Abstract
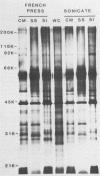
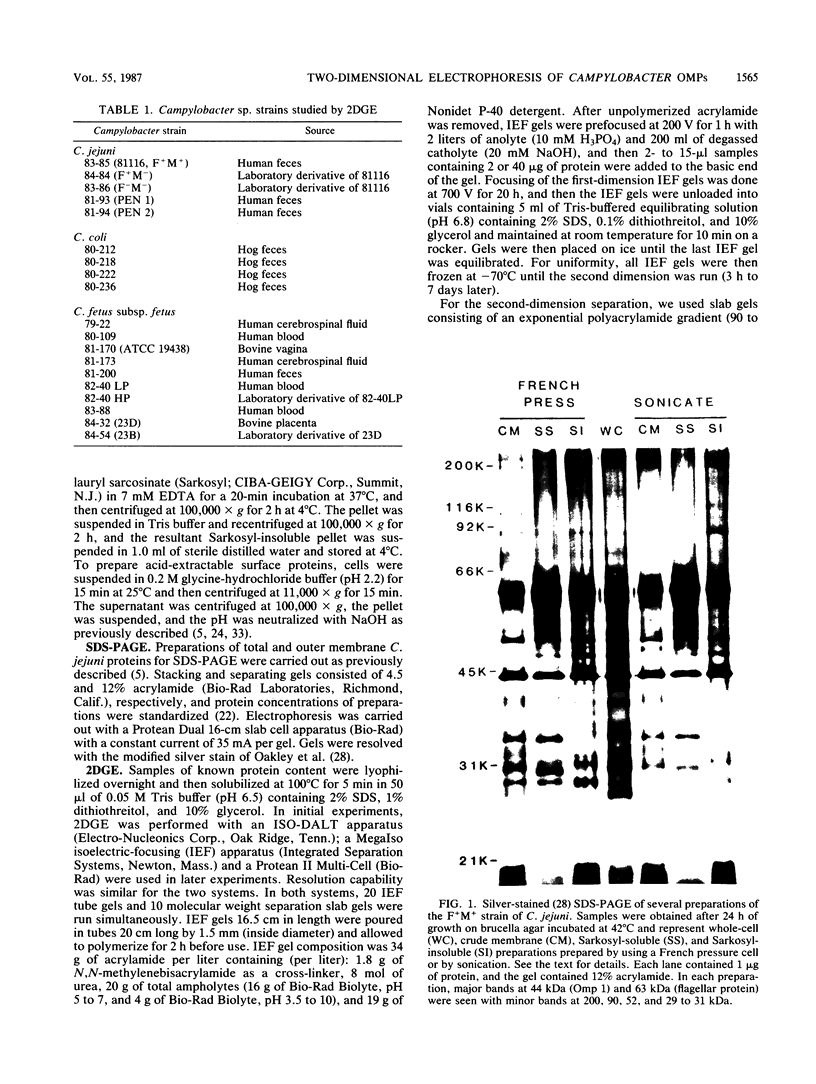
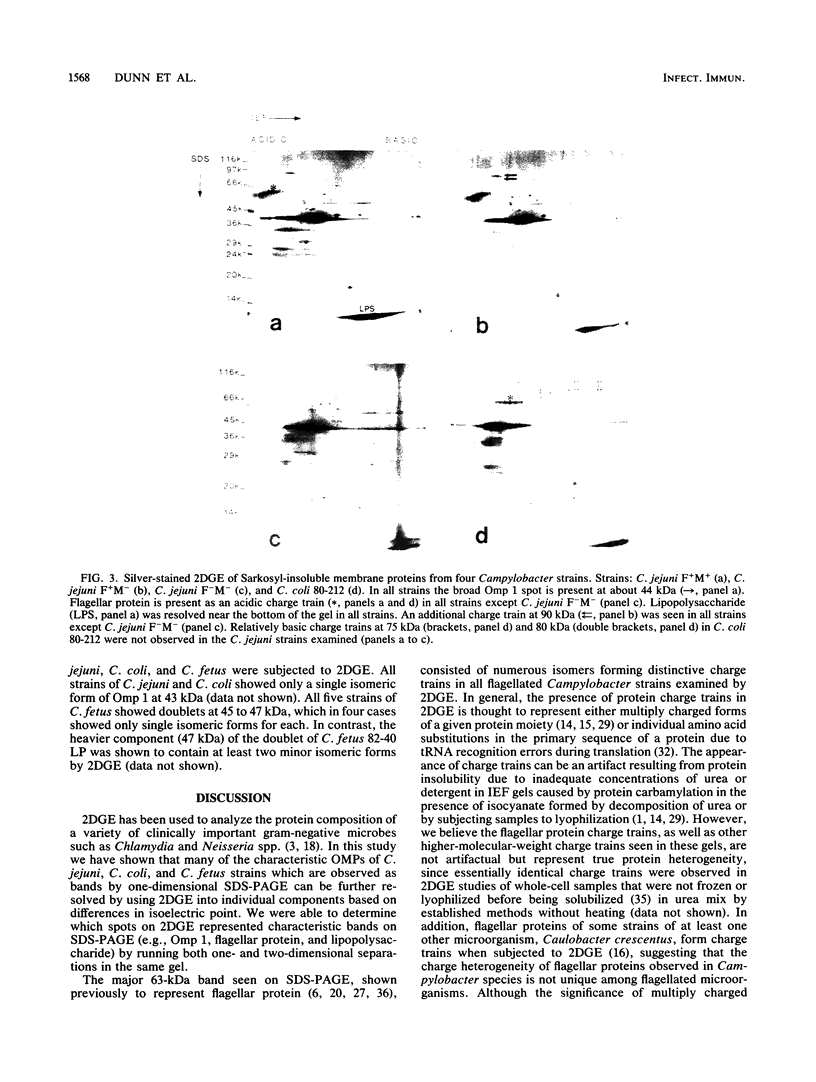
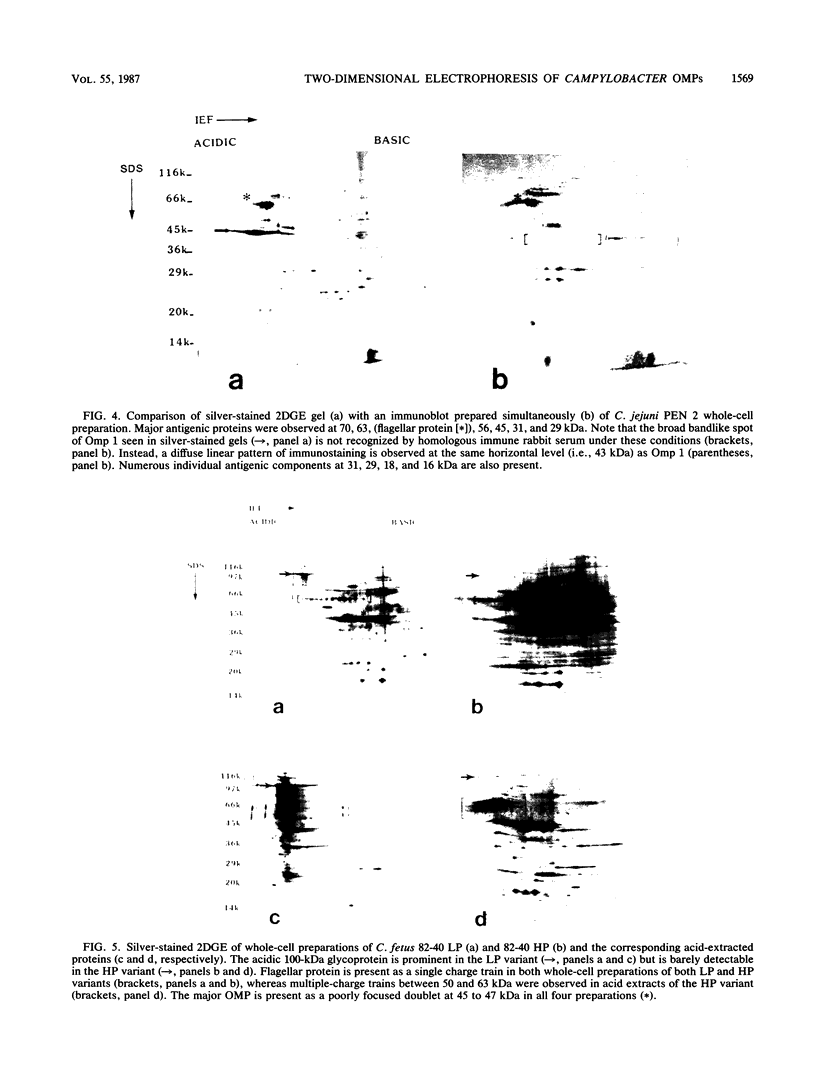
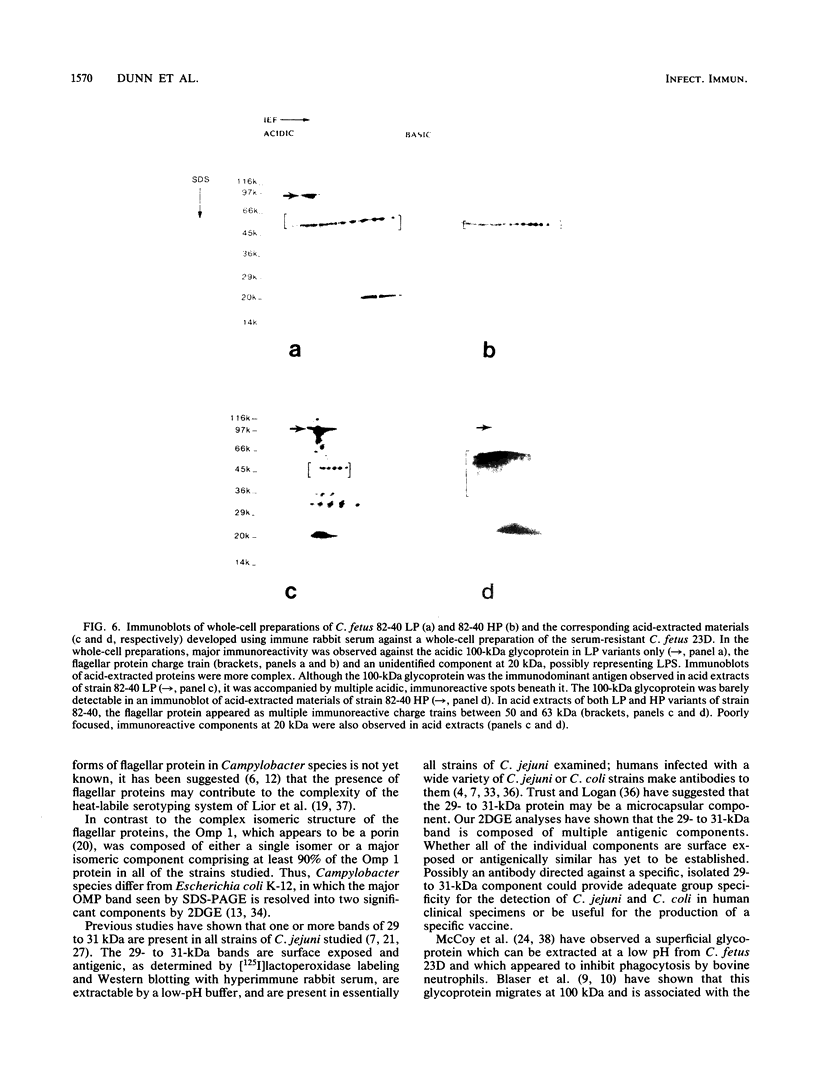
We characterized outer membrane proteins (OMPs) from selected Campylobacter jejuni, C. coli, and C. fetus strains by two-dimensional gel electrophoresis (2DGE), using isoelectric focusing and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and by immunoblotting with immune rabbit serum. The flagellar band with a molecular mass of 63 kilodaltons (kDa) demonstrated previously by one-dimensional SDS-PAGE was shown by 2DGE to consist of one or several charge trains, depending upon the species, strain, and type of preparation studied; each of the individual peptides was found to be antigenic by immunoblotting. In contrast, in all of the strains studied, the major OMP (43 to 44 kDa) of C.jejuni and C. coli consisted of a single isomeric form which was weakly immunogenic. Several minor proteins (29 to 31 kDa) were found to be strongly immunogenic by immunoblotting. C. fetus strains possessed two major OMPs of 45 to 47 kDa, each of which consisted of either a single isomer or a major isomer comprising at least 90% of the major OMP. Serum-resistant strains of C. fetus possessed an acid-labile 100-kDa glycoprotein (pI, 4.1) which was markedly diminished or absent in serum-sensitive strains. These 2DGE analyses provide information that is useful in taxonomic and epidemiologic studies and for the purification of surface antigens for the development of campylobacter vaccines and may also facilitate the identification of specific virulence factors.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Nikaido K. Two-dimensional gel electrophoresis of membrane proteins. Biochemistry. 1976 Feb 10;15(3):616–623. doi: 10.1021/bi00648a026. [DOI] [PubMed] [Google Scholar]
- Anderson N. L., Taylor J., Scandora A. E., Coulter B. P., Anderson N. G. The TYCHO system for computer analysis of two-dimensional gel electrophoresis patterns. Clin Chem. 1981 Nov;27(11):1807–1820. [PubMed] [Google Scholar]
- Batteiger B. E., Newhall W. J., 5th, Jones R. B. Differences in outer membrane proteins of the lymphogranuloma venereum and trachoma biovars of Chlamydia trachomatis. Infect Immun. 1985 Nov;50(2):488–494. doi: 10.1128/iai.50.2.488-494.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser M. J., Duncan D. J. Human serum antibody response to Campylobacter jejuni infection as measured in an enzyme-linked immunosorbent assay. Infect Immun. 1984 May;44(2):292–298. doi: 10.1128/iai.44.2.292-298.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser M. J., Hopkins J. A., Berka R. M., Vasil M. L., Wang W. L. Identification and characterization of Campylobacter jejuni outer membrane proteins. Infect Immun. 1983 Oct;42(1):276–284. doi: 10.1128/iai.42.1.276-284.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser M. J., Hopkins J. A., Perez-Perez G. I., Cody H. J., Newell D. G. Antigenicity of Campylobacter jejuni flagella. Infect Immun. 1986 Jul;53(1):47–52. doi: 10.21236/ada265460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser M. J., Hopkins J. A., Vasil M. L. Campylobacter jejuni outer membrane proteins are antigenic for humans. Infect Immun. 1984 Mar;43(3):986–993. doi: 10.1128/iai.43.3.986-993.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser M. J., Reller L. B. Campylobacter enteritis. N Engl J Med. 1981 Dec 10;305(24):1444–1452. doi: 10.1056/NEJM198112103052404. [DOI] [PubMed] [Google Scholar]
- Blaser M. J., Smith P. F., Hopkins J. A., Heinzer I., Bryner J. H., Wang W. L. Pathogenesis of Campylobacter fetus infections: serum resistance associated with high-molecular-weight surface proteins. J Infect Dis. 1987 Apr;155(4):696–706. doi: 10.1093/infdis/155.4.696. [DOI] [PubMed] [Google Scholar]
- Blaser M. J., Smith P. F., Kohler P. F. Susceptibility of Campylobacter isolates to the bactericidal activity of human serum. J Infect Dis. 1985 Feb;151(2):227–235. doi: 10.1093/infdis/151.2.227. [DOI] [PubMed] [Google Scholar]
- Buck G. E., Smith J. S., Parshall K. A. Composition of the antigenic material removed from Campylobacter jejuni by heat. J Clin Microbiol. 1984 Dec;20(6):1094–1098. doi: 10.1128/jcm.20.6.1094-1098.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra I., Shales S. W. Comparison of the polypeptide composition of Escherichia coli outer membranes prepared by two methods. J Bacteriol. 1980 Oct;144(1):425–427. doi: 10.1128/jb.144.1.425-427.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda A., Asada M., Koyasu S., Yoshida H., Yaginuma K., Okada Y. Regulation of polar morphogenesis in Caulobacter crescentus. J Bacteriol. 1981 Jan;145(1):559–572. doi: 10.1128/jb.145.1.559-572.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hébert G. A., Hollis D. G., Weaver R. E., Lambert M. A., Blaser M. J., Moss C. W. 30 years of campylobacters: biochemical characteristics and a biotyping proposal for Campylobacter jejuni. J Clin Microbiol. 1982 Jun;15(6):1065–1073. doi: 10.1128/jcm.15.6.1065-1073.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson P., Thornley M. J., Thompson R. J. A study by high-resolution two-dimensional polyacrylamide gel electrophoresis of relationships between Neisseria gonorrhoeae and other bacteria. J Gen Microbiol. 1984 Dec;130(12):3189–3201. doi: 10.1099/00221287-130-12-3189. [DOI] [PubMed] [Google Scholar]
- Lior H., Woodward D. L., Edgar J. A., Laroche L. J., Gill P. Serotyping of Campylobacter jejuni by slide agglutination based on heat-labile antigenic factors. J Clin Microbiol. 1982 May;15(5):761–768. doi: 10.1128/jcm.15.5.761-768.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan S. M., Trust T. J. Molecular identification of surface protein antigens of Campylobacter jejuni. Infect Immun. 1983 Nov;42(2):675–682. doi: 10.1128/iai.42.2.675-682.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan S. M., Trust T. J. Outer membrane characteristics of Campylobacter jejuni. Infect Immun. 1982 Dec;38(3):898–906. doi: 10.1128/iai.38.3.898-906.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Marshall T., Williams K. M., Vesterberg O. Two-dimensional electrophoresis of proteins in human serum: improved resolution by use of narrow pH gradients and prolonged electrophoresis. Clin Chem. 1984 Dec;30(12 Pt 1):2008–2013. [PubMed] [Google Scholar]
- Mills S. D., Bradbury W. C. Human antibody response to outer membrane proteins of Campylobacter jejuni during infection. Infect Immun. 1984 Feb;43(2):739–743. doi: 10.1128/iai.43.2.739-743.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachamkin I., Hart A. M. Western blot analysis of the human antibody response to Campylobacter jejuni cellular antigens during gastrointestinal infection. J Clin Microbiol. 1985 Jan;21(1):33–38. doi: 10.1128/jcm.21.1.33-38.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell D. G., McBride H., Pearson A. D. The identification of outer membrane proteins and flagella of Campylobacter jejuni. J Gen Microbiol. 1984 May;130(5):1201–1208. doi: 10.1099/00221287-130-5-1201. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Perez Perez G. I., Blaser M. J. Lipopolysaccharide characteristics of pathogenic campylobacters. Infect Immun. 1985 Feb;47(2):353–359. doi: 10.1128/iai.47.2.353-359.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez G. I., Hopkins J. A., Blaser M. J. Antigenic heterogeneity of lipopolysaccharides from Campylobacter jejuni and Campylobacter fetus. Infect Immun. 1985 May;48(2):528–533. doi: 10.1128/iai.48.2.528-533.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautelin H., Kosunen T. U. An acid extract as a common antigen in Campylobacter coli and Campylobacter jejuni strains. J Clin Microbiol. 1983 Apr;17(4):700–701. doi: 10.1128/jcm.17.4.700-701.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Ito K., Yura T. Membrane proteins of Escherichia coli K-12: two-dimensional polyacrylamide gel electrophoresis of inner and outer membranes. Eur J Biochem. 1977 Sep;78(2):557–567. doi: 10.1111/j.1432-1033.1977.tb11769.x. [DOI] [PubMed] [Google Scholar]
- Wenman W. M., Chai J., Louie T. J., Goudreau C., Lior H., Newell D. G., Pearson A. D., Taylor D. E. Antigenic analysis of Campylobacter flagellar protein and other proteins. J Clin Microbiol. 1985 Jan;21(1):108–112. doi: 10.1128/jcm.21.1.108-112.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter A. J., McCoy E. C., Fullmer C. S., Burda K., Bier P. J. Microcapsule of Campylobacter fetus: chemical and physical characterization. Infect Immun. 1978 Dec;22(3):963–971. doi: 10.1128/iai.22.3.963-971.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]