Abstract
Vertically transmitted endosymbiotic bacteria, such as Wolbachia, Cardinium and Rickettsia, modify host reproduction in several ways to facilitate their own spread. One such modification results in parthenogenesis induction, where males, which are unable to transmit the bacteria, are not produced. In Hymenoptera, the mechanism of diploidization due to Wolbachia infection, known as gamete duplication, is a post-meiotic modification. During gamete duplication, the meiotic mechanism is normal, but in the first mitosis the anaphase is aborted. The two haploid sets of chromosomes do not separate and thus result in a single nucleus containing two identical sets of haploid chromosomes. Here, we outline an alternative cytogenetic mechanism for bacterial endosymbiont-induced parthenogenesis in Hymenoptera. During female gamete formation in Rickettsia-infected Neochrysocharis formosa (Westwood) parasitoids, meiotic cells undergo only a single equational division followed by the expulsion of a single polar body. This absence of meiotic recombination and reduction corresponds well with a non-segregation pattern in the offspring of heterozygous females. We conclude that diploidy in N. formosa is maintained through a functionally apomictic cloning mechanism that differs entirely from the mechanism induced by Wolbachia.
Keywords: parthenogenesis, apomixis, cytogenetics, Neochrysocharis formosa, Rickettsia
1. Introduction
Some vertically transmitted bacteria increase in frequency by manipulating host reproduction in ways that enhance their own transmission, but do not necessarily benefit their host (for review see Bourtzis & Miller 2003). Such manipulations range from cytoplasmic incompatibility, male killing and feminization, to induction of thelytokous parthenogenesis. The latter appears to be most common in the order Hymenoptera, insects that normally reproduce by arrhenotoky where unfertilized haploid and fertilized diploid eggs develop into males and females, respectively. In numerous species, however, unmated females may produce diploid daughters from unfertilized eggs through thelytokous parthenogenesis (Luck et al. 1993). In such cases, thelytoky may be coded for by nuclear genes of the wasp itself or by its endosymbionts (Stouthamer 1997), such as Wolbachia and Rickettsia in the Proteobacteria, and Cardinium in the Sphingobacteria (Stouthamer et al. 1990; Zchori-Fein et al. 2001; Hagimori et al. 2006).
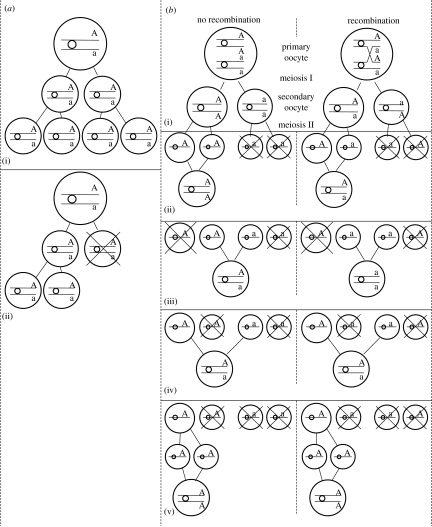
Cytogenetic mechanisms involving thelytokous diploidization can be divided into meiotic, considered most common (Suomalainen et al. 1987), and post-meiotic modifications (Stouthamer 1997). A schematic of different mechanisms in thelytokous diploidization is given in figure 1: a(ii) apomictic parthenogenesis (e.g. Vavre et al. 2004), b(ii) automictic terminal fusion (e.g. Rössler & DeBach 1973), b(iii) automictic central fusion (e.g. Belshaw & Quicke 2003), b(iv) automictic random fusion (e.g. Lampert et al. 2007) and b(v) automictic gamete duplication (e.g. Pannebakker et al. 2004). In most species, meiosis is suppressed and the division has a mitotic character. However, the mechanism of diploidization in Hymenoptera infected with endosymbiotic bacteria, especially Wolbachia, is a post-meiotic modification. The restoration of the diploid number takes place in the first mitotic division when in the anaphase two haploid sets of chromosomes fail to separate and result in a single diploid nucleus containing two identical sets of chromosomes. This diploidization process is called gamete duplication and is shown in figure 1b(v).
Figure 1.
Different cytological mechanisms in apomictic and automictic parthenogenesis and their impact on the transition to homozygosity of a heterozygous locus whether crossing over between the locus and the centromere occurs or not (modified from Pearcy et al. 2006). Large and medium circles represent nuclei. Horizontal lines represent chromatids, with small circles showing the location of the centromere and letters representing alleles at a given locus. The parent is heterozygous (Aa) and the parthenogenesis causes inbreeding when its progeny becomes homozygote (AA or aa). (a(i)) Typical apomixis, (a(ii)) apomixis in Vavre et al. (2004), (b(i)) automixis, (b(ii)) automictic terminal fusion, (b(iii)) automictic central fusion, (b(iv)) automictic random fusion and (b(v)) automictic gamete duplication.
The eulophid parasitoid wasp Neochrysocharis formosa (Westwood) is one of the most important natural enemies of leafminers, Liriomyza trifolii (Burgess) and Liriomyza sativae Blanchard, in Japan (Saito et al. 1996; Tokumaru & Abe 2006). Neochrysocharis formosa has thelytokous and arrhenotokous strains in the field (Arakaki & Kinjo 1998). Thelytokous reproduction in N. formosa is known to be induced by Rickettsia (Hagimori et al. 2006). To date, the cytological mechanism of diploidization by Rickettsia has not been reported.
Here, we compare Rickettsia-induced thelytoky with that of uninfected arrhenotokous individuals using genetic analysis of microsatellite markers and chromosome behaviour analysis in young eggs. We report a new cytogenetic mechanism for symbiont-induced thelytoky in Hymenoptera.
2. Material and methods
(a) Insect collection
Rickettsia-infected thelytokous lines were established from a stock culture originating from the western districts of Japan at Sumitomo Chemical Co. Ltd and from the collections at Fukuyama, Hiroshima, Japan, in autumn 2004. An uninfected arrhenotokous line was established from collections in Nagaokakyo, Kyoto, Japan, in autumn 2005. All cultures were maintained on mining third-instar larvae of L. sativae.
(b) Development of microsatellite primers
Methods used for the isolation of microsatellites were based on the 5′ anchored polymerase chain reaction (PCR) technique (Fisher et al. 1996). DNA was extracted from a Sumitomo thelytokous line individual by crushing with a clean plastic rod in 30 μl of a Tris–EDTA buffer (5N NaCl, 0.5 mM EDTA (pH 8.0) and 1 M Tris–HCl (pH 8.0)) incubated with 2 μl of proteinase K (0.5 mg ml−1) at 37°C for 0.5 h. The homogenate was boiled at 99.9°C for 3 min to inactivate the proteinase K and was used as a template for PCR.
Initial reaction was performed using 33 μl PCRs: 1 μl template DNA; 1.5 U of Taq polymerase (PE Applied Biosystems, Tokyo, Japan); 0.66 μl of 10 mM dNTPs; 2.6 μl of 10 pmol μl−1 PCT4 primer (5′-KKVRVRV(CT)6-3′; Fisher et al. 1996); 3.3 μl of 10×PCR buffer with MgCl2; and 26.2 μl of sterile water. PCR amplification was carried out in an ABI thermocycler (PE Applied Biosystems PCR System 9700, PE Applied Biosystems) with the following programme: an initial denaturing step at 92°C for 1 min; 30 cycles of 92°C for 1 min; an annealing step at 55°C for 1 min, 72°C for 1 min 30 s; and a final extension step of 72°C for 1 min 30 s. The PCR included a negative control (sterile water instead of DNA) to detect contamination. PCR products were resolved on a 2 per cent agarose gel, stained with ethidium bromide and visualized under an UV transilluminator.
The PCR products were cloned according to the p-GEMT Easy Vector system protocol (Promega, Tokyo, Japan). Based on colony PCR analysis, recombinant clones larger than 400 bp were isolated, purified and directly sequenced using M13M4 and M13RV universal primers. A dye terminator-labelled cycle sequencing reaction was conducted with BigDye DNA Sequencing Kit v. 3.1 (PE Applied Biosystems). The temperature profile was 1 min at 96°C followed by 25 cycles of 10 s at 96°C, 5 s at 50°C and 4 min at 60°C. Reaction products were analysed using an ABI PRISM 3130xl Genetic Analyzer (PE Applied Biosystems). Partial sequences were edited and assembled with the Contig Express program in Vector NTI Advance v. 10.1 (Invitrogen InforMax, Frederick, MD, USA).
For the detection of microsatellites in sequences, we used a TROLL program (Martins et al. 2006). Each sequence contained at least two microsatellite repeats on each end of the insert. Some microsatellite clones contained an additional internal microsatellite, implying microsatellites clustered in some genomic regions. Primers were designed to amplify regions containing microsatellite repeats using primer 3 (Rozen & Skaletsky 2000). PIG-tail was attached at the 5′ end of the reverse primer to enhance the 3′ adenosine overhang and to avoid typing error due to variability in non-templated addition of nucleotides at the 3′ end of the PCR products (Brownstein et al. 1996). The forward primer was dye-labelled with NED (PE Applied Biosystems). Resulting forward and reverse primers were named NFTH9-F (5′-AAC TTC TCG CGG CTC ATT TA-3′) and NFTH9-R (5′-GTT TAC GAT CTC CCG TGC TGA TAA-3′). The microsatellite sequence has been deposited in GenBank (accession number AB428375).
(c) Allele segregation and mode of parthenogenesis in N. formosa
We treated four adult female N. formosa, two from the Sumitomo thelytokous line and the other two from the Hiroshima thelytokous line, with antibiotic tetracycline hydrochloride; provided them with hosts and collected their progeny, as per Hagimori et al. (2006). The segregation of the microsatellite markers was tested in the male offspring of these antibiotic-treated females. In addition, eight adult females from the Hiroshima thelytokous line without antibiotic treatment were allowed to oviposit into hosts and their progeny were collected. DNA was extracted from all progeny and genotyped for the microsatellite locus NFTH9.
To assess allele segregation under thelytokous parthenogenesis, we determined the proportion of homozygous female offspring produced by heterozygous mothers, R. We compared R-values with theoretical expectations (r) for five different modes of thelytokous parthenogenesis (as a convention, we use lower case letters to denote parameters and capital letters for the corresponding estimators): apomixis (r=0); automixis with gamete duplication (r=1); terminal fusion (r=1/3–1); fusion of two products of the first meiotic division, here referred to as random fusion (r=1/3); and central fusion (r=0–1/3). Fisher's exact tests were used to determine which mode of parthenogenesis was consistent with the observed rate of transition to homozygosity for the locus. When r comprised a range of values, the value closest to the observed R was used for the test. The statistical software package R (R Development Core Team 2007, http://www.R-project.org) was used for all statistical analyses.
PCR amplifications were performed using an ABI thermocycler (PE Applied Biosystems PCR System 9700, PE Applied Biosystems) and each PCR consisted of 33 μl: 1 μl template DNA; 1.5 U of Taq polymerase (Applied Biosystems, Foster City, CA, USA); 0.66 μl of 10 mM dNTPs; each 1.3 μl of 10 pmol μl−1 NFTH9-F and NFTH9-R primers; 3.3 μl of 10 × PCR buffer with MgCl2; and 23.6 μl of sterile water. Cycling parameters were as follows: 92°C for 1 min; 30 cycles of 1 min denaturation at 92°C; 1 min annealing at 55°C; and 1 min 30 s extension at 72°C. The final extension was conducted at 72°C for 1 min 30 s. For genotyping, the PCR products were electrophoresed along with GeneScan LIZ 500 internal size standard on an ABIPRISM 3130xl DNA sequencer (Applied Biosystems). Allele sizes were assigned against the internal size standard and individuals were genotyped using Genotype v. 4.0 software (Applied Biosystems).
(d) Egg collection and cytological techniques
For investigation of meiotic stages in eggs prior to oviposition, we provided arrhenotokous and thelytokous adult N. formosa females with L. sativae larvae to stimulate egg development in their ovaries. Eggs were dissected from N. formosa females on a microscope depression slide in a drop of Drosophila Ringer's solution (18 mM KCl, 46 mM NaCl, 3 mM CaCl2, 10 mM Tris/HCl (pH 7.2)). Most of the solution was removed by absorption and the eggs were transferred to a glass vial containing Carnoy's fixative (99.5% ethanol : chloroform : acetic acid=6 : 5 : 2) for at least 1 day. The eggs were then transferred to a slide and stained with DAPI (4′,6-diamidino-2-phenylindole; 1.5 μg ml−1) in Vectashield (Vector Laboratories, Burlingame, CA, USA) and covered with a coverslip. They were stored at room temperature in the dark for at least 1 day to allow sufficient stain penetration into them. They were squashed prior to examination under an Olympus VANOX AH2-FL microscope (Olympus, Tokyo, Japan), equipped with epifluorescence at 200×, 400× or 1000× magnification. Images were collected using an HCC-3800, a 3-CCD colour camera from Flovel Co. Ltd (Tokyo, Japan).
To observe meiotic and mitotic stages in more mature eggs, three N. formosa females were allowed to oviposit into a third-instar L. sativae larvae for 30 min. Fly larvae containing parasitoid eggs were kept for each of 30 min time intervals from 0 to 120 min after oviposition. The eggs were dissected from hosts, fixed and stained, as described above. Over 70 eggs were examined for each N. formosa strain.
3. Results
(a) Allele segregation
Infected females were all heterozygous for the NFTH9 locus. The male offspring produced by these females showed the expected segregation with each male carrying a single allele, and the segregation of the two alleles in the male population does not differ significantly from 0.5 (table 1). The proportion of homozygous female offspring produced by 10 heterozygous infected mothers (R) was 0.00 (n=98) (tables 1 and 2). The value was significantly different for a locus from those expected under automixis with terminal fusion, gamete duplication or random fusion. By contrast, it was not different from the values expected under apomixis and automictic parthenogenesis with central fusion. Subsequently, we did cytogenetic observations to distinguish between these two potential outcomes. Under central fusion, the expectation is the presence of two polar bodies at the end of meiosis, while in apomixes, at best a single polar body will be present.
Table 1.
Genotype of 14 N. formosa females and their parthenogenetically produced progeny for the microsatellite locus NFTH9. (Alleles are indicated by their length in base pairs (bp), with males being haploid and therefore carrying only one allele per locus. Females 1–6 were treated with tetracycline, while the others were not. Numbers in parentheses indicate the number of progeny with that genotype.)
| from | female | tetracycline treatment | parental genotype (bp) | progeny genotype | |
|---|---|---|---|---|---|
| female | malea | ||||
| Sumitomo | 1,2,3,4 | yes | 219/234 | 219 (22) 234 (14) | |
| Hiroshima | 5,6 | yes | 219/224 | 219/224 (14) | 219 (10) 224 (9) |
| Hiroshima | 7,8,9,10,11,12,13,14 | no | 219/224 | 219/224 (84) | |
The segregation for the two alleles in the males does not differ significantly from the expected 1 : 1(p>0.05).
Table 2.
Observed rates of transition to homozygosity during parthenogenesis and consistency with different modes of parthenogenesis. Nt, number of offspring from a heterozygous mother; No, number of transitions to homozygosity; R, observed rate of transition to homozygosity; r, expected rate (or range of rates) of transition to homozygosity (see table 1 in Pearcy et al. 2006). Fisher's exact tests of consistency of R-values with r; n.s., not significant; ***highly significant (p<0.001). When r is a range, the test was performed considering the r closed to R within the range.
| automixy | |||||||
|---|---|---|---|---|---|---|---|
| Nt | No | R | gamete duplication (r=1) | terminal fusion (r=1/3–1) | central fusion (r=0–1/3) | random fusion (r=1/3) | apomixy (r=0) |
| 98 | 0 | 0.00 | *** | *** | n.s. | *** | n.s. |
(b) Cytogenetic observation of an arrhenotokous strain
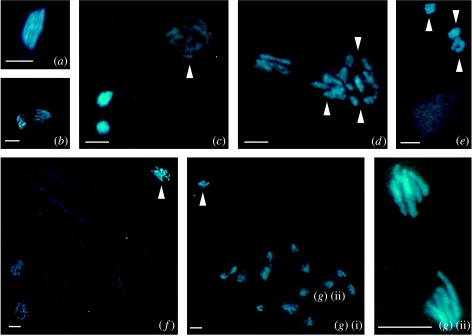
Cytological analysis of arrhenotokous adult females revealed that mature eggs in their ovaries are in first meiotic metaphase (figure 2a). The chromosomes at this stage are packed together and it is impossible to count them with certainty (figure 2a). The first division is reductional, resulting in a first polar body with five chromosomes (dyads) on the periphery and a second set of about five chromosomes (dyads) of the second meiotic division pre-destined to form the pronucleus, and the second polar body, somewhat inward from the surface (figure 2b). This second set divides parallel to the periphery and results in two groups of five chromosomes, while the first polar body is delayed at interphase (figure 2c). The second division results in one set of five chromosomes forming the second polar body close to the two sets of the first polar body, and another set that forms the female pronucleus (figure 2d), which takes on an interphase appearance and migrates away from the polar bodies in no distinct direction, remaining in the anterior region (figure 2e). The pronucleus starts with synchronous mitotic divisions parallel to the surface, typically from 2 h onwards after oviposition (figure 2f). Chromosomes in the eggs could not be captured photographically in one plane due to their small size and the high density of the yolk. In 90 to 120 min old eggs, the number of chromosomes is easier to count when they have a haploid number (figure 2g(i)(ii)).
Figure 2.
Meiosis and mitosis in unfertilized eggs of an arrhenotokous strain of N. formosa: (a) first metaphase, (b) first anaphase, (c) polar body (out of focus), divided first polar body and female pronucleus, (d,e) second polar body, divided first polar body and female pronucleus, (f) haploid telophase of first somatic mitosis and (g(i)(ii)) haploid telophase in embryo. Arrow head indicates polar nuclei. Scale bar represents 10 μm.
(c) Cytogenetic observation of a thelytokous strain
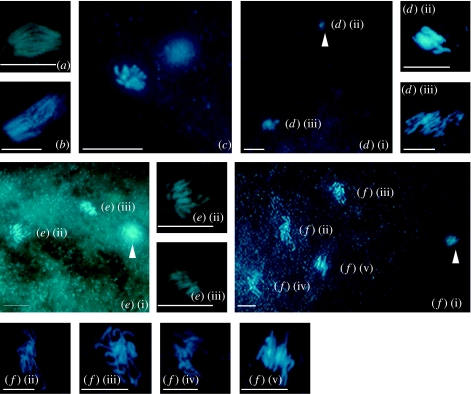
Cytological analysis of thelytokous adult females revealed that mature eggs in their ovaries are also in the first meiotic metaphase (figure 3a). In newly laid eggs, we observed condensed chromosomes at first metaphase (figure 3b). The clumped chromosome mass did not allow us to study chromosome pairing. In 0 to 30 min old eggs, the nuclei decondense and orient towards the equatorial plate (figure 3c). Although individual chromosomes were difficult to assess, we were able to count approximately 10 chromosomes in the complement, a diploid chromosome number also known from arrhenotokous strains.
Figure 3.
Meiosis and mitosis in a thelytokous strain of N. formosa: (a,b) first metaphase, (c) first telophase, (d(i)–(iii)) second metaphase and first polar body, (e(i)–(iii)) diploid telophase of first somatic mitosis and (f(i)–(v)) diploid telophase of second somatic mitosis. Arrow head indicates polar nuclei. Scale bar represents 10 μm.
In the metaphase of meiosis II, one set of chromosomes (figure 3d(ii)) lies close to the periphery of the egg and forms the first polar body, while the other set, with approximately 10 discernable chromosomes, lies in the central part of the egg (figure 3d(iii)). After a short lasting interphase, the central nucleus went through a mitotic division with 10 doubled chromosomes at metaphase and a set of chromatids at anaphase (figure 3e(i)–(iii)), indicating that meiosis is replaced by a single equational division followed by the expulsion of a single polar body. We observed more than 70 eggs, and none of the two nuclei that entered interphase (figure 3e(i)–(iii)) resulted in a second polar body. Instead, they again divided mitotically giving rise to four nuclei (figure 3f(i)–(v)). We never observed cell complements with the haploid chromosome number (n=5), indicating that meiosis is exclusively non-reductional. This cytological mechanism is similar to that of a non-bacteria-induced thelytoky in the parasitoid wasp Trichogramma cacoeciae and is also known as apomictic cloning (figure 1a(ii); Vavre et al. 2004).
4. Discussion
Our results suggest that meiosis in the N. formosa thelytokous strain is achiasmatic, with only a single equational division and the expulsion of a single polar body, and heterozygous females do not segregate for microsatellite markers in their offspring. This mechanism is typically apomictic and differs from parthenogenesis-induced Wolbachia systems in parasitoids where diploidy is restored through automixis resulting in homozygous females in a single generation (Stille & Dävring 1980; Stouthamer & Kazmer 1994; Gottlieb et al. 2002; Pannebakker et al. 2004). This is, therefore, a new cytogenetic mechanism of parthenogenesis induced by endosymbiotic bacteria in Hymenoptera. In mites, Weeks & Breeuwer (2001) reported that the mechanism of parthenogenesis in Bryobia praetiosa would be functionally apomictic and not gamete duplication, based on the data that all of the progeny produced by heterozygous mothers maintained heterozygosity in all the three microsatellite loci they examined. Unfortunately, their experiments used only molecular techniques and they never observed chromosome behaviour, so we could not judge whether the mechanism is apomixis or automictic parthenogenesis with central fusion. We suggest a future comparison of their mechanism with that of thelytokous N. formosa in detail.
In all the cases established within the Hymenoptera to date, bacterial infection by Wolbachia results in gamete duplication for the restoration of diploidy (Stille & Dävring 1980; Stouthamer & Kazmer 1994; Gottlieb et al. 2002; Pannebakker et al. 2004). This mechanism has been thought to be functionally restricted to haplodiploid systems (Stouthamer 1997; Stouthamer et al. 1999), because many species with a haplodiploid sex determination routinely inbreed. Consequently, the transition from normal sexual reproduction to bacterium-induced parthenogenesis does not result in a strong inbreeding depression as would be expected for species that commonly outbreed. However, the mechanism of the Rickettsia-induced parthenogenesis described here maintains heterozygosity, and consequently takes away one of the barriers involved in the transition from sexual to thelytokous reproduction for species that commonly outbreed, such as most diplodiploid species. It is interesting to note that apomictic parthenogenesis is the most common form of parthenogenesis within diplodiploid arthropods (Suomalainen et al. 1987) and has been found in some Hymenoptera where parthenogenesis is not associated with microbial infection (Vavre et al. 2004). Our results suggest that in the future we may detect the presence of parthenogenesis-inducing symbionts in diplodiploid species.
Within the insect order Hymenoptera to which the parasitoids, wasps, bees, ants and sawflies belong, two different sex determination mechanisms are known, one the so-called complementary sex determination (CSD; Cook 1993) and another referred to as the ‘chalcidoid’ system (Luck et al. 1993). Wasps that have the CSD system generally do not inbreed because in addition to normal inbreeding depression, they also suffer from an additional sex determination-induced inbreeding depression. Diploid individuals homozygous for the sex determination locus generally become diploid males that are sterile or die in the larval stage. It is interesting to note that although there are many cases of thelytoky in Hymenoptera with CSD, no cases of Wolbachia-induced thelytoky have been found. A survey of thelytokous Hymenoptera belonging to the families with CSD may result in additional cases of Rickettsia-induced thelytoky.
This cytogenetic mechanism may have important consequences for the infected populations. Under gamete duplication, there is a constant selection against recessive lethal mutations in genes expressed in females; however, the apomictic mechanism found here should allow the accumulation of recessive mutants in the genome of infected wasps. Such recessive lethals should have little effect on the fitness of the females since the mutations will not be expressed; however feeding antibiotics to such thelytokous females will result in the death of their recessive inherited haploid offspring. Consequently, we expect that a complete reproductive dependence of the host on its apomixes-inducing bacterial symbiont will evolve rapidly.
Acknowledgments
We thank Dr Jun Abe for his suggestion of developing microsatellite primers and Dr Andrew Davies for critical reading of the original manuscript. The authors also thank Sumitomo Chemical Co. Ltd for providing N. formosa samples. This research is partially supported by grant-in-aid for JSPS Fellows to T.A.-H. and a NSF-FIBR grant no. 0328363 to R.S.
References
- Arakaki N, Kinjo K. Notes on the parasitoid fauna of the serpentine leafminer Liriomyza trifolii (Burgess) (Diptera: Agromyzidae) in Okinawa, southern Japan. Appl. Entomol. Zool. 1998;33:577–581. [Google Scholar]
- Belshaw R, Quicke D.L.J. The cytogenetics of thelytoky in a predominantly asexual parasitoid wasp with covert sex. Genome. 2003;46:170–173. doi: 10.1139/g02-112. doi:10.1139/g02-112 [DOI] [PubMed] [Google Scholar]
- Bourtzis K, Miller T.A. CRC Press; Boca Raton, FL: 2003. Insect symbiosis. p. 347. [Google Scholar]
- Brownstein M.J, Carpten J.D, Smith J.R. Modulation of non-templated nucleotide addition by tag DNA polymerase: primer modifications that facilitate genotyping. Biotechniques. 1996;20:1004–1010. doi: 10.2144/96206st01. [DOI] [PubMed] [Google Scholar]
- Cook J.M. Sex determination in Hymenoptera: a review of models and evidence. Heredity. 1993;71:421–435. doi:10.1038/hdy.1993.157 [Google Scholar]
- Fisher P.J, Gardner R.C, Richardson T.E. Single locus microsatellites isolated using 5′ anchored PCR. Nucleic Acids Res. 1996;24:4369–4371. doi: 10.1093/nar/24.21.4369. doi:10.1093/nar/24.21.4369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb Y, Zchori-Fein E, Werren J.H, Karr T.L. Diploidy restoration in Wolbachia-infected Muscidifurax uniraptor (Hymenoptera: Pteromalidae) J. Invertebr. Pathol. 2002;81:166–174. doi: 10.1016/s0022-2011(02)00149-0. doi:10.1016/S0022-2011(02)00149-0 [DOI] [PubMed] [Google Scholar]
- Hagimori T, Abe Y, Date S, Miura K. The first finding of a Rickettsia bacterium associated with parthenogenesis induction among insects. Curr. Microbiol. 2006;52:97–101. doi: 10.1007/s00284-005-0092-0. doi:10.1007/s00284-005-0092-0 [DOI] [PubMed] [Google Scholar]
- Lampert K.P, Lamatsch D.K, Fischer P, Epplen J.T, Nanda I, Schmid M, Schartl M. Automictic reproduction in interspecific hybrids of poeciliid fish. Curr. Biol. 2007;17:1948–1953. doi: 10.1016/j.cub.2007.09.064. doi:10.1016/j.cub.2007.09.064 [DOI] [PubMed] [Google Scholar]
- Luck R.F, Stouthamer R, Nunney L. Sex determination and sex ratio patterns in parasitic Hymenoptera. In: Wrench D.L, Ebbert M.A, editors. Evolution and diversity of sex ratio in haplodiploid insects and mites. Chapman & Hall; New York, NY: 1993. pp. 442–476. [Google Scholar]
- Martins W, de Sousa D, Proite K, Guimaraes P, Moretzsohn M, Bertioli D. New softwares for automated microsatellite marker development. Nucleic Acids Res. 2006;34:E31. doi: 10.1093/nar/gnj030. doi:10.1093/nar/gnj030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannebakker B.A, Pijnacker L.P, Zwaan B.J, Beukeboom L.W. Cytology of Wolbachia-induced parthenogenesis in Leptopilina clavipes (Hymenoptera: Figitidae) Genome. 2004;47:299–303. doi: 10.1139/g03-137. [DOI] [PubMed] [Google Scholar]
- Pearcy M, Hardy O, Aron S. Thelytokous parthenogenesis and its consequences on inbreeding in an ant. Heredity. 2006;96:377–382. doi: 10.1038/sj.hdy.6800813. doi:10.1038/sj.hdy.6800813 [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R Foundation for Statistical Computing; Vienna, Austria: 2007. R: a language and environment for statistical computing. See http://www.R-project.org. [Google Scholar]
- Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- Rössler Y, DeBach P. Genetic variability in a thelytokous form of Aphytis mytilaspidis (Le Baron) (Hymenoptera: Aphelinidae) Hilgardia. 1973;42:149–175. [Google Scholar]
- Saito T, Ikeda F, Ozawa A. Effect of pesticides on parasitoid complex of serpentine leafminer Liriomyza trifolii (Burgess) in Shizuoka Prefecture. Jpn J. Appl. Entomol. Zool. 1996;40:127–133. [Google Scholar]
- Stille B, Dävring L. Meiosis and reproductive strategy in the parthenogenetic gall wasp Diplolepis rosae (L.) (Hymenoptera: Cynipidae) Hereditas. 1980;92:353–362. [Google Scholar]
- Stouthamer R. Wolbachia-induced parthenogenesis. In: O'Neill S.L, Hoffmann A.A, Werren J.H, editors. Influential passengers: inherited microorganisms and invertebrate reproduction. Oxford University Press; New York, NY: 1997. pp. 102–124. [Google Scholar]
- Stouthamer R, Kazmer D.J. Cytogenetics of microbe-associated parthenogenesis and its consequence for gene-flow in Trichogramma wasps. Heredity. 1994;73:317–327. doi:10.1038/hdy.1994.139 [Google Scholar]
- Stouthamer R, Luck R.F, Hamilton W.D. Antibiotics cause parthenogenetic Trichogramma to revert to sex. Proc. Natl Acad. Sci. USA. 1990;87:2424–2427. doi: 10.1073/pnas.87.7.2424. doi:10.1073/pnas.87.7.2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stouthamer R, Breeuwer J.A.J, Hurst G.D.D. Wolbachia pipientis: microbial manipulator of arthropod reproduction. Annu. Rev. Microbiol. 1999;53:71–102. doi: 10.1146/annurev.micro.53.1.71. doi:10.1146/annurev.micro.53.1.71 [DOI] [PubMed] [Google Scholar]
- Suomalainen E, Saura A, Lokki J. CRC Press; Boca Raton, FL: 1987. Cytology and evolution in parthenogenesis. [Google Scholar]
- Tokumaru S, Abe Y. Hymenopterous parasitoids of leafminers, Liriomyza sativae Blanchard, L. trifolii (Burgess), and L. bryoniae (Kaltenbach) in Kyoto prefecture. Jpn J. Appl. Entomol. Zool. 2006;50:341–345. doi:10.1303/jjaez.2006.341 [Google Scholar]
- Vavre F, de Jong J.H, Stouthamer R. Cytogenetic mechanism and genetic consequences of thelytoky in the wasp Trichogramma cacoeciae. Heredity. 2004;93:592–596. doi: 10.1038/sj.hdy.6800565. doi:10.1038/sj.hdy.6800565 [DOI] [PubMed] [Google Scholar]
- Weeks A.R, Breeuwer J.A.J. Wolbachia-induced parthenogenesis in a genus of phytophagous mites. Proc. R. Soc. B. 2001;268:2245–2251. doi: 10.1098/rspb.2001.1797. doi:10.1098/rspb.2001.1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zchori-Fein E, Gottlieb Y, Kelly S.E, Brown J.K, Wilson J.M, Karr T.L, Hunter M.S. A newly discovered bacterium associated with parthenogenesis and a change in host selection behavior in parasitoid wasps. Proc. Natl Acad. Sci. USA. 2001;98:12 555–12 560. doi: 10.1073/pnas.221467498. doi:10.1073/pnas.221467498 [DOI] [PMC free article] [PubMed] [Google Scholar]