Abstract
Epithelial odontogenic tumors are rare jaw pathologies that raise clinical diagnosis and prognosis dilemmas notably between ameloblastomas and clear cell odontogenic carcinomas (CCOCs). In line with previous studies, the molecular determinants of tooth development—amelogenin, Msx1, Msx2, Dlx2, Dlx3, Bmp2, and Bmp4—were analyzed by RT-PCR, ISH, and immunolabeling in 12 recurrent ameloblastomas and in one case of CCOC. Although Msx1 expression imitates normal cell differentiation in these tumors, other genes showed a distinct pattern depending on the type of tumor and the tissue involved. In benign ameloblastomas, ISH localized Dlx3 transcripts and inconstantly detected Msx2 transcripts in epithelial cells. In the CCOC, ISH established a lack of both Dlx3 and Msx2 transcripts but allowed identification of the antisense transcript of Msx1, which imitates the same scheme of distribution between mesenchyme and epithelium as in the cup stage of tooth development. Furthermore, while exploring the expression pattern of signal molecules by RT-PCR, Bmp2 was shown to be completely inactivated in the CCOC and irregularly noticeable in ameloblastomas. Bmp4 was always expressed in all the tumors. Based on the established roles of Msx and Dlx transcription factors in dental cell fates, these data suggest that their altered expression is a proposed trail to explain the genesis and/or the progression of odontogenic tumors. (J Histochem Cytochem 57:69–78, 2009)
Keywords: epithelial odontogenic tumors, Dlx, Msx, antisense transcript, RT-PCR, in situ hybridization
Odontogenic tumors are rare jaw pathologies (Kramer et al. 1992). Although they are habitually not accompanied by malignancy, they raise clinical problems such as (a) a regional invasiveness that may require entire maxilla removal and therefore a complex reconstruction, (b) a high recurrence rate implicating reiterative surgeries, (c) an eventual transformation into malignant tumors, and (d) very rarely, kidney and lung metastasis (Akrish et al. 2007). They constitute a heterogenous group of epithelial, mesenchymal, and mixed tumors.
They are classified according to their histological type, anatomical site, and degree of malignancy (Akrish et al. 2007), together with the morphological similarity of tumor cells with the different stages of dental development (bud, cap, bell) and cell differentiation (Pindborg et al. 1972). Although some of them are easily diagnosed, such as the mixed odontogenic tumors (Papagerakis et al. 1999), it is often difficult to establish an accurate diagnosis for epithelial tumors (Carlson and Marx 2006; Pippi 2006). An inappropriate treatment can result from this difficulty to discriminate ameloblastomas from clear cell odontogenic carcinomas (CCOCs). Despite the accumulation of meticulous morphological and clinical observations over the years, more accurate tools, based on gene expression, are needed to discriminate between these rare heterogeneous entities (Lim et al. 2006; Hall et al. 2007).
Genetic mutations, classically reported in cancer cells, such as K-ras and β-catenin, are rare in odontogenic tumors (Sekine et al. 2003; Kumamoto et al. 2004; Miyake et al. 2006). Somatic mutations were identified in the enamel-related ameloblastin gene (Toyosawa et al. 2000; Perdigao et al. 2004). Animal models have shown a tight correlation between knockout expression of ameloblastin and odontogenic tumors (Fukumoto et al. 2004). Similarly, the expression of a mutated version of another enamel-related gene in amelogenin gene knockout background mice led to the development of epithelial odontogenic tumors (Gibson et al. 2007).
Few studies have been devoted to differentiate molecular expression patterns in benign and malignant odontogenic tumors. They have pointed out abnormal patterns for apoptotic molecules, suggesting an abnormal turnover of tumor cells (Kumamoto and Ooya 1999). In addition, a cDNA microarray identified a set of repressed genes in malignant odontogenic tumors compared with benign ameloblastomas (Carinci et al. 2003a). Among them, transcription factors seemed to be involved in processes underlying malignancy (Carinci et al. 2003b). These include factors playing a key role in positioning and controlling the shape of teeth during normal development.
Msx and Dlx homeoproteins are encoded by transcriptional factors involved in normal tooth development and therefore their alteration is likely to lead to malignancy. Their expression is regulated by several molecular signals including bone morphogenetic proteins Bmp2 and Bmp4 (Vainio et al. 1993; Chen et al. 1996; Bei and Maas 1998). Bmp4 has the ability to induce and/or maintain the expression of Msx1 and Dlx2 in mesenchyme during early tooth development (Bei and Maas 1998), whereas Bmp2 induces Dlx3 expression in other cells (Park and Morasso 2002). The mammalian Msx gene family includes Msx1 and Msx2, which have been well characterized with respect to their DNA binding and transcriptional properties.
Targeted inactivation of the Msx1 gene in transgenic mice leads to an arrest of tooth development at the bud stage (Satokata and Maas 1994). Msx2 mutant mice display tooth and alveolar bone defects (Satokata et al. 2000; Aioub et al. 2007). Several human gene mutations and their respective related phenotypes share similarity with those observed in animal models (Vastardis et al. 1996; Price et al. 1998; Van den Boogaard et al. 2000; Dong et al. 2005; Stephanopoulos et al. 2005).
The Dlx gene family is composed of six genes arranged in three clusters. During normal development, all Dlx genes are expressed in the dental ectomesenchyme. The Dlx2 and Dlx3 genes are also expressed in the epithelium (Lezot et al. 2000; Zhao et al. 2000; Ghoul-Mazgar et al. 2005). Disruption of Dlx gene expression induces malformed and poorly mineralized crowns in Dlx5 mutants (Depew et al. 1999) and a lack of maxillary molars in double Dlx1/Dlx2 mutants (Weiss et al. 1994).
The overall hypothesis merging from these studies is that odontogenic tumors are related to abnormal cell signaling. Therefore, we hypothesized that genes implied in dental-specific signals are also involved in the tumoral pathway. Because the expression levels of these genes are different in malignant odontogenic tumors and ameloblastomas (Carinci et al. 2003a), we limited our selection to transcription factors (Msx, Dlx, and Bmp) controlling odontogenesis. In our study, we also focused on recurrent tumors that (a) have an established diagnosis and (b) may differentiate to transform into malignant tumors.
Materials and Methods
The study protocol was approved by the Research Ethic Committee (Helsinki Declaration of 1975, as revised in 1983).
Tissue Preparation
Patients and Tissue Specimens
Samples were derived from recurrent odontogenic tumors of 13 patients at the department of Stomatology and Maxillofacial Surgery (Pitié Salpêtrière University Hospital). Specimens of 12 ameloblastomas and one case of CCOC are summarized in Table 1. Tumors were divided into three parts. The first part was fixed in paraformaldehyde PBS before being embedded in paraffin for pathological diagnosis according to the WHO histological typing of odontogenic tumors (Kramer et al. 1992). The second part was frozen in liquid nitrogen for RNA extraction and RT-PCR analysis. The third part was quick-frozen for ISH. Sufficient carcinoma tissues allowed fixation in Karnovsky solution (4% paraformaldehyde, 1% glutaraldehyde) for ultrastructural analysis and performance of IHC examination.
Table 1.
Clinical features and behavior of the tumors studied
| Tumor | Sex | Tumor localization | Age (years) | First histological diagnosis | Age (years) and diagnosis at first recurrence | Age (years) and diagnosis at the following recurrences | Rec | Final treatment |
|---|---|---|---|---|---|---|---|---|
| CCOT | Female | Rt posterior maxilla | 63 | Ameloblastoma | 64 Clear cell odontogenic carcinoma | 67 Clear cell odontogenic carcinoma | 2 | Rt maxillectomy and radiotherapy |
| A1 | Male | Rt posterior mandible | 57 | Ameloblastoma | 64 Follicular and cystic ameloblastoma | – | 1 | Rt uninterruptive mandibulectomy |
| A2 | Male | Rt posterior mandible | 16 | Plexiform ameloblastoma | 42 Follicular and cystic ameloblastoma | 50, 55, 62 Follicular and cystic ameloblastoma with calcifications | 4 | Rt mandibulectomy |
| A3 | Male | Lt posterior mandible | 36 | Ameloblastoma | 39 Plexiform and follicular ameloblastoma | 42 Plexiform and follicular ameloblastoma 52 Plexiform ameloblastoma | 3 | Enucleation |
| A4 | Male | Rt and Lt posterior mandible | 45 | Keratocyst and ossifying fibroma | 46 Follicular and cystic ameloblastoma | 49 Follicular and cystic ameloblastoma | 2 | Enucleation |
| A5 | Female | Lt posterior mandible | 29 | Cystic ameloblastoma | 33 Cystic ameloblastoma | 43, 46 Cystic ameloblastoma | 3 | Enucleation |
| A6 | Female | Lt posterior mandible | 66 | Follicular ameloblastoma | 67 Follicular ameloblastoma | 71 Follicular ameloblastoma | 2 | Enucleation |
| A7 | Female | Rt posterior mandible | 38 | Keratocyst | 40 Follicular ameloblastoma | – | 1 | Enucleation |
| A8 | Female | Rt posterior and anterior mandible | 30 | Follicular and cystic ameloblastoma | – | – | 0 | Interruptive mandibulectomy |
| A9 | Female | Rt posterior maxillae | 24 | Ameloblastoma | 31 Follicular and cystic ameloblastoma | – | 1 | Rt maxillectomy |
| A10 | Male | Rt posterior and anterior mandible | 35 | Ameloblastoma | – | – | 0 | Rt mandibulectomy |
| A11 | Male | Rt posterior maxillae | 19 | Epidermoid carcinoma on biopsy, then Plexiform and peripheral ameloblastoma after surgery | – | – | 0 | Rt maxillectomy |
| A12 | Male | Rt posterior mandible | 33 | Keratocyst | 34 Cystic ameloblastoma | – | 1 | Enucleation |
Rt, right; Lt, left; Rec, number of recurrency.
Fetal Tissue Specimen
A 9-week-old human embryonic whole orofacial tissue (EOFT), excluding brain, was frozen in liquid nitrogen for RNA extraction and RT-PCR analysis.
RT-PCR Analysis
Total RNAs were isolated from fresh-frozen tissues using TRI-REAGENT protocol according to the manufacturer's instructions (Euromedex; Strasbourg, France). RNA integrity was checked by agarose gel electrophoresis. For RT-PCR analysis, 2 μg of total RNA was reverse-transcribed with an oligo(dT) primer according to the manufacturer's instructions (Invitrogen; San Diego, CA). PCR conditions were as follows: 35 cycles of denaturation at 94C for 30 sec, annealing, and elongation at 72C for each pair of primers (Table 2). The PCR products were run onto a 2% agarose gel containing ethidium bromide.
Table 2.
Primers for RT-PCR assays
| Gene | Sense primer | Antisense primer | Expective size (bp) |
|---|---|---|---|
| Msx1 | 5′-AAGTTCCGCCAGAAGCAGTA-3′ | 5′-TCAGGTGGTACATGCTGTAG-3′ | 328 |
| Msx2 | 5′-CCTCGGTCAAGTCGGAAAATTC-3′ | 5′-CGTATATGGATGCTGCTTGC-3′ | 400 |
| Dlx1 | 5′-CTACGTCAACTCGGTCAGCA-3′ | 5′-GGCAGAGCTAGGTACTGAGT-3′ | 258 |
| Dlx2 | 5′-TCCTACCAGTACCAAGCCA-3′ | 5′-AAGCACAAGGTGGAGAAGC-3′ | 430 |
| Dlx3 | 5′-AAGGTCCGAAAGCCGCGTA-3′ | 5′-CTGCTGCTGTAAGTGGGGT-3′ | 414 |
| Dlx5 | 5′-TGGCAAACCAAAGAAAGTTC-3′ | 5′-AATAGAGTGTCCCGGAGG-3′ | 475 |
| Dlx6 | 5′-GAAAACGGGGAAATCAGGTT-3′ | 5′-ATCATCTGTGGTCTCTGCAT-3′ | 420 |
| Dlx7 | 5′-TAACAAGCTCCTGAAGCAG-3′ | 5′-ATTCACATCATCTGAGGC-3′ | 210 |
| Bmp2 | 5′-AGGTTAGTGAATCAGAATAC-3′ | 5′-TCACTGAAGTCCACATACAA-3′ | 340 |
| Bmp4 | 5′-CGAAGAACATCTGGAGAACA-3′ | 5′-CACTCCCTTGAGGTAACGAT-3′ | 420 |
Preparation of Probes
The human embryonic Dlx3 PCR product (414 bp) was analyzed in a 2% agarose gel, and the amplified fragments were subcloned into bacterial expression vector, pCR2.1 (Invitrogen). In-frame cloning was confirmed by sequencing and Northern blotting of the 9-week-old embryonic whole orofacial tissues as described by Ghoul-Mazgar et al. (2005). Sense and antisense Dlx3 RNA digoxigenin-labeled probes were synthesized after linearization with BamH1 using T7 and T3 RNA polymerases, respectively. Msx1 sense and antisense RNA digoxigenin-labeled probes were synthesized from a Bluescript-SK(+) plasmid containing 350 bp of exon 2 of the mouse Msx1 gene after linearization with BamH1 or HindIII endonuclease, using T7 and T3 RNA polymerases, respectively (Roche Diagnostics; Meylan, France).
Msx2 sense and antisense RNA digoxigenin-labeled probes (850 bp) were synthesized from pSP72 plasmid after linearization with HindIII and BglII using Sp6 and T7 RNA polymerase, respectively (Roche Diagnostics).
Amelogenin sense and antisense RNA probes were prepared from full-length RT cDNA (from W.T. Bonnass and C. Robinson, Leeds, UK), subcloned into Bluescript plasmid, and labeled with digoxigenin-UTP by in vitro transcription using T7 and/or T3 RNA polymerase (Boehringer-Mannheim; Meylan, France) used for ISH.
ISH
ISH was performed as previously described (Hotton et al. 1995; Ghoul-Mazgar et al. 2005) with minor modifications: cryostat sections were hybridized with 30 μl of digoxigenin-labeled probes diluted 1:200, and the reaction was shown by antidigoxigenin Fab alkaline phosphatase–conjugated fragments (Roche Diagnostics). Histoenzymatic staining was performed for 2–18 hr depending on the tissue and the stage of development. Sections were dehydrated, mounted under a coverslip, and photographed. Tissue sections were observed and photographed on a Leica Orthoplan microscope (Leica; Solms, Germany).
IHC
Serial frozen sections (8 μm) were performed with cryostat (Bright Instrument Company; Huntington, UK). Immunolocalizations were performed with rabbit polyclonal antibodies raised against purified bovine amelogenin [gift of S. Sasaki, Tokyo, Japan, and described by Nishikawa et al. (1990)]. Sections were treated with 0.3% hydrogen peroxide in 0.1 M Tris-HCl (pH 7.6) for 10 min to inhibit endogenous peroxidase activity. After being rinsed with the Tris-HCl solution, sections were incubated overnight at 4C in Tris-HCl containing 1:30 non-immune goat serum (Nordic; Tilburg, The Netherlands) to block nonspecific binding sites. The primary antibody was applied at a 1:1000 dilution for 1 hr at room temperature. Sections were rinsed with 1% BSA in Tris-HCl and incubated with biotinylated anti-rabbit secondary antibodies (Sigma; La Verpillière, France) at a 1:200 dilution for 30 min. After incubation in 1:300 diluted-peroxydase (Sigma) for 30 min, the immunoreactive sites were visualized by the oxidization of tetrachloride 3-3′ diaminobenzidine (Sigma) 0.5 mg/ml in Tris-HCl by adding 0.03% H2O2 to the solution. Sections were lightly counterstained with Harris hematoxylin stain (Sigma). Sections were washed in distilled water, dehydrated, and mounted in DePex (Gurr; Osi, France). Irrelevant rabbit antibodies (Sigma) from 1:25 to 1:100 were used as negative controls replacing the primary antibodies.
Transmission Electron Microscopy (TEM)
The CCOC specimen was fixed in Karnovsky solution (4% paraformaldehyde, 1% glutaraldehyde) for 1 hr. After several washes in sodium cacodylate buffer (pH 7.4), the specimen was postfixed for 1 hr in osmium tetroxide diluted in 0.2 M sodium cacodylate buffer. The specimen was dehydrated in graded series of ethanol and left overnight in a mixture of absolute ethanol and epon 1:1. The following day, the specimen was embedded in Epon-Araldite and incubated at 60C for 1 day. Semi-thin sections were cut with a diamond knife, mounted on glass slides, stained with methylene blue (Azur II), and examined under light microscopy for orientation purposes. Ultrathin sections were obtained, collected on copper grids, and stained with 5% uranyl acetate in water for 4 min and lead citrate for 2 min. The sections were examined under a TEM Philips CM-12 (Philips; Amsterdam, The Netherlands).
Results
Histopathological Characterization of the CCOCs
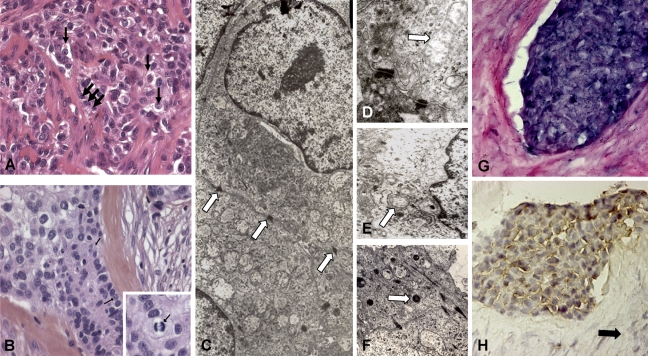
The histological aspect of the recurrent carcinoma showed solid islands and strands of cells with clear cytoplasm in most areas (Figures 1A and 1B). Some tumor islands showed peripheral palisading. The tumor islands and strands were separated by mature fibrous septae. Pleomorphism and mitotic activity were occasionally observed. Periodic acid-Schiff and Alcian blue stains remained undetected.
Figure 1.
Structural and molecular results of the clear cell odontogenic carcinoma (CCOC). Hematoxylin–eosin–stained section of the clear cell odontogenic carcinoma shows the presence of epithelial nests with peripheral palisade cubical cells (A, black thick arrows) surrounding small compact interfaces. The presence of clear cells (B, black thin arrows) with atypia and mitosis, associated with hyalinized stroma containing giant cells, makes the diagnosis of CCOC more evident. Islands and strands of the clear cells are supported by a fibrous connective tissue stroma (B). Higher magnification of tumor cells shows a clear cytoplasm with uniform vesicular nuclei. (Inset: mitosis.) Desmosomes (C, arrows), endoplasmic reticulum, free ribosomes, glycogen rosettes, mitochondria (D, arrow), plasma membrane microvilli (E, arrow), and lysosomes (F, arrow) are ultrastructurally noted. Many cells exhibit a paucity of cytoplasmic organelles with prominent vacuolization. In the epithelial cells, amelogenin transcripts (G) and protein (H) are, respectively, detected by ISH and IHC. No amelogenin expression was detected in stromal cells (H, black arrow).
The ultrastructural analysis of the recurrent tumor showed several cellular features: plasma membrane microvilli, numerous desmosomes, a small endoplasmic reticulum, abundant free ribosomes, glycogen rosettes, and lysosomes. Many cells showed paucity of cytoplasmic organelles with prominent vacuolization (Figures 1C–1F). Amelogenin expression was studied at the RNA (ISH) and protein (immunocytochemistry) levels. Amelogenin RNA was detected in all epithelial islands at a high magnification compared with those of stroma cells (Figure 1G). Amelogenin proteins seemed to be sequestered in the cytoplasm of epithelial cells because no labeling was noted in the extracellular stromal compartment (Figure 1H).
Msx and Dlx Homeogenes and BMP Expression Are Dysregulated in CCOCs
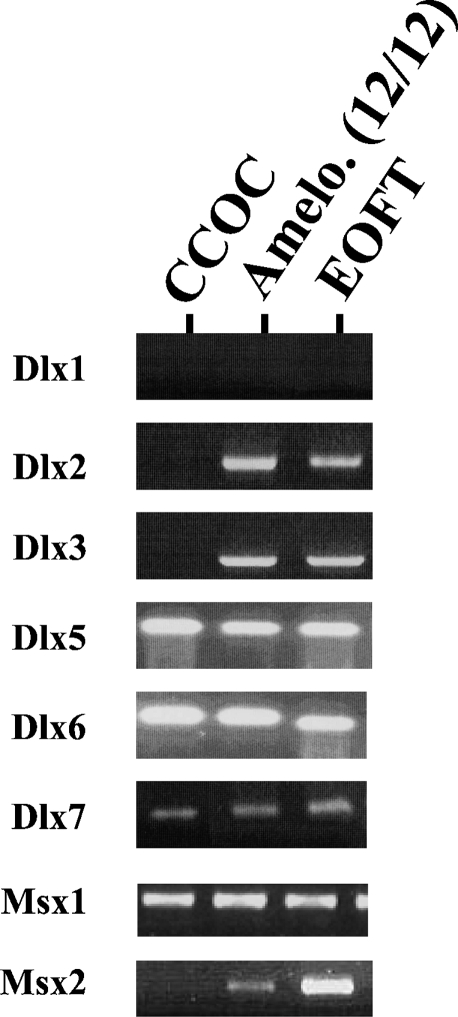
RT-PCR analysis failed to show significant differences in Dlx1, Dlx5, Dlx6, Dlx7, and Msx1 mRNA expression between all the epithelial odontogenic tumors and the human embryonic orofacial tissue (Figure 2). In contrast, Msx2, Dlx2, and Dlx3 mRNA remained undetectable in the unique malignant tumor (CCOC). These transcripts were always present in all the ameloblastoma samples and the orofacial embryonic tissues studied (triplicate assay).
Figure 2.
RT-PCR analysis of Msx and Dlx gene expression in odontogenic tumors. Msx and Dlx gene expression was analyzed in human embryonic oro-facial tissues of a 9-week-old human embryo (EOFT), 12 cases of ameloblastomas, and 1 case of CCOC by RT-PCR. Agarose gel analysis showed similar gene profile among EOFT and ameloblastomas, whereas Dlx2, Dlx3, and Msx2 remain undetected in the CCOC (triplicate essay). Dlx1 expression gene was not detected in all tissue samples.
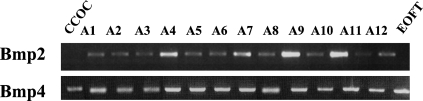
Moreover, the RT-PCR exploration of BMP expression (Figure 3) failed to detect Bmp2 transcript in the CCOC (triplicate assay). However, this transcript was variably detected in all the other recurrent benign ameloblastomas and orofacial embryonic tissues. Comparatively, the Bmp4 transcript was regularly detected in all tissue samples studied.
Figure 3.
RT-PCR analysis of Bmp2 and Bmp4 gene expression in odontogenic tumors. Transcript expression was analyzed by RT-PCR in the CCOC, 12 cases of ameloblastomas (A1–A12), and EOFTs of a 9-week-old embryo. Agarose gel analysis showed an expected size but an irregular band intensity for Bmp2 in the ameloblastomas studied. The Bmp2 band remained undetectable in the CCOC (triplicate essay). Bmp4 transcripts seem to be regularly expressed at the expected size in all the tissue samples studied.
Exploration of Msx2 and Dlx3 Homeogene Expression in Odontogenic Tumors
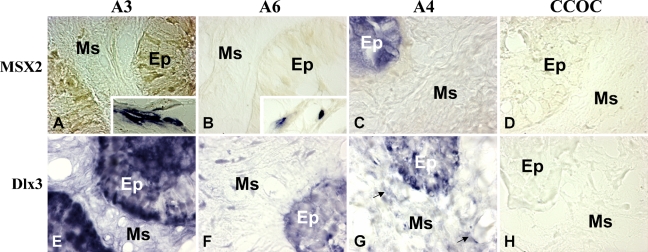
To localize Msx2 and Dlx3 homeogene–expressing cells in tumors, mRNAs of these genes were assessed by ISH (Figure 4). Different aspects were observed and summarized for three cases. Msx2 expression was inconstantly described. In fact, Msx2 transcript was detected in the epithelial cells of some ameloblastomas, as shown in Figure 4B, but only in some peripheral tumor cells, as described in Figures 4A and 4C. The Dlx3 transcript was mostly detected in all ameloblastomas, as shown in Figures 4E–4G. However, it was detected in some ameloblastomas, exclusively in the epithelial cells, as shown in Figure 4E. In other cases, it was observed in both epithelial and mesenchymal cells, as shown in Figures 4F and 4G. Neither Msx2 nor Dlx3 transcripts were detected in the clear cell odontogenic carcinoma (Figures 4D and 4H).
Figure 4.
Detection of Msx2 and Dlx3 transcripts in odontogenic tumors by ISH. Analysis of the epithelial (Ep) and mesenchymal (Ms) compartments of the tumors showed that Msx2 transcripts are not expressed in some tumors as shown for ameloblastoma 3 (A), ameloblastoma 6 (B), and the CCOC (D). However, these transcripts were detected in the peripheral non-tumoral cells of ameloblastoma 3 and 6 (A,B, inset) and only in the epithelial compartment of ameloblastoma 4 (C). Concerning the Dlx3 transcript, it was always detected in the epithelial compartments of the ameloblastomas (E–G) but failed to be detected in the CCOC (H). Some ameloblastomas, as shown for ameloblastoma 4, also express the transcript in the mesenchymal part (G, arrow).
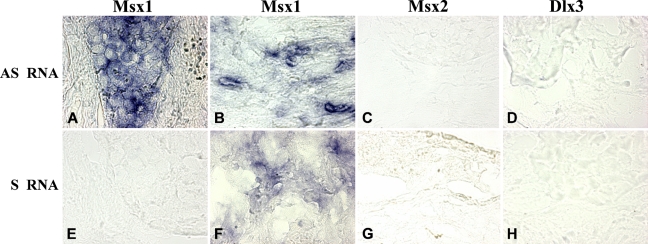
Msx1 antisense transcripts (Figure 5) were assessed by ISH using sense riboprobes. This method detected antisense transcripts of Msx1 in the odontogenic carcinoma. This transcript was localized in the cytoplasm of the epithelial (Figure 5A) and fibroblastic stroma cells (Figure 5B). However, the sense transcripts of Msx1 detected by the antisense riboprobe were only detected in the cytoplasm of fibroblastic stroma cells (Figure 5F). We did not detect signal for the sense and antisense transcripts of Dlx3 and Msx2 in this carcinoma.
Figure 5.
In situ localization of the antisense transcripts of Msx1 and the sense transcripts of Msx2 and Dlx3 in the CCOC. A specific Msx1 sense riboprobe detects antisense transcripts in the CCOC. They are localized in the cytoplasm of all the epithelial (A) and some of the mesenchymal (B) cells. No antisense transcripts were detected in this tumor using sense riboprobes of Msx2 (C) and Dlx3 (D). In contrast, specific antisense riboprobes detect the sense transcripts. In this carcinoma, no sense transcripts were detected for Msx1 either in the epithelial (E) or in the mesenchymal (F) compartments. Msx2 (G) and Dlx3 transcripts (H) were not expressed in this tumor.
Discussion
Because malignant odontogenic tumors are rare neoplasms, structural, ultractructural, and histochemical analyses were used here to confirm the diagnosis of the clear cell odontogenic tumor (Eversole et al. 1985; Kumamoto et al. 1998). Our findings therefore provide evidence that the CCOC studied here showed not only ultrastructural features (Eversole et al. 1985) but also an amelogenin expression pattern in the epithelial cells of this tumor at the RNA and protein levels as previously described (Kumamoto et al. 2001), dispelling any doubt about the histopathological diagnosis.
Although disruptions of Msx2 (Takahashi et al. 1996), Dlx3 (Roberson et al. 2001), and Dlx7 (Neufing et al. 2003; Hollington et al. 2004) homeogene expression were shown in extraoral non-dental epithelial tumors, few studies have investigated these homeogenes in odontogenic tumorigenesis.
Such a study was performed here, based on the importance of these homeogenes in the control of cell fate. At the cellular level, Msx and Dlx play diverse roles. Msx1 overexpression induces the dedifferentiation of multinuclear myotubes into myoblasts and even their transdifferentiation into another cell type such as the osteoblasts under appropriate culture conditions (Odelberg et al. 2000). Msx1 is therefore related to the maintenance of cell plasticity by inhibiting the expression of specific master genes: MyoD in muscular (Odelberg et al. 2000) and Runx2 in osteo-odontogenic (Blin-Wakkach et al. 2001) cells. In the tooth germ, Msx2 was described in the enamel knot, which is considered to be a signaling center and which undergoes cell apoptosis (Vaahtokari et al. 1996). In fact, during tooth development, epithelial cells at the tip of the tooth bud stop proliferating and form the enamel knot that organizes the development of the crown shape by signal-regulated epithelial proliferation (McCollum and Sharpe 2001). Msx2 was shown to induce apoptosis in vivo (Takahashi et al. 1998), particularly during dental development (Jernvall et al. 1998) through Bmp4 proapoptotic signalization (Graham et al. 1994; Israsena and Kessler 2002). On the other hand, experimental Dlx3 overexpression in the epidermal basal cell layer is associated with premature keratinocyst differentiation (Morasso et al. 1996). It may therefore be proposed that the cell–cell communication and the growth factors that drive Msx and Dlx gene expression are essential for the control of cell fate during development, notably in the tooth germ, and may be of great interest in tumoral cell physiopathology.
Indeed, dysregulation of homeobox-containing genes is becoming increasingly recognized as an underlying mechanism of tumorigenesis (Hassan et al. 2006; Shames et al. 2006; Chang et al. 2007; Takahashi et al. 2007). This fact has been described for several members of the Hox homeobox gene cluster: HoxA9 in acute myeloid leukemia (Lawrence et al. 1996) and HoxA13 in T-cell acute lymphoblastic leukemia (Nakamura et al. 1996; Su et al. 2006). The critical role of non-Hox homeobox genes has also been described in hematopoiesis and leukemic transformation (Owens et Hawley 2002). Precisely, the entire family of Dlx genes was found to be reduced in the context of the acute lymphoblastic leukemia observed in vivo and in vitro (Ferrari et al. 2003b). In other tissues, it was shown that a decrease in Dlx4 expression is associated with colorectal carcinogenesis (Hollington et al. 2004). Concerning the Msx family, Msx2 was described in various cell lines derived from human tumors, particularly in carcinoma-derived cell lines (Takahashi et al. 1996). Although Msx1 was shown to induce apoptosis in cancer cells (Park et al. 2001,2005), Msx2 was shown to exert repressive effects on tumoral cells through specific induced apoptotic pathways (Hamada et al. 2005). All these data suggest the existence of the Msx and Dlx effect in tumorigenesis, albeit with potential opposite effects on cell behavior depending on the types of tumors, cells, and Msx-Dlx member.
This study provided an additional set of data on the expression patterns of Msx and Dlx transcripts in previously unexplored tumors. Benign ameloblastomas and non-tumoral tissues (EOFT) seem to have a similar profile by RT-PCR analysis. Non-tumoral tissues as odontogenic epithelium and mesenchyme in human tooth germs showed expression for the Msx1 sense or antisense transcripts. As described by ISH, the antisense RNA was exclusively observed in epithelial cells. However, it was coexpressed with sense RNA in mesenchymal cells (Blin-Wakkach et al. 2001). Thus, Msx1 sense/antisense RNA distribution in tumors imitated the physiological situation. In contrast, Msx2 ISH showed (a) an absence of expression in the stroma of benign tumors that contrasts with normal mesenchymal Msx2 expression in mouse (Aioub et al 2007) and human (Davideau et al. 1999) dental development and (b) variable expression in the epithelial cell islands depending on the tumors. Interestingly, a lack of Msx2 and Dlx expression in CCOCs was observed here. This observation of the affected Msx and Dlx homeogene expression is in line with a cDNA microarray that compared ameloblastomas with non-tumoral odontogenic tissues (Heikinheimo et al. 2002). This study showed underexpression of several transcription factors and transforming growth factor β1. It may therefore be proposed that abnormal Msx2 gene regulation is a candidate underlying epithelial cell differentiation in ameloblastomas and CCOCs. These observations are in line with a wide spectrum of human pathologies in Msx2−/− mice, from eruption defects to amelogenesis imperfecta and odontogenic tumors (Suda et al. 2006; Aioub et al. 2007). Contrary to what was described during normal development, Msx2 may inhibit several apoptotic signalizations in tumoral cells (Hamada et al. 2005). Regulation of Msx2 gene expression in some epithelial tumoral cells may be incriminated in the low or high recurrence levels (Malewski et al. 2005; Depondt et al. 2008).
Dlx2 and Dlx3 are expressed during tooth morphogenesis (Zhao et al. 2000) and have been shown to play a key role during cell differentiation (Lezot et al. 2000; Ghoul-Mazgar et al. 2005) and apoptosis (Ferrari et al. 2003a). In addition, this study showed a lack of Dlx2, Dlx3, Msx2, and Bmp2 expression, specifically in CCOC, compared with ameloblastomas and the normal situation.
In early tooth development, Bmp4 induces Msx1 signaling pathways (Bei and Maas 1998). Msx and Dlx homeoprotein expression is regulated by several molecular signals including bone morphogenetic proteins Bmp2 and Bmp4 (Vainio et al. 1993; Chen et al. 1996; Bei and Maas 1998; Luo et al. 2001). Bmp and their associated molecules were described in odontogenic tumors as ameloblastomas (Kumamoto and Ooya 2006). Dysregulation in Bmp signaling is suggested in our study by the evident absence of Bmp2 transcript expression in the CCOC and not of Bmp4 transcripts. Bmp2 is downregulated at the time of terminal differentiation of ameloblasts, suggesting that the differentiation process is affected in the CCOC cells. Bmp2 not only stimulates expression of Msx1 and Msx2, but it also induces Dlx2 expression (Xu et al. 2001) and transactivates Dlx3 (Park and Morasso 2002). The lack of Bmp2 may be responsible for the absence of these two homeogenes in the case of CCOC.
Epithelial odontogenic tumors are rare neoplasms arising from remnants of the odontogenic epithelium. Their pathogenesis is still unknown. To detail cell cycling perturbations in these tumors, a greater number of sample specimen is needed, but this is complicated by the disease scarcity. For the first time, we showed dysregulated Msx and Dlx gene expression between benign epithelial odontogenic tumors and one case of a rare malignant epithelial odontogenic tumor. Functional invalidation of these molecules may be explained as (a) a result of earlier disturbance events or (b) a causal event of malignant conversion. More genetic studies of these Msx and Dlx signaling molecules in odontogenic tumors should be conducted. Molecular exploration of other cases of malignant odontogenic tumors is needed to confirm our findings.
Acknowledgments
We thank Benoît Robert (Institut Pasteur Paris), Paul T. Sharpe (London), William T. Bonnass, and Colin Robinson for providing the Msx1, Msx2, and amelogenin probes, respectively; Satochi Sasaki (Tokyo) for amelogenin antibodies; and Samir Boukottaya (Dental Faculty of Monastir) for English assistance.
References
- Aioub M, Lezot F, Molla M, Castaneda B, Robert B, Goubin G, Nefussi JR, et al. (2007) Msx2−/− transgenic mice develop compound amelogenesis imperfecta, dentinogenesis imperfecta and periodental osteopetrosis. Bone 41:851–859 [DOI] [PubMed] [Google Scholar]
- Akrish S, Buchner A, Shoshani Y, Vered M, Dayan D (2007) Ameloblastic carcinoma: report of a new case, literature review, and comparison to ameloblastoma. J Oral Maxillofac Surg 65:777–783 [DOI] [PubMed] [Google Scholar]
- Bei M, Maas R (1998) FGFs and BMP4 induce both Msx1-independent and Msx1-dependent signalling pathways in early tooth development. Development 125:4325–4333 [DOI] [PubMed] [Google Scholar]
- Blin-Wakkach C, Lezot F, Ghoul-Mazgar S, Hotton D, Monteiro S, Teillaud C, Pibouin L, et al. (2001) Endogenous Msx1 antisense transcript: in vivo and in vitro evidences, structure and potential involvement in skeleton development in mammals. Proc Natl Acad Sci USA 98:7336–7341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carinci F, Francioso F, Piattelli A, Rubini C, Fioroni M, Evangelisti R, Arcelli D, et al. (2003a) Genetic expression profiling of six odontogenic tumors. J Dent Res 82:551–557 [DOI] [PubMed] [Google Scholar]
- Carinci F, Volinia S, Rubini C, Fioroni M, Francioso F, Arcelli D, Pezzetti F, et al. (2003b) Genetic profile of clear cell odontogenic carcinoma. J Craniofac Surg 14:356–362 [DOI] [PubMed] [Google Scholar]
- Carlson ER, Marx RE (2006) The ameloblastoma: primary, curative surgical management. J Oral Maxillofac Surg 64:484–494 [DOI] [PubMed] [Google Scholar]
- Chang YT, Hsu C, Jeng YM, Chang MC, Wei SC, Wong JM (2007) Expression of the caudal-type homeodomain transcription factor CDX2 is related to clinical outcome in biliary tract carcinoma. J Gastroenterol Hepatol 22:389–394 [DOI] [PubMed] [Google Scholar]
- Chen Y, Bei M, Woo I, Satokata I, Maas R (1996) Msx1 controls inductive signalling in mammalian tooth morphogenesis. Development 122:3035–3044 [DOI] [PubMed] [Google Scholar]
- Davideau JL, Demri P, Hotton D, Gu TT, MacDougall M, Sharpe P, Forest N, et al. (1999) Comparative study of Msx2, Dlx5 and Dlx7 gene expression during early human tooth development. Pediatr Res 46:650–656 [DOI] [PubMed] [Google Scholar]
- Depew M, Liu J, Long J, Presley R, Meneses J, Pedersen R, Rubenstein J (1999) Dlx5 regulates regional development of the branchial arches and sensory capsules. Development 126:3831–3846 [DOI] [PubMed] [Google Scholar]
- Depondt J, Shabana el-H, Walker F, Pibouin L, Lezot F, Berdal A (2008) Links Nasal inverted papilloma expresses the muscle segment homeobox gene Msx2: possible prognostic implications. Hum Pathol 39:350–358 [DOI] [PubMed] [Google Scholar]
- Dong J, Amor D, Aldred MJ, Gu T, Escamilla M, MacDougall M (2005) Dlx3 mutation associated with autosomal dominant amelogenesis imperfecta with taurodontism. Am J Med Genet 133:138–141 [DOI] [PubMed] [Google Scholar]
- Eversole LR, Belton CM, Hansen LS (1985) Clear cell odontogenic tumor: histochemical and ultrastructural features. J Oral Pathol 14:603–614 [DOI] [PubMed] [Google Scholar]
- Ferrari N, Paleari L, Palmisano GL, Tammaro P, Levi G, Albini A, Brigati C (2003a) Induction of apoptosis by fenretinide in tumor cell lines correlates with Dlx2, Dlx3 and Dlx4 gene expression. Oncol Rep 10:973–977 [PubMed] [Google Scholar]
- Ferrari N, Palmisano GL, Paleari L, Basso G, Mangioni M, Fidanza V, Albini A, et al. (2003b) DLX genes as targets of ALL-1: DLX 2,3,4 down-regulation in t(4;11) acute lymphoblastic leukemias. J Leukoc Biol 74:302–305 [DOI] [PubMed] [Google Scholar]
- Fukumoto S, Kiba T, Hall B, Iehara N, Nakamura T, Longenecker G, Krebsbach PH, et al. (2004) Ameloblastin is a cell adhesion molecule required for maintaining the differentiation state of ameloblasts. J Cell Biol 167:973–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoul-Mazgar S, Hotton D, Lézot F, Blin-Wakkach C, Asselin A, Sautier JM, Berdal A (2005) Expression pattern of Dlx3 during cell differentiation in mineralized tissues. Bone 37:799–809 [DOI] [PubMed] [Google Scholar]
- Gibson CW, Yuan ZA, Li Y, Daly B, Suggs C, Aragon MA, Alawi F, et al. (2007) Transgenic mice that express normal and mutated amelogenins. J Dent Res 86:331–335 [DOI] [PubMed] [Google Scholar]
- Graham A, Francis-West P, Brickell P, Lumsden A (1994) The signalling molecule BMP4 mediates apoptosis in the rhombencephalic neural crest. Nature 372:684–686 [DOI] [PubMed] [Google Scholar]
- Hall JM, Weathers DR, Unni KK (2007) Ameloblastic carcinoma: an analysis of 14 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 103:799–807 [DOI] [PubMed] [Google Scholar]
- Hamada S, Satoh K, Kimura K, Kanno A, Masamune A, Shimosegawa T (2005) MSX2 overexpression inhibits gemcitabine-induced caspase-3 activity in pancreatic cancer cells. World J Gastroenterol 11:6867–6870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan MQ, Tare RS, Lee SH, Mandeville M, Morasso MI, Javed A, van Wijnen AJ, et al. (2006) BMP2 commitment to the osteogenic lineage involves activation of Runx2 by DLX3 and a homeodomain transcriptional network. J Biol Chem 281:40515–40526 [DOI] [PubMed] [Google Scholar]
- Heikinheimo K, Jee KJ, Niini T, Aalto Y, Happonen RP, Leivo I, Knuutila S (2002) Gene expression profiling of ameloblastoma and human tooth germ by means of a cDNA microarray. J Dent Res 81:525–530 [DOI] [PubMed] [Google Scholar]
- Hollington P, Neufing P, Kalionis B, Waring P, Bentel J, Wattchow D, Tilley WD (2004) Expression and localization of homeodomain proteins Dlx4, HB9 and HB24 in malignant and benign human colorectal tissues. Anticancer Res 24:955–962 [PubMed] [Google Scholar]
- Hotton D, Davideau JL, Bernaudin JF, Berdal A (1995) In situ hybridization of calbindine-D-28k transcripts in undecalcified sections of the rat continuously erupting incisor. Connect Tissue Res 32:137–143 [DOI] [PubMed] [Google Scholar]
- Israsena N, Kessler JA (2002) Msx2 and p21(CIP1/WAF1) mediate the proapoptotic effects of bone morphogenetic protein-4 on ventricular zone progenitor cells. J Neurosci Res 69:803–809 [DOI] [PubMed] [Google Scholar]
- Jernvall J, Aberg T, Kettunen P, Keranen S, Thesleff I (1998) The life history of an embryonic signaling center: BMP-4 induces p21 and is associated with apoptosis in the mouse tooth enamel knot. Development 125:161–169 [DOI] [PubMed] [Google Scholar]
- Kramer IRH, Pindborg JJ, Shear M (1992) WHO Histological Typing of Odontogenic Tumours. 2nd ed. Berlin, Springer-Verlag
- Kumamoto H, Izutsu T, Ohki K, Takahashi N, Ooya K (2004) p53 gene status and expression of p53, MDM2, and p14 proteins in ameloblastomas. J Oral Pathol Med 33:292–299 [DOI] [PubMed] [Google Scholar]
- Kumamoto H, Kawamura H, Ooya K (1998) Clear cell odontogenic tumor in the mandible: report of a case with an immunohistochemical study of epithelial cell markers. Pathol Int 48:618–622 [DOI] [PubMed] [Google Scholar]
- Kumamoto H, Ooya K (1999) Immunohistochemical analysis of bcl-2 family proteins in benign and malignant ameloblastomas. J Oral Pathol Med 28:343–349 [DOI] [PubMed] [Google Scholar]
- Kumamoto H, Ooya K (2006) Expression of bone morphogenetic proteins and their associated molecules in ameloblastomas and adenomatoid odontogenic tumors. Oral Dis 12:163–170 [DOI] [PubMed] [Google Scholar]
- Kumamoto H, Takahashi N, Ooya K (2004) K-ras gene status and expression of Ras/mitogen-activated protein kinase (MAPK) signalling molecules in ameloblastomas. J Oral Pathol Med 33:360–367 [DOI] [PubMed] [Google Scholar]
- Kumamoto H, Yoshida M, Ooya K (2001) Immunohistochemical detection of amelogenin and cytokeratin 19 in epithelial odontogenic tumors. Oral Dis 7:171–176 [PubMed] [Google Scholar]
- Lawrence HJ, Sauvageau G, Humphries RK, Largman C (1996) The role of HOX homeobox genes in normal and leukemic hematopoiesis. Stem Cells 14:281–291 [DOI] [PubMed] [Google Scholar]
- Lezot F, Davideau JL, Thomas B, Sharpe P, Forest N, Berdal A (2000) Epithelial Dlx2 homeogene expression and cementogenesis. J Histochem Cytochem 48:277–284 [DOI] [PubMed] [Google Scholar]
- Lim J, Ahn H, Min S, Hong SD, Lee JI, Hong SP (2006) Oligonucleotide microarray analysis of ameloblastoma compared with dentigerous cyst. J Oral Pathol Med 35:278–285 [DOI] [PubMed] [Google Scholar]
- Luo T, Matsuo-Takasaki M, Lim JH, Sargent TD (2001) Differential regulation of Dlx gene expression by a BMP morphogenetic gradient. Int J Dev Biol 45:681–684 [PubMed] [Google Scholar]
- Malewski T, Milewicz T, Krzysiek J, Gregoraszczuk EL, Augustowska K (2005) Regulation of Msx2 gene expression by steroid hormones in human nonmalignant and malignant breast cancer explants cultured in vitro. Cancer Invest 23:222–228 [DOI] [PubMed] [Google Scholar]
- McCollum MA, Sharpe PT (2001) Developmental genetics and early hominid craniodental evolution. Bioessays 23:481–493 [DOI] [PubMed] [Google Scholar]
- Miyake T, Tanaka Y, Kato K, Tanaka M, Sato Y, Ijiri R, Inayama Y, et al. (2006) Gene mutation analysis and immunohistochemical study of beta-catenin in odontogenic tumors. Pathol Int 56:732–737 [DOI] [PubMed] [Google Scholar]
- Morasso MI, Markova NG, Sargent TD (1996) Regulation of epidermal differentiation by a distal-less homeodomain gene. J Cell Biol 135:1879–1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Largaespada DA, Shaughnessy JD, Jenkins NA, Copeland NG (1996) Cooperative activation of Hoxa and Pbx1-related genes in murine myeloid leukamias. Nat Genet 12:149–153 [DOI] [PubMed] [Google Scholar]
- Neufing PJ, Kalionis B, Horsfall DJ, Ricciardelli C, Stahl J, Vivekanandan S, Raymond W, et al. (2003) Expression and localization of homeodomain proteins DLX4/HB9 in normal and malignant human breast tissues. Anticancer Res 23:1479–1488 [PubMed] [Google Scholar]
- Nishikawa S, Takagi T, Sasa S (1990) Immunocytochemical localization of amelogenin in rat incisor ameloblasts using ultrathin frozen sections. J Electron Microsc (Tokyo) 39:404–407 [PubMed] [Google Scholar]
- Odelberg SJ, Kollhoff A, Keating MT (2000) Dedifferentiation of mammalian myotubes induced by msx1. Cell 103:1099–1109 [DOI] [PubMed] [Google Scholar]
- Owens BM, Hawley RG (2002) HOX and non-HOX homeobox genes in leukemic hematopoiesis. Stem Cells 20:364–379 [DOI] [PubMed] [Google Scholar]
- Papagerakis P, Peuchmaur M, Hotton D, Ferkdadji L, Delmas P, Sasaki S, Tagaki T, et al. (1999) Aberrant gene expression in epithelial cells of mixed odontogenic tumors. J Dent Res 78:20–30 [DOI] [PubMed] [Google Scholar]
- Park GT, Morasso M (2002) Bone morphogenetic protein-2 (BMP2) transactivates Dlx3 through Smad1 and Smad4: alternative mode for Dlx3 induction in mouse keratinocytes. Nucleic Acids Res 30:515–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Park K, Kim S, Lee JH (2001) Msx1 gene overexpression induces G1 phase cell arrest in human ovarian cancer cell line OVCAR3. Biochem Biophys Res Commun 281:1234–1240 [DOI] [PubMed] [Google Scholar]
- Park K, Kim K, Rho SB, Choi K, Kim D, Oh SH, Park J, et al. (2005) Homeobox Msx1 interacts with p53 tumor suppressor and inhibits tumor growth by inducing apoptosis. Cancer Res 65:749–757 [PubMed] [Google Scholar]
- Perdigao PF, Gomez RS, Pimenta FJ, De Marco L (2004) Ameloblastin gene (AMBN) mutations associated with epithelial odontogenic tumors. Oral Oncol 40:841–846 [DOI] [PubMed] [Google Scholar]
- Pindborg JJ, Kramer IRH, Torloni H (1972) Histologic Typing of the Odontogenic Tumors, Jaw, Cysts and Allied Lesions. Geneva, World Health Organization
- Pippi R (2006) Benign odontogenic tumours: clinical, epidemiological and therapeutic aspects of a sixteen years sample. Minerva Stomatol 55:503–513 [PubMed] [Google Scholar]
- Price JA, Bowden DW, Wright JT, Pettenati MJ, Hart TC (1998) Identification of a mutation in Dlx3 associated with tricho-dento-osseous (TDO) syndrom. Hum Mol Genet 7:563–569 [DOI] [PubMed] [Google Scholar]
- Roberson MS, Meermann S, Morasso MI, Mulvaney-Musa JM, Zhang T (2001) A role for the homeobox protein Distal-less 3 in the activation of the glycoprotein hormone alpha subunit gene in choriocarcinoma cells. J Biol Chem 276:10016–10024 [DOI] [PubMed] [Google Scholar]
- Satokata I, Ma L, Ohshima H, Bei M, Woo I, Nishizawa K, Maeda T, et al. (2000) Msx2 deficiency in mice causes pleiotropic defects in bone growth and ectodermal oragan formation. Nat Genet 24:391–395 [DOI] [PubMed] [Google Scholar]
- Satokata I, Maas R (1994) Msx1 deficient mice exhibit cleft palate and abnormalities of craniofacial and tooth development. Nat Genet 6:348–356 [DOI] [PubMed] [Google Scholar]
- Sekine S, Sato S, Takata T, Fukuda Y, Ishida T, Kishino M, Shibata T, et al. (2003) Beta-catenin mutations are frequent in calcifying odontogenic cysts, but rare in ameloblastomas. Am J Pathol 163:1707–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shames DS, Girard L, Gao B, Sato M, Lewis CM, Shivapurkar N, Jiang A, et al. (2006) A genome-wide screen for promoter methylation in lung cancer identifies novel methylation markers for multiple malignancies. PLoS Med 3:486–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephanopoulos G, Garefalaki ME, Lyroudia K (2005) Genes and related proteins involved in amelogenesis imperfecta. J Dent Res 84:1117–1126 [DOI] [PubMed] [Google Scholar]
- Su X, Drabkin H, Clappier E, Morgado E, Busson M, Romana S, Soulier J, et al. (2006) Transforming potential of the T-cell acute lymphoblastic leukemia-associated homeobox genes HOXA13, TLX1, and TLX3. Genes Chromosomes Cancer 45:846–855 [DOI] [PubMed] [Google Scholar]
- Suda N, Kitahara Y, Ohyama K (2006) A case of amelogenesis imperfecta, cleft lip and palate and polycystic kidney disease. Orthod Craniofac Res 9:52–56 [DOI] [PubMed] [Google Scholar]
- Takahashi C, Akiyama N, Matsuzaki T, Takai S, Kitayama H, Noda M (1996) Characterization of a human MSX-2cDNA and its fragment isolated as a transforming suppressor gene against v-Ki-ras oncogene. Oncogene 12:2137–2146 [PubMed] [Google Scholar]
- Takahashi K, Nuckolls GH, Tanaka O, Semba I, Takahashi I, Dashner R, Shum L, et al. (1998) Adenovirus-mediated ectopic expression of Msx2 in even-numbered rhombomeres induces apoptotic elimination of cranial neural crest cells in ovo. Development 125:1627–1635 [DOI] [PubMed] [Google Scholar]
- Takahashi O, Hamada J, Abe M, Hata S, Asano T, Takahashi Y, Tada M, et al. (2007) Dysregulated expression of HOX and ParaHOX genes in human esophageal squamous cell carcinoma. Oncol Rep 17:753–760 [PubMed] [Google Scholar]
- Toyosawa S, Fujiwara T, Ooshima T, Shintani S, Sato A, Ogawa Y, Sobue S, et al. (2000) Cloning and characterization of the human ameloblastin gene. Gene 256:1–11 [DOI] [PubMed] [Google Scholar]
- Vaahtokari A, Aberg T, Thesleff I (1996) Apoptosis in the developing tooth: association with an embryonic signalling center and suppression by EGF and FGF-4. Development 122:121–129 [DOI] [PubMed] [Google Scholar]
- Vainio S, Karavanova I, Jowett A, Thesleff I (1993) Identification of BMP4 as a signal mediating secondary induction between epithelial and mesenchymal tissues during early tooth development. Cell 75:45–58 [PubMed] [Google Scholar]
- Van den Boogaard MJ, Dorland M, Beemer FA, Van Amstel HK (2000) Msx1 mutation is associated with orofacial clefting and tooth agenesis in humans. Nat Genet 24:342–343 [DOI] [PubMed] [Google Scholar]
- Vastardis H, Karimbux N, Guthua SW, Seidman JG, Seidman CE (1996) A human MSX1 homeodomain missense mutation causes selective tooth agenesis. Nat Genet 13:417–421 [DOI] [PubMed] [Google Scholar]
- Weiss KM, Bollekens J, Ruddle FH, Takashita K (1994) Distal-less and other homeobox genes in the development of the dentition. J Exp Zool 270:273–284 [DOI] [PubMed] [Google Scholar]
- Xu SC, Harris MA, Rubenstein JL, Mundy GR, Harris SE (2001) Bone morphogenetic proteine-2 (BMP-2) signaling to the col2alpha 1 gene in chondroblasts requires the homeobox gene Dlx2. DNA Cell Biol 20:359–365 [DOI] [PubMed] [Google Scholar]
- Zhao Z, Stock DW, Buchanan AV, Weiss KM (2000) Expression of Dlx genes during the development of the murine dentition. Dev Genes Evol 210:270–275 [DOI] [PubMed] [Google Scholar]