Abstract
The School Based Asthma Therapy (SBAT) trial builds on a pilot study in which we found that school-based administration of preventive asthma medications for inner-city children reduced asthma symptoms. However, the beneficial effects of this program were seen only among children not exposed to environmental tobacco smoke (ETS). The current study is designed to establish whether this intervention can be enhanced by more stringent adherence to asthma guidelines through the addition of symptom-based medication dose adjustments, and whether smoke-exposed children benefit from the intervention when it is combined with an ETS reduction program. The intervention consists of both administration of preventive asthma medications in school (with dose adjustments according to NHLBI guidelines) and a home-based ETS reduction program utilizing motivational interviewing principles. This paper describes the methodology, conceptual framework, and lessons learned from the SBAT trial. Results of this study will help to determine whether this type of comprehensive school-based program can serve as a model to improve care for urban children and reduce disparities.
Keywords: asthma, children, preventive care, schools, environmental tobacco smoke, adherence
Introduction
Asthma is one of the most common chronic illnesses of childhood (1–3), and hospitalization rates are on the rise (4–6). Asthma causes morbidity from ongoing and recurrent symptoms as well as impairment of quality of life and functional impairment, including limitation of activity, absenteeism from school, and missed days of work for caretakers. In the United States, impoverished children and children from minority ethnic and racial backgrounds suffer disproportionately from asthma (7–10). There are numerous studies available that document disparities in medication use, health care utilization, and asthma-related outcomes in minority patients (11–15).
Experts believe that many of the problems caused by asthma could be averted if the disease was managed according to established guidelines (16). The NHLBI has convened several expert panels to prepare guidelines for the diagnosis and management of asthma. One of the key recommendations in the guidelines is that all patients with persistent asthma symptoms (whether mild, moderate, or severe) receive daily preventive anti-inflammatory medications (17–20). However, studies indicate that many children in the United States who should receive preventive medications are not receiving them (11, 21–23). The greatest underuse of preventive medications occurs among poor children living in the inner city (12, 13, 24).
Environmental tobacco smoke (ETS) has been associated with worsening asthma symptoms and decreased pulmonary function in young children with asthma (25–29). Children exposed to maternal smoking have higher requirements for medications and more frequent visits to the emergency department (30–32). This is particularly pertinent for young urban children, since they experience greater exposure to environmental tobacco smoke compared to other children (33–35). In addition, data suggest that cigarette smoking may reduce the anti-inflammatory action of glucocorticoids (36), making management of symptoms more difficult (37, 38). Thus, the influence of environmental tobacco smoke exposure warrants consideration in asthma research and management.
There currently are no practical, systemwide interventions for urban children with asthma that assure that children take the medications they should be receiving. The School-Based Asthma Therapy (SBAT) trial is designed to improve adherence to the guidelines for preventive asthma care through a comprehensive school-based program. This study builds on our pilot study of school-based administration of preventive asthma medications, including 180 urban children from 54 schools in the Rochester City School district (39). We found that parents of children receiving preventive asthma medications through school had a greater improvement in quality of life compared with parents of children in the usual-care group (change score, 0.63 vs. 0.24; p = .047); also, children in the school-based care group versus the usual-care group missed less school because of asthma (mean total days missed, 6.8 vs. 8.8; p = .047) and experienced more symptom-free days during the early winter months (mean days per two-week period, 9.2 vs. 7.3; p = .02). However, all of these significant findings were produced by differences among children who were not exposed to secondhand smoke, and there was essentially no effect of the intervention for the children exposed to smoke in the home.
The SBAT trial builds on this work by testing both administration of preventive asthma medications in school (with dose adjustments according to NHLBI guidelines) and a home-based ETS reduction program for smoke-exposed children. We hypothesize that children receiving the intervention will experience less asthma-related morbidity compared to children receiving usual care. This paper describes the methodology and conceptual framework of the SBAT trial. Results of this study will help to determine whether this type of comprehensive school-based program can serve as a model to improve care for urban children and reduce disparities.
Study Population
We are currently recruiting children 3–10 years of age attending preschool or elementary school in the Rochester City School District. This includes more than 50 sites. A total of 530 children will be recruited into the study over three years. All children whose parents have indicated that they have asthma on their school screening forms are identified. Parents of these children receive a telephone survey to determine if the child meets study criteria.
In order to be eligible for the intervention, children must have physician-diagnosed asthma (based on parent report with validation from the child’s physician) with mild persistent to severe symptoms (based on NHLBI guidelines [18, 20]). The child must attend school in the Rochester City School District preschools or elementary schools. In addition, the physician for each child must sign an authorization form to enroll the child stating that they agree with the child’s need for a daily inhaled corticosteroid. While the majority of children in the Rochester community have a usual care provider, any child without a primary care provider (PCP) is offered assistance in identifying a physician and may be included in the study if their new provider authorizes their participation. We exclude families of children with other significant medical conditions, including congenital heart disease, cystic fibrosis, or other chronic lung disease, that could interfere with the assessment of asthma-related outcome measures. Enrollment and randomization occur in a rolling fashion from the first day of school until the second week of November.
We use home visits to elicit informed consent and to obtain baseline measurements. Baseline evaluations include an assessment of asthma severity, standard demographic, family, and health history variables, exposure to ETS by interview survey using validated tools, salivary cotinine measurements, measurement of pulmonary function (using the PiKo-1 device from Pulmonary Data Services, Inc. [Louisville, CO] for peak expiratory flow [PEF] and forced expiratory volume in the first second [FEV1] of expiration), and completion of a home environmental assessment for asthma triggers. An asthma symptom diary is given to families for the tracking of asthma symptoms during the study.
Design
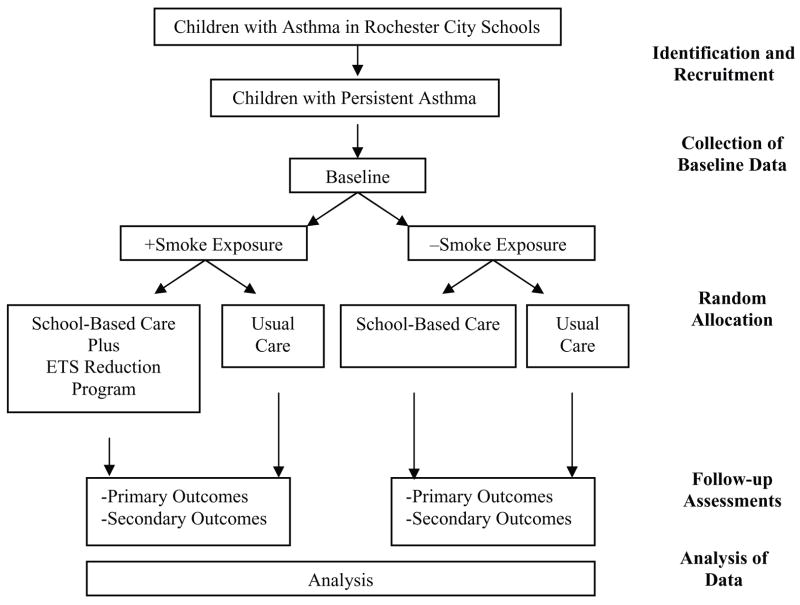
Following completion of the baseline assessment, each child is stratified based on the presence or absence of spoke exposure in the home (by parent report) and then randomized into either the school-based care group, where preventive asthma medications are provided by the school nurse, or the usual care group where medications are not provided by the schools (Figure 1). Two hundred and sixty-five subjects in both the smoke exposed and unexposed groups will be randomized.
Figure 1.
Summary of study design, N =530.
Families of smoke-exposed children in the school-based care group also receive an ETS reduction program (described below). After randomization, children are followed prospectively and systematically for the remainder of the school year (seven to eight months). The primary outcome measure is the number of symptom-free days averaged over the peak asthma season (November to February). This outcome measure is consistent with the symptom monitoring suggested by the national guidelines for asthma care, has been suggested as an appropriate surrogate marker for asthma control (40) and corresponds to the time frame when we saw greatest benefit in the pilot program (39). Secondary outcome measures include clinical outcomes (e.g., symptoms, health care use), functional outcomes (e.g., functional severity, absenteeism, and caregiver quality of life), and both direct and indirect costs (e.g., productivity costs, medical costs).
Intervention Group (School-Based)
The parent or guardian of children in the school-based care group is given a canister of preventive medication (either fluticasone propionate or fluticasone propionate with salmeterol) with a spacer and mask as appropriate, and shown the proper administration technique. The family uses this inhaler for medication doses on weekend days and other days in which the child does not attend school. A second medication canister is delivered to the child’s school nurse for use on the days in which the child attends school. The school nurses also are shown the proper administration technique and are instructed to deliver one dose of medication to the child during the school day. Nurses track medication delivery on a daily medication administration sheet. While adherence to medication administration is assured by the nurse on the days the child attends school, adherence is encouraged but not assured on days the child does not attend school.
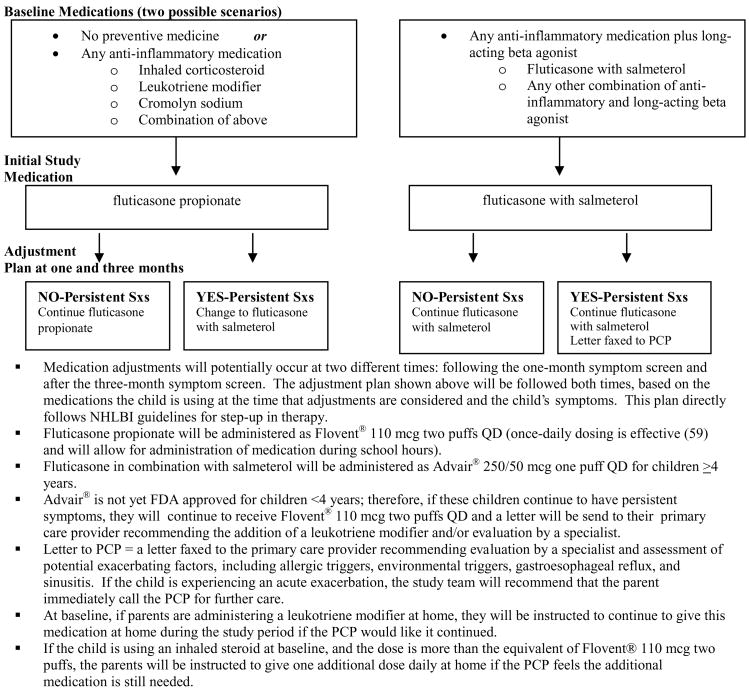
Assessment for possible medication dose adjustments occurs twice during the study period: following the first monthly follow-up interview and following the three-month follow-up interview. The symptom information from each of these interviews is relayed to the study team and medication adjustments are made for children who continue to have persistent symptoms. Figure 2 shows the protocol for initial medication choice and dose adjustments. Information regarding changes in the child’s regimen is relayed promptly to the child’s physician by facsimile and to the family by telephone, and both parties must agree prior to the implementation of the adjusted dose.
Figure 2.
Medication schematic.
Families of smoke-exposed children in the school-based care group receive an ETS reduction program aimed at motivating smoking cessation among family members and decreasing their child’s exposure to ETS. Previous studies suggest that face-to-face ETS reduction interventions for parents of children with asthma can yield substantial reductions in ETS exposure (41, 42). Specifically, motivational interviewing (MI), a patient-centered counseling technique to build intrinsic motivation for change (43), has shown promise in helping caregivers of children with asthma quit smoking and reduce their children’s exposure to ETS (44, 45). The current study utilizes MI principles and is adapted from previous intervention programs by Borrelli and colleagues that focus on motivating parents of children with asthma to quit smoking (45). Patients are not confronted about the need for change; rather, the MI counselor helps the patient explore their reasons for engaging in the risky behavior (e.g., smoking, ETS exposure) and attempts to elicit the patient’s own concerns about the risky behavior. The MI practitioner acts as a collaborator and consultant who provides guidance to the patient in their decision making.
The intervention focuses on reducing total household ETS exposure by using MI principles to: (1) counsel the primary caregiver about how to reduce ETS in the home if they are ready, willing, and able to do so, (2) motivate the caregiver to reduce ETS in the home if they are not ready to do so, and (3) provide brief smoking cessation counseling tailored to smokers’ readiness to quit. Regarding the latter, if the primary caregiver is not a smoker, the cessation intervention is done with the smoker who spends the most time with the child. Information on the child’s cotinine level is given using a feedback graph and is used to enhance the caregiver’s perception of risk to the child’s health. The intervention consists of a 30–40-minute home-based counseling session two to three weeks after the baseline evaluation, one session given to the primary caregiver focusing on reducing household ETS and a separate session for the smoker in the household. A total of two follow-up phone calls (10–15 minutes each) are provided to both the primary caregiver and the smoker one and three months after the face-to-face counseling sessions. The intervention nurses for this program were trained in motivational interviewing over the course of two days by clinical psychologists with extensive experience in MI (Borrelli and colleagues), and several methods are used to assure treatment fidelity (e.g., routine review of audiotaped MI sessions with feedback from trainers, scoring of sessions according to the Motivational Treatment Integrity System (MITI), and additional in-person training to boost skills).
Usual Care Group
Families of children in the usual care group are encouraged to promptly contact their primary care provider to discuss the child’s persistent asthma symptoms. These families are responsible for filling prescriptions from their physicians and administering medications daily to the child. To assure prompt care and safety for children in both groups, any child experiencing an acute asthma exacerbation at the time of a home visit or follow-up phone call is referred immediately to their PCP for care. In addition, we instruct school nurses to contact the parent of any child presenting to the nurses office with acute symptoms and refer them promptly to their PCP.
Theoretical Framework
The framework for this intervention was adapted from Wagner’s Chronic Care Model (CCM) (46–48). This model currently is being implemented in a number of health care systems as an organizing framework for collaborative quality-improvement efforts (49). The CCM serves as a framework to identify the essential elements of a health care system that encourage high-quality chronic disease care. The model is designed to apply to a variety of chronic illnesses, health care settings, and populations (50).
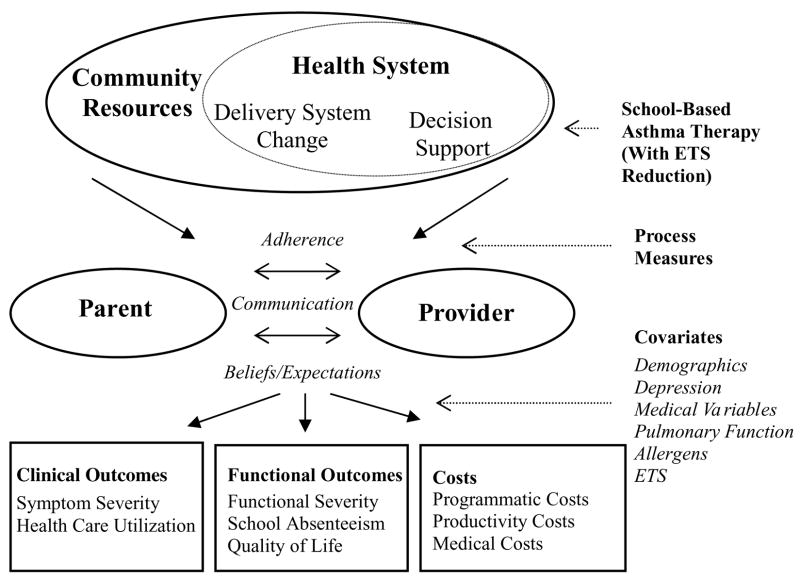
Figure 3 shows an adaptation of the CCM as it relates to the SBAT trial. Three main components of the CCM are incorporated into the intervention, including delivery system change and decision support within the health system, and community resources to support the system. These components together serve to coordinate an interlinking of care between the parents and the primary care providers to improve adherence to medications and communication about symptoms, and alter beliefs and expectations in order to improve both clinical and functional outcomes and reduce costs.
Figure 3.
An adaptation of the chronic care model.
The components of the CCM that are incorporated into the intervention include:
Community Resources: The CCM states that the performance of a health care system can be improved by establishing linkages with community resources, and that this is particularly important for vulnerable populations of patients. This school-based asthma intervention utilizes school-based care in the community to assure adherence to recommended medications for poor children with asthma. Such linkages have been proven most effective when connected to primary care (51). In this intervention, the medical home remains unchanged, the PCP directs the patient’s care, and the PCP is informed of, and must approve of, all intervention components prior to their implementation. Interaction between the family and the PCP is encouraged in the context of community-based care delivery.
Health System–Delivery System Change: Wagner suggests that effective chronic illness management involves changes to the existing care-delivery models. This study tests a model of delivery of preventive asthma medications through schools. In additional support of this model, there is evidence in other therapeutic settings that treatment that is directly observed can be effective (52–55). This intervention tests collaboration with the school nurses to provide directly observed administration of preventive asthma medications.
Health System–Decision Support: Simply educating practitioners about guidelines does little to change practice behavior (56). The CCM suggests that implementation of guidelines requires collaborative support from specialists and support tools and prompts. We are implementing care according to national guidelines by notifying parents and physicians of the child’s symptoms and recommending medications as supported by the national guidelines for asthma care. In addition, we incorporate a feedback mechanism to the PCP and the family about the child’s symptoms with appropriate recommendations for medication adjustments based on the child’s symptoms.
All of these components serve to facilitate the delivery of primary preventive care for children with asthma and allow for productive interactions between the parents and practice team involved in the child’s care. The specific processes potentially influenced by this intervention are adherence to preventive medications, communication between families and PCPs about the child’s symptoms, and beliefs and expectations about the child’s illness and medications. Changes in any of these processes could lead to improved clinical and functional outcomes for the children and their families, as well as reduced costs. The model also incorporates covariates that may modify an individual child’s response to the intervention. These include family demographic variables, maternal depression, medical variables and pulmonary function, and measures of environmental allergens, particularly ETS.
Potential Limitations and Rationale for the Structure of the Two Treatment Arms and the ETS Reduction Program
School-Based Care Group
This study focuses on preventive medication delivery as the primary intervention to be tested. We do not incorporate education regarding asthma management into the intervention for the school-based care group because other studies have shown that asthma education is not sufficient to produce the magnitude of difference that we are testing, and our pilot data suggest that medical management alone may produce substantial results. For the children in the school-based care group, medication adherence will only be assured on the days in which the child attends school. On other days, parents or caretakers will be responsible for medication administration. We have avoided implementing more intensive intervention for the parents on these days (e.g. reminder phone calls) in order to maintain the relative simplicity of the school-based program.
Usual Care Group
We designed this study as a health services trial to evaluate the impact of a system change rather than a single parameter change on the effectiveness of care. Therefore, this precludes altering the system of care for the children in the usual care group to avoid the intrinsic bias of adopting a new routine process. There are, by necessity, inherent differences between the usual care and school-based care groups beyond the location (school vs. home) of drug administration. In the usual care group, these include the requirement to obtain prescriptions from the physician, fill the prescription at the pharmacy, and administer the medication to the child at home. Since the children in the usual care group will have persistent asthma symptoms upon enrollment into the program, we contact their PCP and recommend that the child’s medical management be reviewed, and we encourage the family to make an appointment with their provider.
ETS Reduction Program
Because the school-based intervention was ineffective for children exposed to ETS in the pilot study, we have combined our original intervention with an evidence-based ETS reduction program for families in homes where there is ETS exposure. We plan to deliver the ETS reduction as part of the system change, with a nurse interacting with the family as an adjunct to the school nurse system of medication delivery. Eligibility for the ETS reduction program is based on parent-report of exposure in the home, rather than child cotinine levels. We expect that some children whose parents report no smoke exposure will have elevated cotinine levels. This would lead to a conservative bias in our analysis of the nonexposed subgroup.
Outcomes Assessment and Overview of Analytic Strategy
Outcome measures are assessed by telephone interview, medical record review, and review of school records by an independent group blinded to the subject’s group allocation. Our primary aim is to test whether children receiving a comprehensive school-based intervention will experience less asthma-related morbidity compared to children receiving usual care. The main outcome variable is the number of symptom free days averaged over the peak winter season (November to February). Parents are asked to report the number of days over the prior two weeks that the children had no symptoms of asthma, including coughing, wheezing, or shortness of breath. Other outcome measures are presented in Table 1.
Table 1.
Measurement strategies.
| Variable | Measurement strategy | Time of administration |
|---|---|---|
| Process measures | ||
| Adherence | Parent interview—Horne scale (60) | Baseline, each monthly follow-up |
| Parent/provider communication | Parent interview—AIR Questionnaire (61) | Baseline, final survey |
| Asthma beliefs/expectations | Parent interview—AIR Questionnaire (61) | Baseline, final survey |
| Covariates | ||
| Demographic, medical variables | Parent interview | Baseline |
| Maternal depression | Parent interview—Kessler Psychological Distress Scale (62) | Baseline, final survey |
| Pulmonary function | PEF and FEV1 readings | Baseline |
| Environmental allergens | Environmental assessment at home visit | Baseline survey |
| Secondhand smoke | Parent interview (41,63–66) | Baseline, two-month, final survey |
| Cotinine measurements | Baseline, two-month visit, final survey | |
| Clinical outcomes | ||
| Symptom severity | Parent report of symptoms, based on NHLBI guidelines | Baseline, each monthly follow-up |
| Health care utilization | Parent interview regarding: Health care contacts, chart review | Baseline, each monthly follow-up |
| Functional outcomes | ||
| Functional severity | Parent interview—Child Health Survey for Asthma | Baseline, each monthly follow-up |
| School absenteeism | Parent interview and school record review | Baseline, each monthly follow-up |
| Quality of life | Parent interview—Juniper Scale (67) | Baseline, two-month follow-up, final |
| Cost measures | ||
| Programmatic costs | Program review | Final review |
| Productivity and medical costs | Parent interview—Work days missed/health care utilization | Baseline, each monthly follow-up |
PEF, peak expiratory flow; FEV1, forced expiratory volume in one second.
We will use bivariate and multivariate comparisons to assess the total effect of school-based asthma therapy (relative to usual care) on the outcomes (symptom free days, quality of life, etc). We will adjust for pertinent covariates as depicted in the theoretical framework, including: demographic and background characteristics (age, race, ethnicity, insurance, caretaker’s education), maternal depression, medical variables (prematurity, allergy/eczema), baseline symptoms and pulmonary function measurements (FEV1 categories), presence or absence of allergen exposures, and parent-reported smoke exposure (initial and final). We also plan to determine whether or not certain process measures (medication adherence, parent/provider communication, parental beliefs/expectations) act as mediators in the relationship between the intervention and outcomes. These analyses will aid in our understanding of the pathways by which the intervention impacts the outcomes.
Another aim of the study is to consider the subgroup of smoke-exposed children, and determine whether those who receive school-based asthma therapy with ETS reduction will have less asthma morbidity than those who receive usual care. Since randomization will occur within smoke exposure groups (see Figure 1), we can estimate the intervention effect on asthma outcomes by stratifying on the smoke exposure variable. We will, therefore, repeat the analyses within each of the two ETS exposure groups, and in particular will assess whether smoke-exposed children who receive the intervention have lower asthma morbidity than smoke-exposed children who receive usual care.
We also will perform a cost-effectiveness analysis to assess health and economic benefits of school-based asthma therapy compared to usual care (57). The benefits of this intervention will be measured as the number of symptom-free days during the school year (58). Three main categories of costs (Table 2) to be considered include programmatic costs, productivity costs, and medical costs estimated at the individual child level. The main outcome for the analysis will be an incremental cost-effectiveness ratio, which is the ratio of net program costs to the number of symptom-free days gained.
Table 2.
Types of cost included in analysis.
| Included in analysis from:
|
||
|---|---|---|
| Type of cost | Societal perspective | Medicaid perspectivea |
| Programmatic costs | ||
| Start-up costs (nurse training, developing protocols, parents training) | Yes | No |
| Program delivery costs (nurse and coordinator time) | Yes | No |
| Cost of medication | Yes | Yes |
| Parent productivity/opportunity costs | Yes | No |
| Medical costs | Yes | Yes |
For subpopulation of children who are covered by Medicaid.
Lessons Learned and Conclusion
In the development of this intervention, we are able to offer the following “lessons learned.” First, our collaborations with the community, and in particular in the schools, have allowed us to reach many high-risk children with asthma and approach innovative methods to improve the delivery of preventive care. Since young children spend a large percentage of their time in school, and schools already routinely provide daily medications for other conditions such as attention deficit disorder, the provision of daily asthma preventive medications could be a simple and logical system change to improve adherence. Second, the use of two well-defined frameworks, the Wagner Chronic Care Model and Motivational Interviewing, allows us to have both a community systems focus as well as a focus on individual level factors that may be impeding change. Last, our multifaceted approach, including the school-based delivery of preventive medications, medication dose adjustments, and an ETS reduction intervention, allows us to determine if a change in the system of preventive care can reduce morbidity for high-risk asthmatic children.
We have been granted permission from the school district to continue our work with more than 50 schools to test this new method of care delivery. An evaluation of this intervention is particularly important, since it is relatively simple to administer, potentially could be widely disseminated, and could substantially reduce disparities in morbidity between poor and nonpoor children.
Acknowledgments
This work was funded by a grant from the Halcyon Hill Foundation and the National Heart, Lung, and Blood Institute (HL079954). We thank the SBAT study team for their limitless energy to help children with asthma, Andy MacGowan and the Rochester City School District for their ongoing partnership and support of our work, Ann Marie Brooks for her pulmonary expertise, and Katia Noyes and Robert Holloway for their expertise in cost-effectiveness analysis.
References
- 1.Adams PF, Marano MA. Current estimates from the National Health Interview Survey, 1994. Vital Health Stat. 1995;(193 Pt 1):1–260. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Surveillance for Asthma—US, 1960–1995. MMWR. 1995;47:1022–1025. [Google Scholar]
- 3.NIH. National Heart, Lung, and Blood Institute. Data Fact Sheet Asthma Statistics. Bethesda, Maryland, USA: National Institutes of Health, Public Health Services; 1999. [Google Scholar]
- 4.Centers for Disease Control and Prevention. Asthma mortality and hospitalization among children and adults—United States, 1980–1993. MMWR. 1996;45(17):350–353. [PubMed] [Google Scholar]
- 5.Gergen PJ, Weiss KB. Changing patterns of asthma hospitalization among children: 1979 to 1987. JAMA. 1990;264(13):1688–1692. [PubMed] [Google Scholar]
- 6.Akinbami LJ, Schoendorf KC. Trends in childhood asthma: prevalence, health care utilization, and mortality. Pediatrics. 2002;110(2 Pt 1):315–322. doi: 10.1542/peds.110.2.315. [DOI] [PubMed] [Google Scholar]
- 7.Carr W, Zeitel L, Weiss K. Variations in asthma hospitalizations and deaths in New York City. Am J Pub Health. 1992;82(1):59–65. doi: 10.2105/ajph.82.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lang DM, Polansky M. Patterns of asthma mortality in Philadelphia from 1969 to 1991. N Engl J Med. 1994;331(23):1542–1546. doi: 10.1056/NEJM199412083312302. [DOI] [PubMed] [Google Scholar]
- 9.Weiss KB, Wagener DK. Changing patterns of asthma mortality. Identifying target populations at high risk. JAMA. 1990;264(13):1683–1687. [PubMed] [Google Scholar]
- 10.Weiss KB, Sullivan SD, Lyttle CS. Trends in the cost of illness for asthma in the United States, 1985–1994. J Allergy Clin Immunol. 2000;106(3):493–499. doi: 10.1067/mai.2000.109426. [DOI] [PubMed] [Google Scholar]
- 11.Diaz T, Sturm T, Matte T, et al. Medication use among children with asthma in East Harlem. Pediatrics. 2000;105(6):1188–1193. doi: 10.1542/peds.105.6.1188. [DOI] [PubMed] [Google Scholar]
- 12.Crain EF, Kercsmar C, Weiss KB, Mitchell H, Lynn H. Reported difficulties in access to quality care for children with asthma in the inner city. Arch Pediatr Adolesc Med. 1998;152(4):333–339. doi: 10.1001/archpedi.152.4.333. [DOI] [PubMed] [Google Scholar]
- 13.Eggleston PA, Malveaux FJ, Butz AM, et al. Medications used by children with asthma living in the inner city. Pediatrics. 1998;101(3 Pt 1):349–354. doi: 10.1542/peds.101.3.349. [DOI] [PubMed] [Google Scholar]
- 14.Ortega AN, Gergen PJ, Paltiel AD, Bauchner H, Belanger KD, Leaderer BP. Impact of site of care, race, and Hispanic ethnicity on medication use for childhood asthma. Pediatrics. 2002;109(1):E1. doi: 10.1542/peds.109.1.e1. [DOI] [PubMed] [Google Scholar]
- 15.Akinbami LJ, LaFleur BJ, Schoendorf KC. Racial and income disparities in childhood asthma in the United States. Ambul Pediatr. 2002;2(5):382–387. doi: 10.1367/1539-4409(2002)002<0382:raidic>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 16.NIH. Healthy People 2010. [Accessed 15 Nov 2007]; Available at: http:/hin.nhlbi.nih.gov/2010objs/24REspiratory.htm.
- 17.NIH. National Asthma Education and Prevention Program. Expert Panel Report: Guidelines for the Diagnosis and Management of Asthma. Bethesda, Maryland, USA: National Institutes of Health, National Heart, Lung, and Blood Institute; 1991. NIH Publication No. 91-3042. [Google Scholar]
- 18.NIH. National Asthma Education and Prevention Program. Expert Panel Report II: Guidelines for the Diagnosis and Management of Asthma. Bethesda, Maryland, USA: National Institutes of Health, National Heart, Lung, and Blood Institute; 1997. NIH Publication No. 97-4051. [Google Scholar]
- 19.NIH. National Asthma Education and Prevention Program. NAEPP Expert Panel Report: Guidelines for the Diagnosis and Management of Asthma—Update on Selected Topics 2002. Bethesda, Maryland, USA: National Institutes of Health, National Heart, Lung, and Blood Institute; 2002. NIH Publication No. 02-5075. [Google Scholar]
- 20.NIH. National Asthma Education and Prevention Program. Expert Panel Report III: Guidelines for the Diagnosis and Management of Asthma. Bethesda, Maryland, USA: National Institutes of Health, National Heart, Lung, and Blood Institute; 2007. NIH Publication No. 07-4051. [Google Scholar]
- 21.Ordonez GA, Phelan PD, Olinsky A, Robertson CF. Preventable factors in hospital admissions for asthma. Arch Dis Child. 1998;78(2):143–147. doi: 10.1136/adc.78.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Homer CJ, Szilagyi P, Rodewald L, et al. Does quality of care affect rates of hospitalization for childhood asthma? Pediatrics. 1996;98(1):18–23. [PubMed] [Google Scholar]
- 23.Halterman JS, Aligne CA, Auinger P, McBride JT, Szilagyi PG. Inadequate therapy for asthma among children in the United States. Pediatrics. 2000;105(1 Pt 3):272–276. [PubMed] [Google Scholar]
- 24.Anarella J, Roohan P, Balistreri E, Gesten F. A survey of Medicaid recipients with asthma: perceptions of self-management, access, and care. Chest. 2004;125(4):1359–1367. doi: 10.1378/chest.125.4.1359. [DOI] [PubMed] [Google Scholar]
- 25.Mannino DM, Homa DM, Redd SC. Involuntary smoking and asthma severity in children: data from the Third National Health and Nutrition Examination Survey. Chest. 2002;122(2):409–415. doi: 10.1378/chest.122.2.409. [DOI] [PubMed] [Google Scholar]
- 26.Strachan DP, Cook DG. Health effects of passive smoking. 6. Parental smoking and childhood asthma: longitudinal and case-control studies. Thorax. 1998;53(3):204–212. doi: 10.1136/thx.53.3.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chilmonczyk BA, Salmun LM, Megathlin KN, et al. Association between exposure to environmental tobacco smoke and exacerbations of asthma in children. N Engl J Med. 1993;328(23):1665–1669. doi: 10.1056/NEJM199306103282303. [DOI] [PubMed] [Google Scholar]
- 28.Canadian Paediatric Society Section on Allergy. Secondhand cigarette smoke worsens symptoms in children with asthma. CMAJ. 1986;135(4):321–323. [PMC free article] [PubMed] [Google Scholar]
- 29.Institute of Medicine. Clearing the Air: Asthma and Indoor Air Exposures. Washington, DC: National Academy Press; 2000. [PubMed] [Google Scholar]
- 30.Frischer T, Kuehr J, Meinert R, et al. Maternal smoking in early childhood: a risk factor for bronchial responsiveness to exercise in primary-school children. J Pediatr. 1992;121(1):17–22. doi: 10.1016/s0022-3476(05)82534-x. [DOI] [PubMed] [Google Scholar]
- 31.Weitzman M, Gortmaker S, Walker DK, Sobol A. Maternal smoking and childhood asthma. Pediatrics. 1990;85(4):505–511. [PubMed] [Google Scholar]
- 32.Evans D, Levison MJ, Feldman CH, et al. The impact of passive smoking on emergency room visits of urban children with asthma. Am Rev Respir Dis. 1987;135(3):567–572. doi: 10.1164/arrd.1987.135.3.567. [DOI] [PubMed] [Google Scholar]
- 33.Morkjaroenpong V, Rand CS, Butz AM, et al. Environmental tobacco smoke exposure and nocturnal symptoms among inner-city children with asthma. J Allergy Clin Immunol. 2002;110(1):147–153. doi: 10.1067/mai.2002.125832. [DOI] [PubMed] [Google Scholar]
- 34.Hopper JA, Craig KA. Environmental tobacco smoke exposure among urban children. Pediatrics. 2000;106(4):E47. doi: 10.1542/peds.106.4.e47. [DOI] [PubMed] [Google Scholar]
- 35.Weaver VM, Davoli CT, Murphy SE, et al. Environmental tobacco smoke exposure in inner-city children. Cancer Epidemiol Biomarkers Prev. 1996;5(2):135–137. [PubMed] [Google Scholar]
- 36.Wenzel SE, Szefler SJ, Leung DY, Sloan SI, Rex MD, Martin RJ. Bronchoscopic evaluation of severe asthma. Persistent inflammation associated with high dose glucocorticoids. Am J Respir Crit Care Med. 1997;156(3 Pt 1):737–743. doi: 10.1164/ajrccm.156.3.9610046. [DOI] [PubMed] [Google Scholar]
- 37.Cox G, Whitehead L, Dolovich M, Jordana M, Gauldie J, Newhouse MT. A randomized controlled trial on the effect of inhaled corticosteroids on airways inflammation in adult cigarette smokers. Chest. 1999;115(5):1271–1277. doi: 10.1378/chest.115.5.1271. [DOI] [PubMed] [Google Scholar]
- 38.Chalmers GW, Macleod KJ, Little SA, Thomson LJ, McSharry CP, Thomson NC. Influence of cigarette smoking on inhaled corticosteroid treatment in mild asthma. Thorax. 2002;57(3):226–230. doi: 10.1136/thorax.57.3.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Halterman JS, Szilagyi PG, Yoos HL, et al. Benefits of a school-based asthma treatment program in the absence of secondhand smoke exposure: results of a randomized clinical trial. Arch Pediatr Adolesc Med. 2004;158(5):460–467. doi: 10.1001/archpedi.158.5.460. [DOI] [PubMed] [Google Scholar]
- 40.Szefler SJ. Challenges in assessing outcomes for pediatric asthma. J Allergy Clin Immunol. 2001;107(5 Suppl):S456–S464. doi: 10.1067/mai.2001.114947. [DOI] [PubMed] [Google Scholar]
- 41.Hovell MF, Meltzer SB, Zakarian JM, et al. Reduction of environmental tobacco smoke exposure among asthmatic children: a controlled trial. Chest. 1994;106(2):440–446. doi: 10.1378/chest.106.2.440. [DOI] [PubMed] [Google Scholar]
- 42.Wahlgren DR, Hovell MF, Meltzer SB, Hofstetter CR, Zakarian JM. Reduction of environmental tobacco smoke exposure in asthmatic children. A 2-year follow-up. Chest. 1997;111(1):81–88. doi: 10.1378/chest.111.1.81. [DOI] [PubMed] [Google Scholar]
- 43.Miller WR, Rollnick S. Motivational Interviewing: Preparing People to Change Addictive Behavior. New York: Guilford Press; 1991. [Google Scholar]
- 44.Borrelli B, McQuaid EL, Becker B, et al. Motivating the parents of kids with asthma to quit smoking: preliminary findings from the PAQS project. Paper presented at the Annual Meeting of the Society of Behavioral Medicine; Baltimore, Maryland, USA. 2004. [Google Scholar]
- 45.Borrelli B, McQuaid EL, Becker B, et al. Motivating parents of kids with asthma to quit smoking: the PAQS project. Health Educ Res. 2002;17(5):659–669. doi: 10.1093/her/17.5.659. [DOI] [PubMed] [Google Scholar]
- 46.Wagner EH. Chronic disease management: what will it take to improve care for chronic illness? Eff Clin Pract. 1998;1(1):2–4. [PubMed] [Google Scholar]
- 47.Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. Milbank Q. 1996;74(4):511–544. [PubMed] [Google Scholar]
- 48.Glasgow RE, Orleans CT, Wagner EH. Does the chronic care model serve also as a template for improving prevention? Milbank Q. 2001;79(4):579–612. iv–v. doi: 10.1111/1468-0009.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wagner EH, Austin BT, Davis C, Hindmarsh M, Schaefer J, Bonomi A. Improving chronic illness care: translating evidence into action. Health Aff (Millwood) 2001;20(6):64–78. doi: 10.1377/hlthaff.20.6.64. [DOI] [PubMed] [Google Scholar]
- 50.Robert Wood Johnson Foundation. Improving Chronic Illness Care. [Accessed May 1, 2007]; Available at: http://www.improvingchroniccare.org/change/inaction.html.
- 51.Fisher EB, Auslander WF, Munro JF, Arfken CL, Brownson RC, Owens NW. Neighbors for a smoke free north side: evaluation of a community organization approach to promoting smoking cessation among African Americans. Am J Pub Health. 1998;88(11):1658–1663. doi: 10.2105/ajph.88.11.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Al-Dossary FS, Ong LT, Correa AG, Starke JR. Treatment of childhood tuberculosis with a six month directly observed regimen of only two weeks of daily therapy. Pediatr Infect Dis J. 2002;21(2):91–97. doi: 10.1097/00006454-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 53.Borgdorff MW, Floyd K, Broekmans JF. Interventions to reduce tuberculosis mortality and transmission in low- and middle-income countries. Bull World Health Organ. 2002;80(3):217–227. [PMC free article] [PubMed] [Google Scholar]
- 54.Mitty JA, Stone VE, Sands M, Macalino G, Flanigan T. Directly observed therapy for the treatment of people with human immunodeficiency virus infection: a work in progress. Clin Infect Dis. 2002;34(7):984–990. doi: 10.1086/339447. [DOI] [PubMed] [Google Scholar]
- 55.Steiner KC, Davila V, Kent CK, Chaw JK, Fischer L, Klausner JD. Field-delivered therapy increases treatment for chlamydia and gonorrhea. Am J Pub Health. 2003;93(6):882–884. doi: 10.2105/ajph.93.6.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ockene JK, Zapka JG. Provider education to promote implementation of clinical practice guidelines. Chest. 2000;118(2 Suppl):33S–39S. doi: 10.1378/chest.118.2_suppl.33s. [DOI] [PubMed] [Google Scholar]
- 57.Drummond MF, O’Brien B, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. 3. Oxford/New York: Oxford University Press; 2005. [Google Scholar]
- 58.Sullivan SD, Weiss KB, Lynn H, et al. The cost-effectiveness of an inner-city asthma intervention for children. J Allergy Clin Immunol. 2002;110(4):576–581. doi: 10.1067/mai.2002.128009. [DOI] [PubMed] [Google Scholar]
- 59.LaForce CF, Pearlman DS, Ruff ME, et al. Efficacy and safety of dry powder fluticasone propionate in children with persistent asthma. Ann Allergy Asthma Immunol. 2000;85(5):407–415. doi: 10.1016/S1081-1206(10)62556-2. [DOI] [PubMed] [Google Scholar]
- 60.Horne R, Weinman J, Hankins M. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psych Health. 1999;14:1–24. [Google Scholar]
- 61.Yoos HL, Kitzman H, McMullen A. Barriers to anti-inflammatory medication use in childhood asthma. Ambul Pediatr. 2003;3(4):181–190. doi: 10.1367/1539-4409(2003)003<0181:btamui>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 62.Kessler RC, Barker PR, Colpe LJ, et al. Screening for serious mental illness in the general population. Arch Gen Psychiatry. 2003;60(2):184–189. doi: 10.1001/archpsyc.60.2.184. [DOI] [PubMed] [Google Scholar]
- 63.Hovell MF, Zakarian JM, Wahlgren DR, Matt GE, Emmons KM. Reported measures of environmental tobacco smoke exposure: trials and tribulations. Tob Control. 2000;9(Suppl 3):III22–III28. doi: 10.1136/tc.9.suppl_3.iii22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Matt GE, Wahlgren DR, Hovell MF, et al. Measuring environmental tobacco smoke exposure in infants and young children through urine cotinine and memory-based parental reports: empirical findings and discussion. Tob Control. 1999;8(3):282–289. doi: 10.1136/tc.8.3.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res. 2003;5(1):13–25. [PubMed] [Google Scholar]
- 66.McMillen RC, Winickoff JP, Klein JD, Weitzman M. US adult attitudes and practices regarding smoking restrictions and child exposure to environmental tobacco smoke: changes in the social climate from 2000–2001. Pediatrics. 2003;112(1 Pt 1):e55–e60. doi: 10.1542/peds.112.1.e55. [DOI] [PubMed] [Google Scholar]
- 67.Juniper EF, Guyatt GH, Feeny DH, Ferrie PJ, Griffith LE, Townsend M. Measuring quality of life in the parents of children with asthma. Qual Life Res. 1996;5(1):27–34. doi: 10.1007/BF00435966. [DOI] [PubMed] [Google Scholar]