involved in activation of inflammatory responses (Schenk and Mueller, 2006). Toxoplasma is known to induce several effectors that mimic IBD in the mouse model (Liesenfeld, 2002). For example, over production of Th1 cytokines leads to inflammation and either IL-10 or TGF-β can abrogate this effect (Liesenfeld, 2002). While inflammatory monocytes are protective in the control of acute toxoplasmosis in the studies described here, it is possible that increased infiltration of inflammatory monocytes also sets up a chronic condition that would be more prone to inflammation. Thus, T. gondii infection in the mouse offers an attractive model for studying the role of monocytes in host defense and inflammation.
Experimental Procedures
Parasite Strains and Mouse Infections
The B7 clone of the type II strain ME49 strain (ATCC # 50840) (American Type Culture, Manassas, VA). Collection of T. gondii was used for production of tissue cysts in CD1 outbred mice (Charles River Laboratories, Wilmington, MA). Tissue cysts used in experiments were obtained from mice that were inoculated 1–3 months previously with 5 cysts by i.p. injection. Animals were sacrificed, the brains were removed and homogenized in 1 ml of phosphate buffer saline (PBS), pH 7.2, and tissue cysts were counted based on 3 or more aliquots of 20 uL. To assure uniform inoculum, cysts were disrupted by acidic pepsin acid solution (pH 1.6) from porcine stomach (Sigma Chemical Co., St Louis, MO) for 45 minutes followed by neutralization with 1.2% sodium bicarbonate (pH 8.3), as described previously (Fux et al., 2007).
For challenge studies, 8–12 week old female mice were used for all studies. Mice were infected by oral route with using an inoculum equivalent to a single tissue cyst and survival was followed by 30 days. The precise number of bradyzoites inoculated was not determined. CCR2−/− fully backcrossed onto a C57BL/6 background were obtained from Dr. William Kuziel, as described previously (Robben et al., 2005). MCP-1−/− CCR6+/−, and wild type C57BL/6 mice were obtained from Jackson Labs (JAX Mice and Services, Bar Harbor Maine). CCR6−/− homozygotes were identified by PCR using protocols supplied by Jackson Labs. Age and sex-matched heterozygote or homozygote littermates were used as controls. Infection was confirmed in survivors by western blotting for antibodies against whole parasite lysate, as described previously (Fux et al., 2007).
Animals were maintained in an AAALAC-approved facility overseen by the Institutional Animal Care Committee at Washington University.
Parasite Burdens in the Tissues of Infected Mice
Mice were infected by oral challenge as above and sacrificed on day 3, 6, 9, and 11–12 days post-infection. Parasite numbers in the ileum were enumerated by counting the mean number of parasites per mm2 in the ileum of frozen sections (5 µm thickness) stained with rabbit polyclonal anti-T. gondii followed by Alexa 488 conjugated goat anti-rabbit (Molecular Probes-Invitrogen, Carlsbad, CA). Counts of parasites were done from random microscopic fields (n = 25) from two different distant sections of the same ileum of two separate mice. Values shown are expressed as mean ± s.d. from a representative of two similar experiments.
Cytokine Levels in Serum
Blood was obtained form the orbital plexus at 3, 6, 9, and 11 days post-infection, using Microtainer™ separator tubes (Becton Dickinson, Franklin Lakes, NJ) centrifuged and the sera stocked in −20°C. Sera were diluted and used to detect cytokines and chemokines (IL-12, IFN-γ, IL-6, TNF-α, IL-10 and MCP-1) using the cytokine bead array kit from BD Bioscience (San Jose, CA). Data shown are means ± s.d. (n = 3 animals per group, tested in duplicate) from a representative of three or more experiments.
Histological Studies
Animals were sacrificed at 3, 6, 9 and 11 days post-infection, tissues removed from the ileum, liver, spleen, lungs, and brain and fixed in 10% neutral buffered formalin. Tissues were dehydrated in ethanol, embedded in paraffin, and 5 µm sections were stained with hematoxylin and eosin (H&E). Sections were read blind and scored for severity of necrosis. For immunostaining, tissues were processed in O.C.T. Tissue-Tek (Sakura, Torrance, CA) and frozen at −80°C before being sectioned on a cryomicrotome at 5 µm. Sections were mounted on glass slides and stored at −20°C prior to use.
Immunofluorescence Microscopy
Hybridoma cell lines for the markers F4/80 (HB-198), CD11b (Mac-1α, M1/70) (TIB-128), CD11c (mAb N418)(HB-224), mouse Fc gamma receptors (mAb 2.4G2)(HB-197) were obtained from the ATCC. The mAb RB6-8C5 against Gr1 was obtained from Dr. Emil Unanue (Washington University). The mAb 1A8 that selectively recognizes Ly6G was obtained from BD Biosciences. Rabbit anti iNOS serum was obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Hybridomas were grown in RPMI containing 20% characterized FBS (Hyclone, Logan, UT), 10 mM HEPES, 2 mM L-glutamine and 10 µg/ml of gentamicin. High-titer supernatants were produced in CELLine (Intregra Biosciences AG, Switzerland) flasks according to manufacturer’s directions. In some cases, hybridomas grown in serum-free medium (Invitrogen, Carlsbad, CA) were directly labeled using AlexaFluor labeling kits from Molecular Probes, following the manufacturer’s protocols. Parasites were detected using rabbit or rat anti-whole cell lysate or the mAb DG52 that reacts to the surface protein SAG1.
Frozen sections were acetone fixed for 10 min then rehydrated and blocked by incubation for 10 min in diluent (PBS containing 2.0 % normal goat serum, 1% bovine serum albumin, 0.1% gelatin, 0.05% Tween 20, and 0.05% sodium azide). Sections were incubated in primary antibodies in diluent for 60 min and rinsed in wash buffer (PBS containing 0.5% FBS), and incubated for 60 min with secondary antibodies conjugated to Alexa 488 or Alexa 594 (Molecular Probes). For negative controls, primaries were substituted by isotype-matched controls of the same species. Sections were rinsed in wash buffer and mounted in Vectashield containing DAPI (Vector Labs, Burlingame, CA) or ProLong Gold containing DAPI (Molecular probes). Slides were examined using a Zeiss epifluorescence microscope equipped with an AxioCam CCD camera and Axiovision v4.0 software for image capture (Carl Zeiss, Inc, Peabody. MA). Images were processed using similar linear adjustments for all samples in Photoshop 4.0 (Adobe, San Jose, CA).
Flow Cytometry
Leukocytes isolated from the lamina propria or peritoneal cavity were pretreated on ice for 20 min with mAb 2.4G2 (2.0 µg/ml) to block non-specific binding to Fcγ receptors. Thereafter, cells were incubated on ice for 30 min with fluorescently conjugated antibodies for cell surface markers: Cy5-conjugated anti-Gr1 mAb RB6-8C5 (eBioscience, San Diego, CA), PE or APC- conjugated anti-F4/80 mAb A3-1 (AbD serotec, Raleigh, NC), FITC-conjugated hamster anti-mouse p150/90 (CD11c) (eBioscience), PE-conjugated anti-iNOS (M-19, Santa Cruz), FITC-conjugated anti-Ly6C mAb AL-21 (BD bioscience), PE-conjugated anti-Ly6G mAb 1A8 (BD bioscience), or Cy5-conjugated anti-CD11b mAb Mac-1α (eBioscience). Isotype controls consisted of PE conjugated IgG2b and Cy5-conjugated IgG2b (eBioscience), FITC-conjugated hamster IgG (eBioscience), or FITC-conjugated Rat IgM (BD Pharmingen). Cells were stained with 7-Aminoactinomycin (7-AAD; Sigma-Aldrich), washed and then fixed in 0.2 % paraformaldehyde for 10 minutes. Analysis of stained cells was performed with a FACScanto™ flow cytometer (BD Biosciences). Dead cells were gated out on the basis of 7-AAD staining and isotype controls were used to set appropriate gates. Data were analyzed using FACS diva (BD Bioscience) and FloJo 6.4.7 (Tree Star Inc., Ashland, OR). For all samples approximately 50,000 cells were analyzed to generate plots.
For intracellular cytokine staining, samples were treated for 4 hours with Brefeldin-A (10 µg/ml) (eBioscience) at 37°C. Cells were isolated from the lamina propria, stained with surface markers Ly6C (mAb AL-21 conjugated to FITC) and F4/80 (mAb A3-1 conjugated to APC), washed in PBS, and fixed in 2 % paraformaldehyde (Polyscience, Warrington, PA) in PBS for 10 min at room temperature. Cells were permeabilized in PBS containing 0.5% saponin and 0.5% BSA (Sigma-Aldrich), which was used throughout the staining procedure. Intracellular markers were detected by incubation with antibodies for 30 min, washing in PBS, and analysis by flow cytometry. Intracellular markers were detected with the following antibodies: PE-conjugated anti-TNF (clone MP6-XT22, BD Pharmingen), PE-conjugated anti- IL-12 (clone C17.8, BD Pharmingen) or PE-conjugated anti-iNOS mAb (M-19 Santa Cruz), or isotype matched controls.
Isolation of Leukocytes from the Lamina Propria
Leukocytes were isolated from the lamina propria using a modification of a protocol described previously (Heimesaat et al., 2006). Briefly, ilea of 3 animals were pooled and washed in PBS containing penicillin / streptomycin (10,000 / ml Units penicillin, 10 mg / ml streptomycin). Intestinal fat and Peyer’s patches were removed, intestines were cut longitudinally, and washed multiple times in PBS containing penicillin / streptomycin. Tissue was cut into 0.5 cm pieces and incubated in PBS with 5% FCS and 5 mM EDTA at 37°C for 15 min with gentle shaking. This process was repeated two times to remove epithelial and intraepithelial cells. Pieces of small ileum were then washed for 10 min in DMEM to inactivate EDTA. Thereafter pieces of the small ileum were incubated shaking in DMEM containing 5% FBS, collagenase (50 U/ml, Sigma-Aldrich), dispase (200 U/ml, Sigma-Aldrich), and DNase I (100 µg/ml, Sigma-Aldrich) for 30 min at 37°C. Supernatants were filtered through a 40-µm nylon sieve and washed in DMEM containing 5% FCS and centrifuged to pellet the cells. Cells were resuspended in DMEM containing 10% FBS, washed twice, and used immediately for flow cytometric analysis. Experiments were repeated three or more times with three animals per group.
Adoptive Transfer
To elicit Gr1+ (Ly6C+) monocytes, C57BL/6 mice were injected i.p. treated with freshly egressed parasites (200 parasites in 200µl PBS) of the type II PTG strain (ATCC # 50841) tachyzoites grown in HFF cells. On day 4 post-infection, peritoneal cells were harvested and washed in FACS buffer (PBS containing 2% FBS, 0.1mM EDTA). Cells were counted in a hemacytometer and treated with pyrimethamine (5 µM) for 30 minutes to eliminate parasites. Thereafter cells were treated with mAb 2.4G2 to block anti-Fcγ receptors (2.0 µg/ml for 20 min). Cells were incubated with anti-Gr1 (mAb RB6 - 8C5 conjugated to Cy5) and anti-F4/80 mAb (mAb A3-1conjugated to PE) and double positive (Gr1+ F4/80+) and single positive (Gr1− F4/80+) cells sorted by high speed FACSVantage (BD Biosciences). Cells were counted and transferred by i.v. inoculation of 1 × 106 in the tail vein of CCR2 −/− mice three days after oral infection with T. gondii, as described above. Mortality of mice was observed until day 25 after oral infection. Alternatively, on day 9 after oral infection terminal ileum were collected, fixed in formalin and processed for histological examination as above. Sections (5 µm) were stained with H&E and examined by light microscopy. Adoptive transfer experiments were repeated four times with 3–4 animals per group.
CFSE Labeling
Gr1+ (Ly6C+) F4/80+ macrophages were collected from the peritoneal cavity of mice as described above. Vybrant CFDA SE Cell tracer Kit (Invitrogen) was used to label the sorted cell population. Briefly, cells (1 × 107 /ml) were washed and incubated with 1 µM CFSE SE (Component A diluted in DMSO) in prewarmed PBS for 15 minutes. After centrifugation cells were resuspended in prewarmed PBS for further 30 minutes incubation. Cells were washed and 1 × 106 transferred in the tail vein of C57BL/6 mice on day 3 after oral infection with T. gondii as described above. The next day (4 days after oral infection), animals were sacrificed, ilea were collected, and processed in O.C.T. fixative. Frozen sections (5µm) were fixed in paraformaldehyde (3.6%) and observed by immunfluorescence microscopy. Experiments were repeated twice with two animals per group.
Statistical analysis
Samples were compared using the unpaired Student t test (two-tailed) using Excel (Microsoft).
Supplementary Material
Four Supplemental Figures can be found with this article online at:
Acknowledgements
We thank Chris Hunter and Oliver Liesenfeld for helpful comments, Julie Nawas, Suzanne Schloemann, Suzanne Hickerson, and Wandy Beatty for expert technical assistance. R.A.D. was supported by a fellowship from CNPq, Brazil. I.R.D. was supported by a fellowship from the DFG Postdoctoral Fellowship Program, Germany. Supported by grants from the NIH to L.D.S. (AI52293 and AI071299) and M.C. (AI071299).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aliberti J, Sousa CR, Schito M, Hieny S, Wells T, Huffnagle GB, Sher A. CCR5 provides a signal for microbial induced production of IL-12 by CD8-α+ dendritic cells. Nat. Immunol. 2000;1:83–87. doi: 10.1038/76957. [DOI] [PubMed] [Google Scholar]
- Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- Barragan A, Sibley LD. Migration of Toxoplasma gondii across biological barriers. Trends Microbiol. 2003;11:426–430. doi: 10.1016/s0966-842x(03)00205-1. [DOI] [PubMed] [Google Scholar]
- Bliss SK, Gavrilescu LC, Alcaraz A, Denkers EY. Neutrophil depletion during Toxoplasma gondii infection leads to impaired immunity and lethal systemic pathology. Infect. Immun. 2001;69:4898–4905. doi: 10.1128/IAI.69.8.4898-4905.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss SK, Marshall AJ, Zhang Y, Denkers EY. Human polymorphonuclear leukocytes produce IL-12, TNF-α, and the chemokines macrophage-inflammatory protein-1α and -1β in response to Toxoplasma gondii antigens. J. Immunol. 1999;162:7369–7375. [PubMed] [Google Scholar]
- Bliss SK, Zhang Y, Denkers EY. Murine neutrophil stimulation by Toxoplasma gondii antigen drives high level production of IFN-γ-independent IL-12. J. Immunol. 1999;163:2081–2088. [PubMed] [Google Scholar]
- Buzoni-Gatel D, Debbabi H, Moretto M, Dimier-Poisson IH, Lepage AC, Bout DT, Kasper LH. Intraepithelial lymphocytes traffic to the intestine and enhance resistance to Toxoplasma gondii oral infection. J. Immunol. 1999;162:5846–5852. [PubMed] [Google Scholar]
- Buzoni-Gatel D, Kasper LH. Innate Immunity in Toxoplasma gondii infection. In: Weiss LM, Kim K, editors. Toxoplasma gondii The model apicomplexan: perspectives and methods. London: Academic Press Elsevier; 2007. pp. 593–607. [Google Scholar]
- Buzoni-Gatel D, Lapage AC, Dimier-Poisson IH, Bout DT, Kasper LH. Adoptive transfer of gut intraepithelial lymphocytes protects against murine infection with Toxoplasma gondii. J. Immunol. 1997;158:5882–5889. [PubMed] [Google Scholar]
- Chardes T, Buzoni-Gatel D, Lepage A, Bernard F, Bout D. Toxoplasma gondii oral infection induces specific cytotoxic CD8 α/β+ Thy-1+ gut intraepithelial lymphocytes, lytic for parasite-infected enterocytes. J. Immunol. 1994;153:4596–4603. [PubMed] [Google Scholar]
- Cook DN, Prosser DM, Forster R, Zhang J, Kuklin NA, Abbondanzo SJ, Niu XD, Chen SC, Manfra DJ, Wiekowski MT, Sullivan LM, Smith SR, Greenberg HB, Narula SK, Lipp M, Lira SA. CCR6 mediates dendritic cell localization, lymphocyte homeostasis, and immune responses in mucosal tissue. Immunity. 2000;12:495–503. doi: 10.1016/s1074-7613(00)80201-0. [DOI] [PubMed] [Google Scholar]
- Courret N, Darche S, Songio P, Milon G, Buzoni-Gatel D, Tardieux I. CD11c and CD11b expressing mouse leukocytes transport single Toxoplasma gondii tachyzoites to the brain. Blood. 2005;107:309–316. doi: 10.1182/blood-2005-02-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J. Leuk. Biol. 2007;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- Del Rio L, Bennouna S, Salinas J, Denkers EY. CXCR2 deficiency confers impaired neutrophil recruitment and increased susceptibility during Toxoplasma gondii infection. J. Immunol. 2001;167:6503–6509. doi: 10.4049/jimmunol.167.11.6503. [DOI] [PubMed] [Google Scholar]
- Drevets DA, Dillon MJ, Schawang JS, Rooijen NV, Ehrchen J, Sunderkotter C, Leenen PJM. The Ly-6Chigh monocyte subpopulation transports Listeria monocytogenes into brain during systemic infection of mice. J. Immunol. 2004;172:4418–4424. doi: 10.4049/jimmunol.172.7.4418. [DOI] [PubMed] [Google Scholar]
- Fux B, Nawas J, Khan A, Gill DB, Su C, Sibley LD. Toxoplasma gondii strains defective in oral transmission are also defective in developmental stage differentiation. Infect. Immun. 2007;75:2580–2590. doi: 10.1128/IAI.00085-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzinelli R, Wysocka M, Hayashi S, Denkers E, Hieny S, Caspar P, Trinchieri G, Sher A. Parasite-induced IL-12 stimulates early INF-γ synthesis and resistance during acute infection with Toxoplasma gondii. J. Immunol. 1994;153:2533–2543. [PubMed] [Google Scholar]
- Geissmann F, Auffrey C, Palframan R, Wirrig C, Ciocca A, Campisis L, Narni-Manichelli E, Lauvau G. Blodd monocytes: distinct subsets, how they relate to dendritic cells, and their possible roles in the regulation of T-cell responses. Immunol. Cell Biol. 2008 doi: 10.1038/icb.2008.19. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principle subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- Heimesaat MM, Berewill S, Fischer D, Fuchs D, Struck D, Niebergall J, Jahn HK, Dunay IR, Moter A, Gescher DM, Schumann RR, Gobel UB, Liesenfeld O. Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. J. Immunol. 2006;177:8785–8795. doi: 10.4049/jimmunol.177.12.8785. [DOI] [PubMed] [Google Scholar]
- Johansson C, Kelsall BL. Phenotype and function of intestinal dendritic cells. Seminars Immunol. 2005;17:284–294. doi: 10.1016/j.smim.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Jung SJA, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell. Biol. 2000;20:4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan IA, Matsuura T, Kasper LH. Interleukin-12 enhances murine survival against acute toxoplasmosis. Infect. Immun. 1994;62:1639–1642. doi: 10.1128/iai.62.5.1639-1642.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuziel WA, Morgan SJ, Dawson TC, Griffin S, Smithies O, Ley K, Maeda N. Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2. Proc. Natl. Acad. Sci.(USA) 1997;94:12053–12058. doi: 10.1073/pnas.94.22.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert H, Hitziger N, Dellacasa I, Svensson M, Barragan A. Induction of dendritic cell migration upon Toxoplasma gondii infection potentiates parasite dissemination. Cell Micro. 2006;8:1611–1623. doi: 10.1111/j.1462-5822.2006.00735.x. [DOI] [PubMed] [Google Scholar]
- LeBorgne M, Etchart N, Groubier A, Lira SA, van Rooijen N, Caux C, Ait-Yahia S, Vicari A, Kaiserlian D, Dubois B. Dendritic cells rapidly recruited into epithelial tissues via CCR6/CCL20 are responsible for CD8+ T cell crosspriming in vivo. Immunity. 2006;24:191–201. doi: 10.1016/j.immuni.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Liesenfeld O. Oral infection of C57BL/6 mice with Toxoplasma gondii: a new model of inflammatory bowel disease? J. Infect. Dis. 2002;185:S96–S101. doi: 10.1086/338006. [DOI] [PubMed] [Google Scholar]
- Luther SA, Cyster JG. Chemokines as regulators of T cell differentiation. Nat. Immunol. 2001;2:102–122. doi: 10.1038/84205. [DOI] [PubMed] [Google Scholar]
- McKee AS, Dzierszinski F, Boes M, Roos DS, Pearce EJ. Functional inactivation of immature dendritic cells by the intracellular parasite Toxoplasma gondii. J. Immunol. 2004;173:2632–2640. doi: 10.4049/jimmunol.173.4.2632. [DOI] [PubMed] [Google Scholar]
- Mordue DG, Sibley LD. A novel population of Gr-1+ activated macrophages induced during acute toxoplasmosis. J. Leukoc. Biol. 2003;74:1015–1025. doi: 10.1189/jlb.0403164. [DOI] [PubMed] [Google Scholar]
- Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat. Rev. Immunol. 2003;3:331–341. doi: 10.1038/nri1057. [DOI] [PubMed] [Google Scholar]
- Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, Littman DR, Reinecker HC. CX3CR1 mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- Robben PM, Mordue DG, Truscott SM, Takeda K, Akira S, Sibley LD. Induction of IL-12 by Toxoplasma gondii depends on the parasite genotype. J. Immunol. 2004;172:3686–3694. doi: 10.4049/jimmunol.172.6.3686. [DOI] [PubMed] [Google Scholar]
- Robben PR, Laregina M, Kuziel WA, Sibley LD. Recruitment of Gr-1+ monocytes is essential for control of acute toxoplasmosis. J Exp. Med. 2005;201:1761–1769. doi: 10.1084/jem.20050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts CW, Gazzinelli RT, Khan IA, Nowakowska D, Esquivel A, Mcleod R. Adaptive immunity and genetics of the host immune response. In: Weiss LM, Kim K, editors. Toxoplasma gondii The model apicomplexan: perspectives and methods. London: Academic Press Elsevier; 2007. pp. 610–720. [Google Scholar]
- Salazar-Gonzalez RM, Niess JH, Zammit DJ, Ravindran R, Srinivasan A, Maxwell JR, Stoklasek T, Yadav R, Williams IR, Gu X, McCormick BA, Pazes MA, Vella AT, Lefrancois L, Reinecker HC, McSorley SJ. CCR6-mediated dendritic cell activation of pathogen-specific T cells in the Peyer's patch. Immunity. 2006;24:623–632. doi: 10.1016/j.immuni.2006.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk M, Mueller C. Adaptations of intestinal macrophages to an antigen-rich environment. Seminars Immunol. 2006;19:84–93. doi: 10.1016/j.smim.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat. Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19:59–70. doi: 10.1016/s1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- Sibley LD. Invasion strategies of intracellular parasites. Science. 2004;304:248–253. doi: 10.1126/science.1094717. [DOI] [PubMed] [Google Scholar]
- Sousa CR, Hieny S, Scharton-Kersten T, Jankovic D, Charest H, Germain RN, Sher A. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J. Exp. Med. 1997;186:1819–1829. doi: 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Orellana MA, Schreiber RD, Remington JS. Interferon-γ: the major mediator of resistance against Toxoplasma gondii. Science. 1988;240:516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- Tacke F, Randolph GJ. Migratory fate and differentiation of blood monocytes. 2007 doi: 10.1016/j.imbio.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Varol C, Lamdsman L, Fogg DK, Grennshtein L, Gildor B, Margalit R, Kalchenko V, Geissmann F, Jung S. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J. Exp. Med. 2007;204:171–180. doi: 10.1084/jem.20061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap GS, Sher A. Effector cells of both nonhemopoietic and hemopoietic origin are required for interferon (IFN)-γ- and tumor necrosis factor (TNF)-α- dependent host resistance to the intracellular pathogen, Toxoplasma gondii. J. Exp. Med. 1999;189:1083–1091. doi: 10.1084/jem.189.7.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarovinsky F, Zhang D, Andersen JF, Bannenberg GL, Serhan CN, Hayden MS, Hieny S, Sutterwala FS, Flavell RA, Ghosh S, Sher A. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science. 2005;308:1626–1629. doi: 10.1126/science.1109893. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Four Supplemental Figures can be found with this article online at: