Abstract
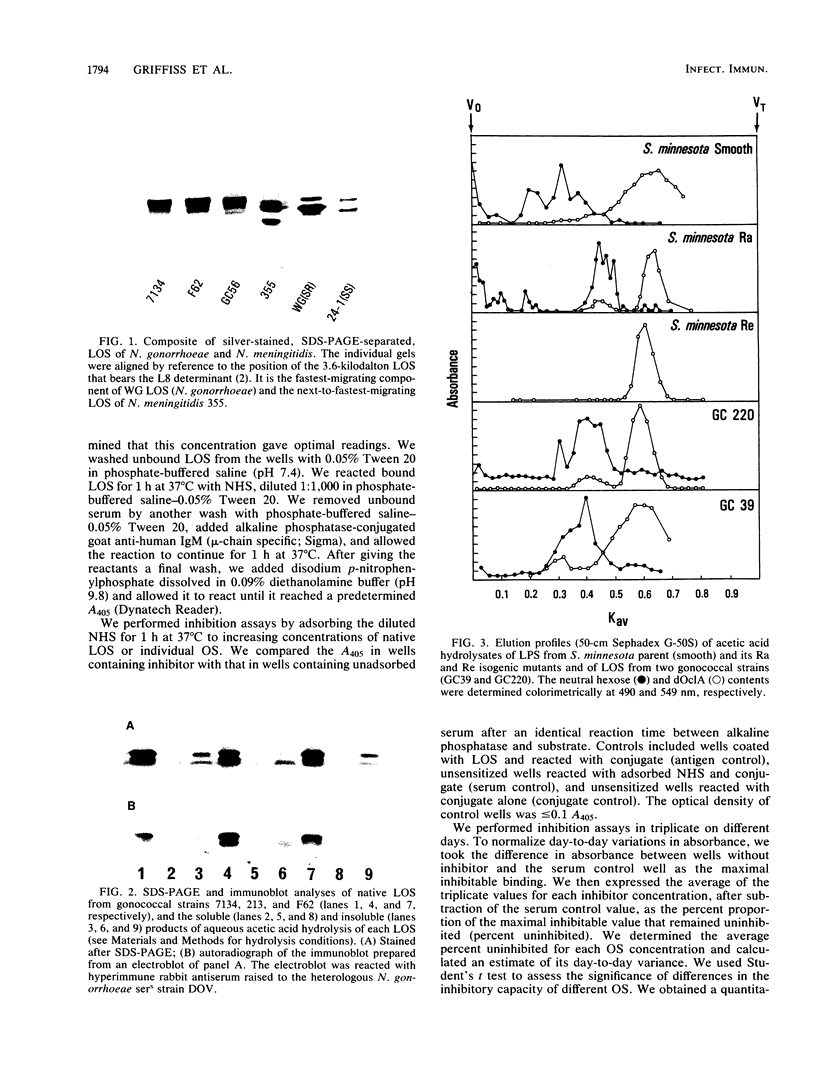
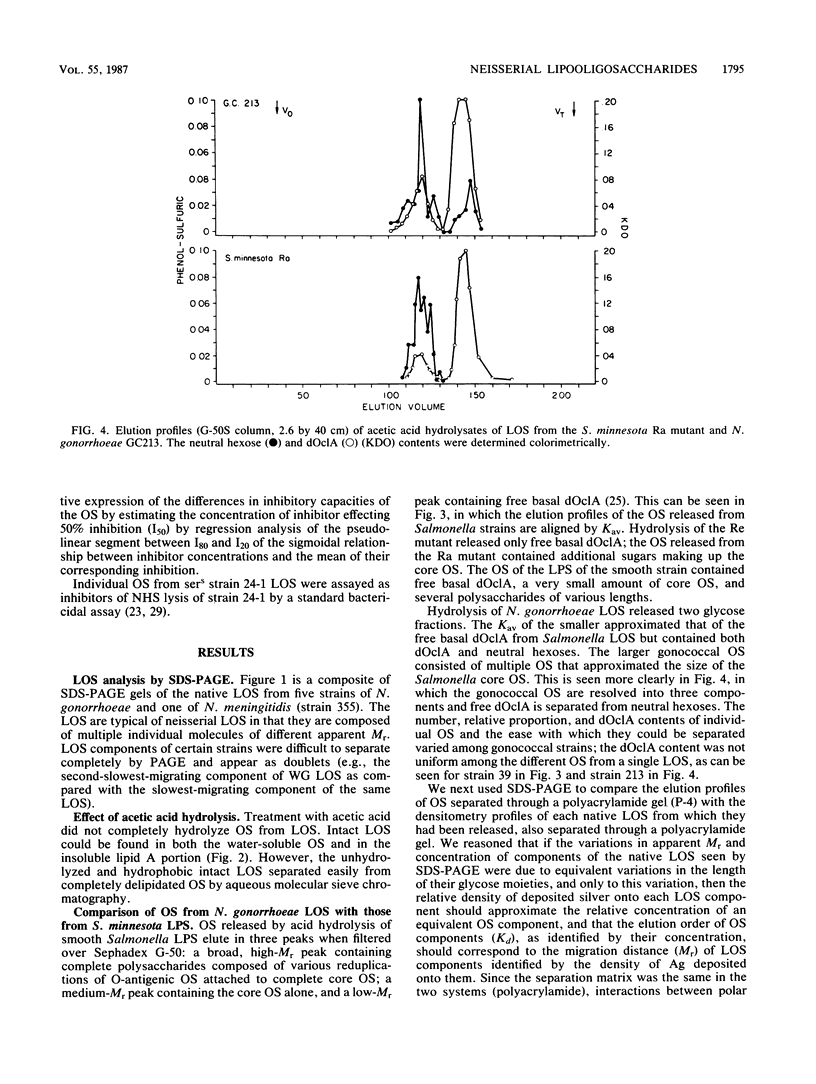
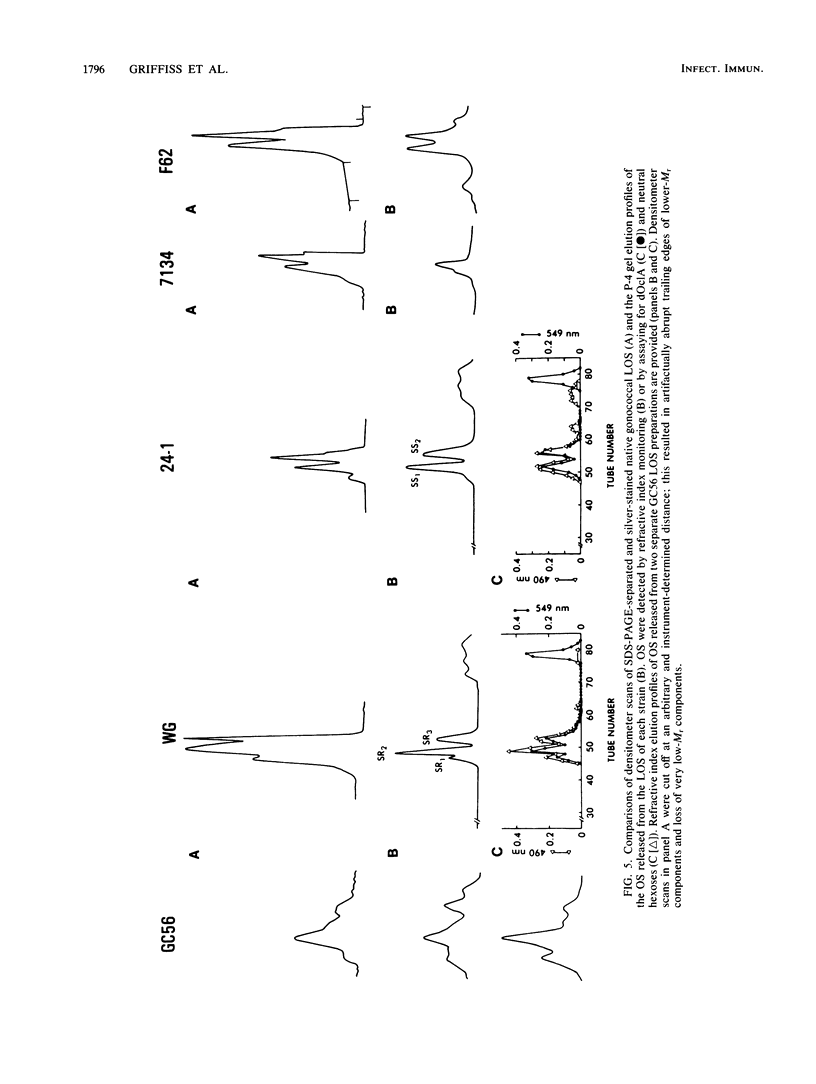
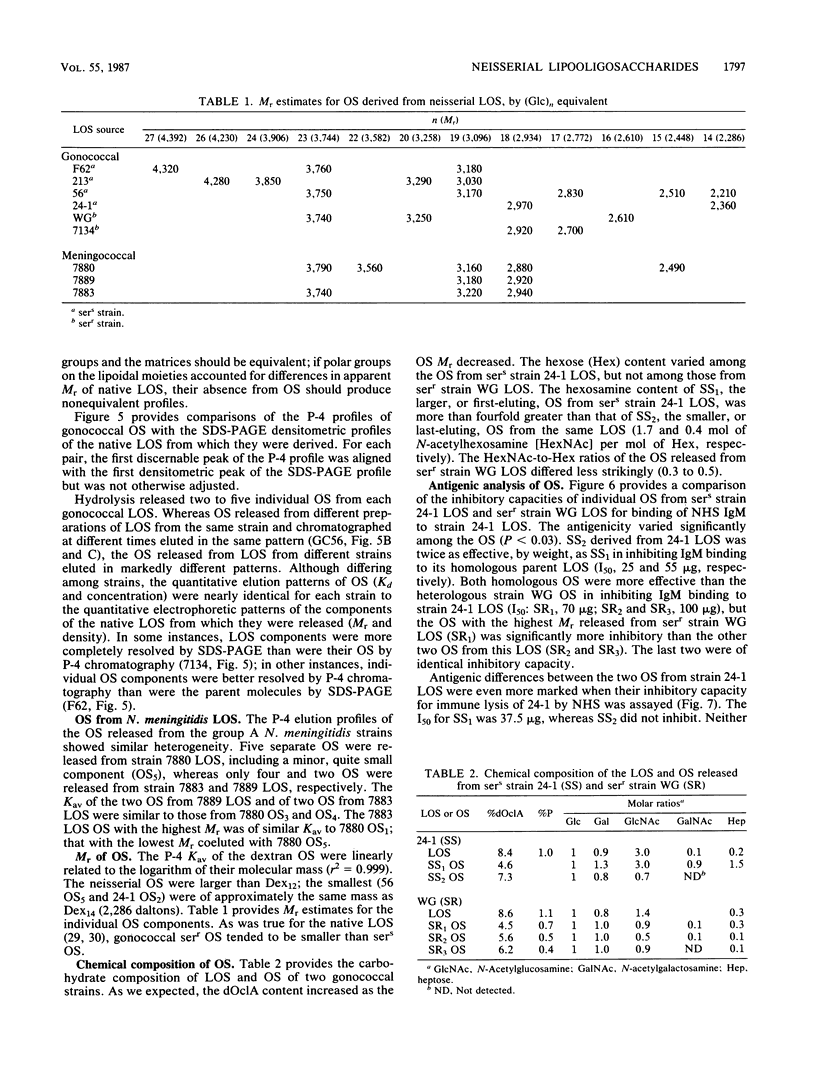
We studied the oligosaccharides (OS) of outer membrane lipooligosaccharides (LOS) of Neisseria gonorrhoeae and Neisseria meningitidis. OS from the LOS of an individual neisserial strain always eluted from Sephadex G-50S as multiple peaks; the polyacrylamide gel elution profiles were nearly identical to the polyacrylamide gel electrophoresis profiles of the sodium dodecyl sulfate-disaggregated native LOS from which the OS were derived. Neisserial OS coeluted with Dex14 to Dex27 dextran oligomers (Mr, 2,210 to 4,320). Monosaccharide composition varied among the several OS released from the LOS of a single strain. The two OS of a gonococcal strain sensitive to normal human serum (NHS) bacteriolysis (sers) varied in their ability to inhibit the binding of NHS immunoglobulin M to their parental LOS. The OS that was rich in hexosamines inhibited NHS immune lysis of its parent strain; the OS that was poor in hexosamines did not. We conclude that structural differences in their OS account for the Mr heterogeneity of the LOS of a strain.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen P. Z., Connelly M. C., Apicella M. A. Interaction of lectins with Neisseria gonorrhoeae. Can J Microbiol. 1980 Apr;26(4):468–474. doi: 10.1139/m80-078. [DOI] [PubMed] [Google Scholar]
- Apicella M. A., Breen J. F., Gagliardi N. C. Degradation of the polysaccharide component of gonococcal lipopolysaccharide by gonococcal and meningococcal sonic extracts. Infect Immun. 1978 Apr;20(1):228–234. doi: 10.1128/iai.20.1.228-234.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram M. A., Griffiss J. M., Broud D. D. Response to antigenic determinants of Neisseria meningitidis lipopolysaccharide investigated with a new radioactive antigen-binding assay. J Immunol. 1976 Mar;116(3):842–846. [PubMed] [Google Scholar]
- Bhatti T., Chambers R. E., Clamp J. R. The gas chromatographic properties of biologically important N-acetylglucosamine derivatives, monosaccharides, disaccharides, trisaccharides, tetrasaccharides and pentasaccharides. Biochim Biophys Acta. 1970 Nov 24;222(2):339–347. doi: 10.1016/0304-4165(70)90122-4. [DOI] [PubMed] [Google Scholar]
- Blumberg K., Liniere F., Pustilnik L., Bush C. A. Fractionation of oligosaccharides containing N-acetyl amino sugars by reverse-phase high-pressure liquid chromatography. Anal Biochem. 1982 Jan 15;119(2):407–412. doi: 10.1016/0003-2697(82)90605-4. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Counts G. W., Gregory D. F., Spearman J. G., Lee B. A., Filice G. A., Holmes K. K., Griffiss J. M. Group A meningococcal disease in the U.S. Pacific Northwest: epidemiology, clinical features, and effect of a vaccination control program. Rev Infect Dis. 1984 Sep-Oct;6(5):640–648. doi: 10.1093/clinids/6.5.640. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Gnehm H. E., Pelton S. I., Gulati S., Rice P. A. Characterization of antigens from nontypable Haemophilus influenzae recognized by human bactericidal antibodies. Role of Haemophilus outer membrane proteins. J Clin Invest. 1985 May;75(5):1645–1658. doi: 10.1172/JCI111872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiss J. M. Epidemiological value of lipopolysaccharide and heat-modifiable outer-membrane protein serotyping of group-A strains of Neisseria meningitidis. J Med Microbiol. 1982 Aug;15(3):327–330. doi: 10.1099/00222615-15-3-327. [DOI] [PubMed] [Google Scholar]
- Jann B., Reske K., Jann K. Heterogeneity of lipopolysaccharides. Analysis of polysaccharide chain lengths by sodium dodecylsulfate-polyacrylamide gel electrophoresis. Eur J Biochem. 1975 Dec 1;60(1):239–246. doi: 10.1111/j.1432-1033.1975.tb20996.x. [DOI] [PubMed] [Google Scholar]
- Jennings H. J., Johnson K. G., Kenne L. The structure of an R-type oligosaccharide core obtained from some lipopolysaccharides of Neisseria meningitidis. Carbohydr Res. 1983 Sep 16;121:233–241. doi: 10.1016/0008-6215(83)84020-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mandrell R., Schneider H., Apicella M., Zollinger W., Rice P. A., Griffiss J. M. Antigenic and physical diversity of Neisseria gonorrhoeae lipooligosaccharides. Infect Immun. 1986 Oct;54(1):63–69. doi: 10.1128/iai.54.1.63-69.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltola H. Group A meningococcal polysaccharide vaccine and course of the group A meningococcal epidemic in Finland. Scand J Infect Dis. 1978;10(1):41–44. doi: 10.3109/inf.1978.10.issue-1.09. [DOI] [PubMed] [Google Scholar]
- Rice P. A., Kasper D. L. Characterization of gonococcal antigens responsible for induction of bactericidal antibody in disseminated infection. J Clin Invest. 1977 Nov;60(5):1149–1158. doi: 10.1172/JCI108867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice P. A., Kasper D. L. Characterization of serum resistance of Neisseria gonorrhoeae that disseminate. Roles of blocking antibody and gonococcal outer membrane proteins. J Clin Invest. 1982 Jul;70(1):157–167. doi: 10.1172/JCI110589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt G., Jann B., Jann K. Immunochemistry of R lipopolysaccharides of Escherichia coli. Different core regions in the lipopolysaccharides of O group 8. Eur J Biochem. 1969 Oct;10(3):501–510. doi: 10.1111/j.1432-1033.1969.tb00717.x. [DOI] [PubMed] [Google Scholar]
- Schneider H., Griffiss J. M. A bactericidal microassay for testing serum sensitivity of Neisseria gonorrhoeae. J Immunol Methods. 1982 Oct 15;54(1):101–105. doi: 10.1016/0022-1759(82)90118-1. [DOI] [PubMed] [Google Scholar]
- Schneider H., Griffiss J. M., Mandrell R. E., Jarvis G. A. Elaboration of a 3.6-kilodalton lipooligosaccharide, antibody against which is absent from human sera, is associated with serum resistance of Neisseria gonorrhoeae. Infect Immun. 1985 Dec;50(3):672–677. doi: 10.1128/iai.50.3.672-677.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H., Griffiss J. M., Williams G. D., Pier G. B. Immunological basis of serum resistance of Neisseria gonorrhoeae. J Gen Microbiol. 1982 Jan;128(1):13–22. doi: 10.1099/00221287-128-1-13. [DOI] [PubMed] [Google Scholar]
- Schneider H., Hale T. L., Zollinger W. D., Seid R. C., Jr, Hammack C. A., Griffiss J. M. Heterogeneity of molecular size and antigenic expression within lipooligosaccharides of individual strains of Neisseria gonorrhoeae and Neisseria meningitidis. Infect Immun. 1984 Sep;45(3):544–549. doi: 10.1128/iai.45.3.544-549.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H., Hammack C. A., Shuman B. A., Griffiss J. M. Stability of expression of Neisseria gonorrhoeae lipooligosaccharides. Infect Immun. 1986 Dec;54(3):924–927. doi: 10.1128/iai.54.3.924-927.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seid R. C., Jr, Schneider H., Bondarew S., Boykins R. A. Quantitation of L-glycero-D-manno-heptose and 3-deoxy-D-manno-octulosonic acid in rough core lipopolysaccharides by partition chromatography. Anal Biochem. 1982 Aug;124(2):320–326. doi: 10.1016/0003-2697(82)90046-x. [DOI] [PubMed] [Google Scholar]
- Takayama K., Qureshi N., Hyver K., Honovich J., Cotter R. J., Mascagni P., Schneider H. Characterization of a structural series of lipid A obtained from the lipopolysaccharides of Neisseria gonorrhoeae. Combined laser desorption and fast atom bombardment mass spectral analysis of high performance liquid chromatography-purified dimethyl derivatives. J Biol Chem. 1986 Aug 15;261(23):10624–10631. [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- WHITE L. A., KELLOGG D. S., Jr NEISSERIA GONORRHOEAE IDENTIFICATION IN DIRECT SMEARS BY A FLUORESCENT ANTIBODY-COUNTERSTAIN METHOD. Appl Microbiol. 1965 Mar;13:171–174. doi: 10.1128/am.13.2.171-174.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollinger W. D., Mandrell R. E. Type-specific antigens of group A Neisseria meningitidis: lipopolysaccharide and heat-modifiable outer membrane proteins. Infect Immun. 1980 May;28(2):451–458. doi: 10.1128/iai.28.2.451-458.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]




