Abstract
To augment the available influenza vaccine supply, a phase III study was conducted to evaluate the immunogenicity, safety, and consistency of a new trivalent inactivated influenza vaccine manufactured by CSL Limited. Healthy adults (ages 18–64) were randomized to receive either a single dose of TIV from multi-dose vials with thimerosal, TIV from pre-filled syringes without thimerosal, or placebo. Of the TIV recipients, 97.8% achieved a post-vaccination titer ≥ 40 against H1N1, 99.9% against H3N2 component, and 94.2% against influenza B. Few local or systemic adverse events were noted after vaccination with either TIV presentation. TIV was well tolerated and immunogenic.
Keywords: Influenza, vaccine, immunogenicity
1. Introduction
Each year in the United States (US), influenza A and B viruses infect between 5% to 20% of the population causing approximately 200,000 hospitalizations and 36,000 deaths[1]. Due to a greater appreciation of the burden of influenza in young children and their role in transmission and the desire to reduce the incidence of influenza in all age and at risk groups, the indications for use of influenza vaccine have recently been expanded[1]. However, since 2000, the influenza vaccine supply in the US has suffered several major disruptions, including repeated manufacturing delays and the unanticipated removal of nearly 50% of the entire U.S. influenza vaccine supply during the 2004–2005 influenza season [2]. These supply reductions highlight the need for multiple manufacturers to supply seasonal influenza vaccine to the US, particularly those from other countries [3]. The National Institutes of Health (NIH) Vaccine Treatment and Evaluation Units (VTEU) conducted a large randomized trial of two presentations of CSL trivalent inactivated influenza vaccine (TIV) for the purposes of licensure. A Biologics License Application (BLA) for the vaccine was subsequently filed on 30th March 2007 and approved on 28th September, 2007.
2. Methods
2.1. Study Design
This study was conducted as a phase III, randomized, double-blinded, placebo-controlled, multi-center trial at nine VTEU sites to evaluate the immunogenicity, safety, and tolerability of CSL influenza vaccine in healthy adults between the ages of 18–64 years. Subjects were excluded if they had a known hypersensitivity to influenza vaccine or an allergy to eggs or other vaccine components; had an underlying illness for which influenza vaccination was routinely recommended; were pregnant or lactating; were acutely ill at the time of vaccination; had a previous history of Guillain-Barré syndrome; were receiving immunosuppressive or immunomodulating agents; or had received a previous influenza vaccine within 6 months prior to the study onset. Subjects who met the entry criteria for the study were stratified into two age groups (18–49 and 50–64 years) and randomized in a 1:1:1:1:1 ratio to receive either one of three lots of influenza vaccine in multi-dose vials (thimerosal-containing), a single lot of vaccine in pre-filled syringes (thimerosal-free), or placebo in multi-dose vials (thimerosal-containing). The three production lots of multi-dose vials were used to assess lot consistency.
2.2. Study Vaccines
The CSL TIV is a purified, inactivated, split-virion vaccine with each dose containing 15 µg of each of the three contemporary influenza hemagglutinin antigens per dose (total 45 µg). The vaccine was the 2006 Southern Hemisphere recommended formulation: A/New Caledonia/20/99 (IVR-116) (H1N1)-like strain, A/New York/55/2004-NYMC X- 157(H3N2)-like strain, and B/Malaysia/2506/2004-like strain. The multi-dose vials contained the following excipients in each 0.5-mL dose; 50 µg of thimerosal, 4.1 mg sodium chloride, 80 µg monobasic sodium phosphate, 300 µg dibasic sodium phosphate, 20 µg potassium phosphate, 20 µg potassium chloride, and 1.5 µg calcium chloride. The pre-filled syringes contained the same excipients, with the exception of thimerosal. Three multi-dose lots were prepared for the study, each formulated from 3 different lots of Monovalent Pooled Harvests from each of the vaccine strains (9 Monovalent Pooled Harvests). One of the multi-dose lots was used to formulate the pre-filled syringe presentation. The placebo contained phosphate buffered saline and the following excipients per 0.5-mL dose; 4.1 mg sodium chloride, 120 µg monobasic sodium phosphate, 245 µg dibasic sodium phosphate, and 50 µg of thimerosal.
2.3. Vaccine Administration
Vaccine was administered by intramuscular injection into the deltoid muscle of the non-dominant arm by an unblinded vaccine administrator. Local and systemic signs and symptoms were assessed in the clinic for at least 30 minutes after vaccination. Subjects were also instructed to maintain a post-vaccination memory aid for four days that solicited local and systemic adverse events (AEs) and another post-vaccination memory aid for 20 additional days for recording unsolicited AEs. The subjects recorded oral temperatures and measured any redness or swelling at the vaccination site at the same time each day. Subjects returned to the clinic with memory aids on Day 5–7 and Day 21–24 to review solicited and unsolicited AEs with clinic staff.
2.4. Laboratory Assays
Serum hemagglutination inhibition (HI) assays were performed by Focus Diagnostics, Cypress, CA on venous blood samples (20 mL) collected from each subject before vaccination and on Day 21 after immunization. Pre- and post-vaccination serum samples were simultaneously assessed in triplicate.
2.5. Statistics
The primary objectives of this study were to demonstrate that the two presentations of vaccine produced 40% seroconversion (defined as an increase in HI antibody titer of at least 4-fold, with a minimum post-vaccination HI titer of 40) and 70% seroprotection (defined as a minimum post-vaccination HI titer of 40) to each of the three individual influenza antigens. Secondary objectives included demonstrating clinical consistency between the three lots of the multi-dose vial presentations and between the multi-dose vial and the pre-filled syringe presentations, and demonstrating the safety and tolerability of the vaccine. Additional analyses were performed to determine the effect of age and history of prior vaccination on immunogenicity.
The sample size was chosen based on the power required to meet the immunogenicity endpoints. The true seroconversion rate was assumed to be at least 45.4%, so that with a total sample size of N=1000, the power for this comparison would exceed 93% for each antigen. Assuming the true seroprotection rate was at least 75%, then with a total sample size of N=1000, the power for this comparison would exceed 93% for each antigen. In addition, with the projected enrollment, the study would have at least 95% power to detect a significant safety event with incidence rates of at least 0.33%.
Consistency of immune responses across the three different vaccine lots was demonstrated by calculating the post-vaccination log titers for the 3 lots, with pre-vaccination log titers serving as covariates. Safety and tolerability were assessed by comparing the proportion of subjects who experienced local (induration, erythema, vaccination site pain, tenderness, and ecchymosis) and systemic adverse events (fever, headache, malaise, myalgia, chills, nausea, and vomiting) during the 4 days following vaccination (Day 0 through Day 4) in each of the study groups.
3. Results
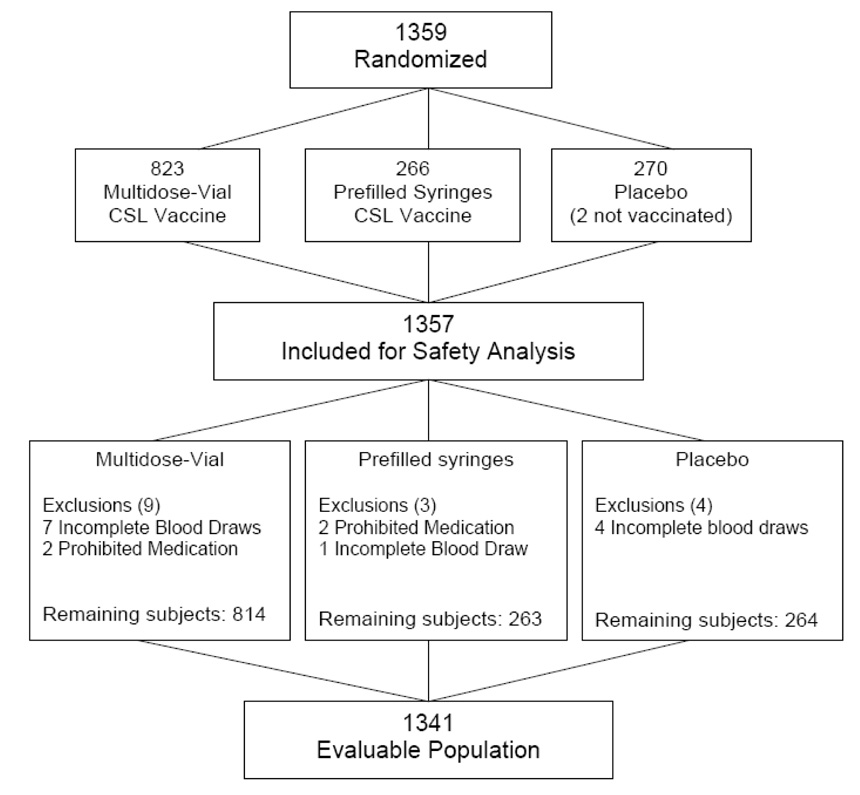
A total of 1359 subjects (see Figure 1), were enrolled and 1357 subjects received either TIV or placebo from 12 June 2006 to 01 August 2006. A total of 823 subjects received the multi-dose lots (Lot 1 n=273, Lot 2 n=275, Lot 3 n=275); 266 subjects received the pre-filled, thimerosal-free syringes, and 268 subjects received placebo. Table 1 demonstrates that the subjects were randomly distributed between the different vaccine formulations by age, gender, and race. All subjects who received any study vaccine or placebo were included in the safety assessment.
Figure 1.
Flow Chart of Subject Disposition
Table 1.
Demographic characteristics of vaccinated subjects
| Multi-dose Vial (Thimerosalcontaining) (Lots 1, 2, & 3) | Pre-filled Syringe (Thimerosal-free) | Placebo | ||
|---|---|---|---|---|
| Number of Subjects Enrolled | 823 | 266 | 268 | |
| Mean Age (years) (min, max) | 38 (18,64) | 38.2 (18,64) | 38.2 (18,64) | |
| Male Gender (N, %) | 303 (36.8%) | 103 (38.7%) | 90 (33.6%) | |
| Race* (%) | ||||
| Hispanic or Latino Ethnicity | 39 (4.7%) | 8 (3.0%) | 5 (1.9%) | |
| Asian | 49 (6%) | 19 (7.1%) | 15 (5.6%) | |
| Black or African-American | 101 (12.3%) | 33 (12.4%) | 31 (11.6%) | |
| White | 667 (81.0%) | 217 (81.6%) | 219 (81.7%) | |
| Other | 23 (2.8%) | 1 (0.4%) | 8 (3.0%) | |
| Reason for Termination (%) | ||||
| Lost to follow-up | 3 (0.4%) | 1 (0.4%) | 1 (0.4%) | |
| Voluntary withdrawal | 0 (0%) | 0 (0%) | 1 (0.4%) | |
Subjects in the study could be counted in more than one race.
A total of 1351 subjects (99.5%) completed the study, and 8 subjects (0.6%) were withdrawn: five subjects were lost to follow-up, one subject withdrew from the study voluntarily, and two subjects were randomized but not vaccinated (Figure 1). There were no withdrawals due to vaccine-associated adverse events.
Subjects without paired pre- and post-vaccination blood samples and individuals who received either steroids or immunomodulating agents after enrollment were excluded, leaving a total evaluable population of 1341 subjects: 814 subjects receiving vaccine from the multi-dose vials (Lot 1 n=270 [98.9%], Lot 2 n=275 [100%], Lot 3 n=269 [97.8%]); 263 subjects (98.9%) receiving vaccine from the pre-filled syringes, and 264 subjects (97.8%) receiving placebo (see Figure 1).
Table 2 presents the immunogenicity data on the 1341 subjects in the evaluable population. The proportion of subjects who achieved seroprotection (post-vaccination titer ≥ 40) was 97.8% (95% CI 96.7%, 98.6%) for the A/New Caledonia strain, 99.9% (95% CI 99.5%, 100.0%) for the A/New York strain, and 94.2% (95% CI 92.7%, 95.6%) for the B/Malaysia strain. All presentations of vaccine yielded comparable immune responses.
Table 2.
Co-primary immunogenicity endpoint* results by presentation, lot, placebo and for all active vaccine formulations
| Multi-dose Vial (Thimerosal Containing) | |||||||
|---|---|---|---|---|---|---|---|
| Influenza Strain | Immunogenicity evaluation | Lot 1 N=270n (%) | Lot 2 N=275 n (%) | Lot 3 N=269 n (%) | Prefilled Syringe (Thimerosal-free) N= 263 n (%) | All active vaccines N=1077 n (%) | Placebo N=264 n (%) |
| H1N1: A/Caledonia/20/99 | Seroconversion or Significant Increase in Titer | 131 (48.5%) |
133 (48.4%) |
132 (49.1%) |
128 (48.7%) |
524 (48.7%) |
6 (2.3%) |
| Seroprotection | 261 (96.7%) |
270 (98.2%) |
262 (97.4%) |
260 (98.9%) |
1053 (97.8%) |
197 (74.6%) |
|
| H3N2:A/New York/55/2004 | Seroconversion or Significant Increase in Titer | 187 (69.3%) |
196 (71.3%) |
203 (75.5%) |
184 (70%) |
770 (71.5%) |
0 (0%) |
| Seroprotection | 270 (100.0%) |
274 (99.6%) |
269 (100.0%) |
263 (100.0%) |
1076 (99.9%) |
190 (72.0) |
|
| B Strain: B/Malaysia/2506/2004 | Seroconversion or Significant Increase in Titer | 194 (71.9%) |
187 (68.0%) |
186 (69.1%) |
184 (70.0%) |
751 (69.7%) |
1 (0.4%) |
| Seroprotection | 258 (95.6%) |
258 (93.8%) |
250 (92.9%) |
249 (94.7%) |
1015 (94.2%) |
124 (47.0%) |
|
Seroconversion or Significant Increase in titer is defined as pre-vaccination HI < 10 increased to HI ≥ 40 postvaccination or fourfold rise in prevaccination titer. Seroprotection is defined as HI ≥ 40.
Table 3 displays post-vaccination sero-protection rates based on age groups of subjects at the time of enrollment. Sero-protection rates were similar in all age groups for the H1N1 and H3N2 strains, but were slightly lower with age for the B strain. Subjects last vaccinated in the previous year were more likely to have had pre-vaccination seroprotective titres compared with subjects who were last vaccinated either 2 years or more ago (Table 4). Pre-vaccination seroprotection rates were generally much lower for the B strain than the other strains.
Table 3.
Percentage of subjects with seroprotective levels of antibodies by influenza strain and age group.
| Influenza Strain | 18–34 years N=507 % (95% CI) | 35–49 years N=280 % (95% CI) | 50–64 years N=294 % (95% CI) |
|---|---|---|---|
| H1N1 | 99.0 (97.7, 99.6) | 97.1 (94.5,98.5) | 96.2 (93.4,97.9) |
| H3N2 | 99.8 (98.9,100.0) | 100.0 (98.6,100.0) | 100.0 (98.7,100.0) |
| B | 96.6 (94.7,97.9) | 93.9 (90.5,96.2) | 90.5 (86.6,93.3) |
Seroprotection is defined as HI ≥ 40
Table 4.
Percent of subjects with prevaccination seroprotective titers by influenza strain and year of the last influenza vaccine received
| Influenza Strain | Last year N=626 % (95% CI) | 2 years ago N=138 % (95% CI) | > 2 years ago N=594 % (95% CI) |
|---|---|---|---|
| H1N1 | 86.9 (84.0,89.3) | 81.2 (73.8,86.8) | 57.1 (53.1,61.0) |
| H3N2 | 92.2 (89.8,94.0) | 73.2 (65.2,79.9) | 54.7 (50.7,58.7) |
| B | 60.7 (56.8,64.5) | 50.0 (41.8,58.2) | 24.6 (21.3,28.2) |
Seroprotection is defined as HI ≥ 40
The solicited adverse events of induration, erythema, pain, tenderness, bruising and myalgia were reported more often after vaccine than placebo (Table 5). In general the solicited adverse event profile was comparable between the thimerosal-free and thimerosal-containing preparations. However, injection site pain (47% vs 37.4%, p=0.0031), and tenderness (68.0% vs 57.1%, p=0.0007) were more frequently seen with the thimerosal-free than the thimerosal-containing preparation. P values were not adjusted for multiple comparisons.
Table 5.
Proportion of subjects reporting solicited adverse events on Days 0–4
| Multi-dose Vial (Thimerosal-containing) N=823 | Pre-filled Syringe (Thimerosal-free) N=266 | Placebo N=268 | ||||
|---|---|---|---|---|---|---|
| Symptom | Any % (95% CI) | Severe % | Any % (95% CI) | Severe % | Any % (95% CI) | Severe % |
| Fever | 1.1 (0.5,2.1) |
0.0 | 1.5 (0.4,3.8) |
0.0 | 0.7 (<0.1,2.7) |
0.0 |
| Headache | 25.2 (22.2,28.3) |
0.2 | 27.1 (21.8,32.8) |
1.1 | 25.7 (20.6,31.4) |
0.4 |
| Malaise | 18.8 (16.2,21.7) |
0.1 | 21.4 (16.7,26.9) |
1.5 | 18.7 (14.2,23.8) |
0.4 |
| Myalgia | 12.2 (10.0,14.6) |
0.0 | 15** (11.0,19.9) |
0.8 | 9.0 (5.8,13.0) |
0.7 |
| Chills | 3.3 (2.2,4.7) |
0.0 | 2.3 (0.8,4.8) |
0.4 | 2.2 (0.8,4.8) |
0.0 |
| Nausea | 5.7 (4.2,7.5) |
0.2 | 8.6 (5.6,12.7) |
0.4 | 8.6 (5.5,12.6) |
0.4 |
| Vomiting | 0.9 (0.3,1.7) |
0.2 | 0.8 (<0.1,2.7) |
0.4 | 0.7 (<0.1,2.7) |
0.4 |
| Induration (swelling) | 10.0** (8.0,12.2) |
0.1 | 6.8** (4.1,10.5) |
1.1† | 0.7 (<0.1,2.7) |
0.0 |
| Erythema (redness) | 17.7** (15.2,20.5) |
0.2 | 12.0 (8.4,16.6) |
0.0‡ | 8.2 (5.2,12.2) |
0.0 |
| Vaccination site pain | 37.4** (34.1,40.8) |
0.0 | 47** (40.9,53.2) |
0.0 | 9.3 (6.1,13.5) |
0.0 |
| Tenderness | 57.1** (53.6,60.5) |
0.0 | 68** (62.1,73.6) |
0.0 | 17.9 (13.5,23.0) |
0.0 |
| Ecchymosis (bruising) | 5.1** (3.7,6.8) |
0.0 | 3.8 (1.8,6.8) |
0.0 | 1.1 (0.2,3.2) |
0.0 |
P <0.05 (P-values are from comparison of each vaccine to placebo.)
Severe induration is defined as > 50mm
Severe eyrthema is defined as > 50mm
Of those subjects who reported AEs, most symptoms were mild to moderate in intensity. During the 20-days post-vaccination period the majority of unsolicited AEs were mild (350) or moderate (191) with induration, erythema, and pain seen significantly more often after vaccine than placebo. A smaller percentage of subjects had unsolicited AEs that were considered severe in intensity (1.9% in the placebo group, 1.1% thimerosal-free preparation, and 0.9% in the thimerosal-containing vials). No severe intensity unsolicited AEs were judged to be associated with vaccine.
One subject, a health care worker who had been previously vaccinated on multiple occasions without adverse events, experienced generalized urticaria, arthralgias, and dermatographism beginning 24 hours after immunization, which has persisted for at least one year and has been diagnosed as serum sickness syndrome. The subject had no prior history of reaction to vaccines, thimerosal or egg protein, nor a family history of allergy. The event was assessed as vaccine associated (Lot 3 multi-dose vial, thimerosal-containing presentation). Another subject became pregnant at approximately the time of vaccination and delivered a healthy baby. There were no vaccine related serious adverse events during the study.
4. Discussion
For the licensure of a new seasonal influenza vaccine, the FDA provides definitive benchmarks that must be achieved [4]. According to these benchmarks, 40% of subjects between the ages of 18 to 65 years must achieve seroconversion and > 70% must achieve seroprotection. The new CSL vaccine studied in this report exceeded these requirements with > 90% of subjects achieving post-immunization titers ≥ 40 for all three vaccine antigens. Further, this immunogenic response was consistent across the three different lots of vaccine and between the two presentations, multi-dose vials and pre-filled syringes. Although this trial did not provide a direct comparison of the study vaccine to already licensed TIV products, achieving these protocol-specified immunogenicity endpoints allowed licensure on September 28, 2007.
These excellent immunogenicity results were shown in healthy adults 18–64 years of age, but the trial did not include elderly adults or adults with a medical history that would be recommended for influenza vaccination. When the three different age groups within the study were compared, each age group achieved a consistently immunogenic response, with a trend for lower seroprotection rates in older age groups to the H1N1 and B strains Additional comparative studies in younger and older individuals of the different TIV vaccines would be necessary to definitively compare the products in other age groups.
This study showed high seroconversion and seroprotection rates associated with the CSL influenza B antigen, similar to the responses to the influenza A antigens. Reported immune responses to the B antigens are quite variable in the literature. Recent data published by Belshe et al[5, 6], have shown similar immune responses to influenza B. However, other reports have demonstrated poorer responses to the B antigen[7, 8]. One reason for the high seroresponse rates in our study may be that 43% of subjects in this study had seroprotective levels of antibodies prior to vaccination.
The CSL inactivated influenza vaccine was well tolerated with few local or systemic adverse events. However, an uncommon adverse event of moderate intensity was noted in one subject, a persistent generalized urticarial reaction diagnosed as serum sickness and assessed to be vaccine-associated. Serum sickness has previously been associated with both pneumococcal and tetanus vaccination[9, 10], but there are no data to assess the frequency of this event. The subject with serum sickness had been exposed to influenza vaccines repeatedly in the past without recalling any prior reactions.
Although the CSL vaccine has not been licensed previously in the United States, over 39 million doses of vaccine have been administered in 16 different countries, including Australia and several countries in Europe. This study demonstrates that the CSL inactivated influenza vaccine was well tolerated and induced comparable antibody titers to those previously reported in studies of US-licensed TIV vaccines [3, 5]. The licensure of an additional influenza vaccine is welcome since it will provide additional doses of vaccine to accommodate potential vaccine shortages and support the expanding recommendations for influenza vaccine in the US population.
Acknowledgements
This work was supported by the NIH contracts: N01 AI 25462 (Vanderbilt), N01 AI 25465 (Baylor College of Medicine), N01 AI 25460 (University of Rochester), N01 AI 25464 (St. Louis University), N01 AI 25459 (Cincinnati Children’s Hospital Medical Center), N01 AI 25461 (University of Maryland) and the following GCRC grants: M01 RR-00095 (Vanderbilt University Hospital), M01 RR00044 (University of Rochester), M01 RR00059 (University of Iowa), and M01 RR00070 (Stanford University) and M01 RR16500 (University of Maryland).
Dr. Talbot received salary support and career development from the NIH/NCRR (5 K12 RR017697–05, Dr. Nancy Brown, PI Vanderbilt Mentored Clinical Research Scholar Program) and from the NIAID (1K23AI074863-01).
Statistical analysis was done by Chiltern International Limited and additional post-hoc analysis was done by CSL Limited.
The CSL brand of influenza virus vaccine (AFLURIA®) was approved by the FDA on September 28, 2007 and will be distributed by CSL Biotherapies Inc., King of Prussia, PA 19406, USA
We would like to thank all the study staff for their help and the volunteers for their participation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fiore AE, Shay DK, Haber P, Iskander JK, Uyeki TM, Mootrey G, et al. Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007. MMWR Recomm Rep. 2007;56(RR6):1–54. [PubMed] [Google Scholar]
- 2.Tiered use of inactivated influenza vaccine in the event of a vaccine shortage. MMWR Morb Mortal Wkly Rep. 2005;54(30):749–750. [PubMed] [Google Scholar]
- 3.Treanor JJ, Campbell JD, Brady RC, Keitel WA, Drame M, Jain VK, et al. Rapid licensure of a new, inactivated influenza vaccine in the United States. Hum Vaccin. 2005;1(6):239–244. doi: 10.4161/hv.1.6.2376. [DOI] [PubMed] [Google Scholar]
- 4.Food and Drug Administration CfBEaR. FDA; Guidance for Industry: Clinical Data Needed to Support the Licensure of Trivalent Inactivated Influenza Vaccines. 2006 March 2006 ed.
- 5.Belshe RB, Newman FK, Cannon J, Duane C, Treanor J, Van Hoecke C, et al. Serum Antibody Responses after Intradermal Vaccination against Influenza. N Engl J Med. 2004;351(22):2286–2294. doi: 10.1056/NEJMoa043555. [DOI] [PubMed] [Google Scholar]
- 6.Belshe RB, Newman FK, Wilkins K, Graham IL, Babusis E, Ewell M, et al. Comparative immunogenicity of trivalent influenza vaccine administered by intradermal or intramuscular route in healthy adults. Vaccine. 2007;25(37–38):6755–6763. doi: 10.1016/j.vaccine.2007.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frey S, Poland G, Percell S, Podda A. Comparison of the safety, tolerability, and immunogenicity of a MF59-adjuvanted influenza vaccine and a non-adjuvanted influenza vaccine in non-elderly adults. Vaccine. 2003;21(27–30):4234–4237. doi: 10.1016/s0264-410x(03)00456-0. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell DK, Ruben FL, Gravenstein S. Immunogenicity and safety of inactivated influenza virus vaccine in young children in 2003–2004. Pediatr Infect Dis J. 2005;24(10):925–927. doi: 10.1097/01.inf.0000180978.66362.d9. [DOI] [PubMed] [Google Scholar]
- 9.Edsall G. Serum Sickness After Tetanus Toxoid Infection. Jama. 1972;220(1):137. [Google Scholar]
- 10.Hengge UR, Scharf RE, Kroon FP, Pfeffer K. Severe serum sickness following pneumococcal vaccination in an AIDS patient. Int J STD AIDS. 2006;17(3):210–211. doi: 10.1258/095646206775809123. [DOI] [PubMed] [Google Scholar]