Abstract
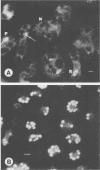
Phagosome-lysosome fusion (P-LF) was studied in cultured mouse resident peritoneal macrophages after phagocytosis of Candida albicans. The macrophages were labeled with acridine orange (AO), the electronopaque colloidal Thorotrast, or both markers. After phagocytosis of heat-killed C. albicans, both markers were delivered to more than 95% of phagosomes. After ingestion of viable C. albicans by labeled cells, delivery of AO to phagosomes was highly suppressed (90%), and yet Thorotrast delivery was almost universal. After phagocytosis and 60 min of incubation, about 10 to 20% of the yeasts were killed, and a similar fraction of phagosomes was stained by the fluorescent marker. The evidence from Thorotrast transfer and assessment of yeast viability indicates that C. albicans largely resists intracellular killing by resident macrophages in the face of entirely uninhibited P-LF. We infer that AO must transfer to nearly all of the phagosomes but that it is evidently recognizable only in those in which the yeasts have been killed or possibly severely injured. This conclusion constitutes yet another limitation in the usefulness of AO for studying P-LF.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. A., Hart P. D. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med. 1971 Sep 1;134(3 Pt 1):713–740. doi: 10.1084/jem.134.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cech P., Lehrer R. I. Heterogeneity of human neutrophil phagolysosomes: functional consequences for candidacidal activity. Blood. 1984 Jul;64(1):147–151. [PubMed] [Google Scholar]
- Goren M. B., D'Arcy Hart P., Young M. R., Armstrong J. A. Prevention of phagosome-lysosome fusion in cultured macrophages by sulfatides of Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2510–2514. doi: 10.1073/pnas.73.7.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goren M. B. Phagocyte lysosomes: interactions with infectious agents, phagosomes, and experimental perturbations in function. Annu Rev Microbiol. 1977;31:507–533. doi: 10.1146/annurev.mi.31.100177.002451. [DOI] [PubMed] [Google Scholar]
- Goren M. B. Some paradoxes of macrophage function. Adv Exp Med Biol. 1983;162:31–50. doi: 10.1007/978-1-4684-4481-0_4. [DOI] [PubMed] [Google Scholar]
- Goren M. B., Swendsen C. L., Fiscus J., Miranti C. Fluorescent markers for studying phagosome-lysosome fusion. J Leukoc Biol. 1984 Sep;36(3):273–292. doi: 10.1002/jlb.36.3.273. [DOI] [PubMed] [Google Scholar]
- Goren M. B., Vatter A. E., Fiscus J. Polyanionic agents as inhibitors of phagosome-lysosome fusion in cultured macrophages: evolution of an alternative interpretation. J Leukoc Biol. 1987 Feb;41(2):111–121. doi: 10.1002/jlb.41.2.111. [DOI] [PubMed] [Google Scholar]
- Hart P. D., Young M. R. Interference with normal phagosome-lysosome fusion in macrophages, using ingested yeast cells and suramin. Nature. 1975 Jul 3;256(5512):47–49. doi: 10.1038/256047a0. [DOI] [PubMed] [Google Scholar]
- Hirsch J. G., Fedorko M. E. Ultrastructure of human leukocytes after simultaneous fixation with glutaraldehyde and osmium tetroxide and "postfixation" in uranyl acetate. J Cell Biol. 1968 Sep;38(3):615–627. doi: 10.1083/jcb.38.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A. The Legionnaires' disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J Exp Med. 1983 Dec 1;158(6):2108–2126. doi: 10.1084/jem.158.6.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. C., Hirsch J. G. The interaction between Toxoplasma gondii and mammalian cells. II. The absence of lysosomal fusion with phagocytic vacuoles containing living parasites. J Exp Med. 1972 Nov 1;136(5):1173–1194. doi: 10.1084/jem.136.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian M. C., Cohn Z. A. Phagosome-lysosome fusion. Characterization of intracellular membrane fusion in mouse macrophages. J Cell Biol. 1980 Jun;85(3):754–765. doi: 10.1083/jcb.85.3.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Cline M. J. Interaction of Candida albicans with human leukocytes and serum. J Bacteriol. 1969 Jun;98(3):996–1004. doi: 10.1128/jb.98.3.996-1004.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Ferrari L. G., Patterson-Delafield J., Sorrell T. Fungicidal activity of rabbit alveolar and peritoneal macrophages against Candida albicans. Infect Immun. 1980 Jun;28(3):1001–1008. doi: 10.1128/iai.28.3.1001-1008.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leijh P. C., van den Barselaar M. T., van Furth R. Kinetics of phagocytosis and intracellular killing of Candida albicans by human granulocytes and monocytes. Infect Immun. 1977 Aug;17(2):313–318. doi: 10.1128/iai.17.2.313-318.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantazis C. G., Kniker W. T. Assessment of blood leukocyte microbial killing by using a new fluorochrome microassay. J Reticuloendothel Soc. 1979 Aug;26(2):155–170. [PubMed] [Google Scholar]
- STRUGGER S. Die Vitalfluorochromierung des Protoplasmas. Naturwissenschaften. 1947;34(9):267–273. doi: 10.1007/BF00589857. [DOI] [PubMed] [Google Scholar]
- Sasada M., Johnston R. B., Jr Macrophage microbicidal activity. Correlation between phagocytosis-associated oxidative metabolism and the killing of Candida by macrophages. J Exp Med. 1980 Jul 1;152(1):85–98. doi: 10.1084/jem.152.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid L., Brune K. Assessment of phagocytic and antimicrobial activity of human granulocytes. Infect Immun. 1974 Nov;10(5):1120–1126. doi: 10.1128/iai.10.5.1120-1126.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. L., Rommel F. A rapid micro method for the simultaneous determination of phagocytic-microbiocidal activity of human peripheral blood leukocytes in vitro. J Immunol Methods. 1977;17(3-4):241–247. doi: 10.1016/0022-1759(77)90106-5. [DOI] [PubMed] [Google Scholar]