Abstract
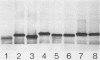
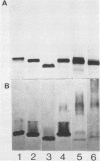
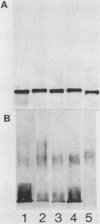
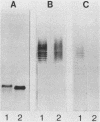
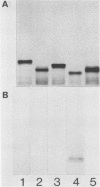
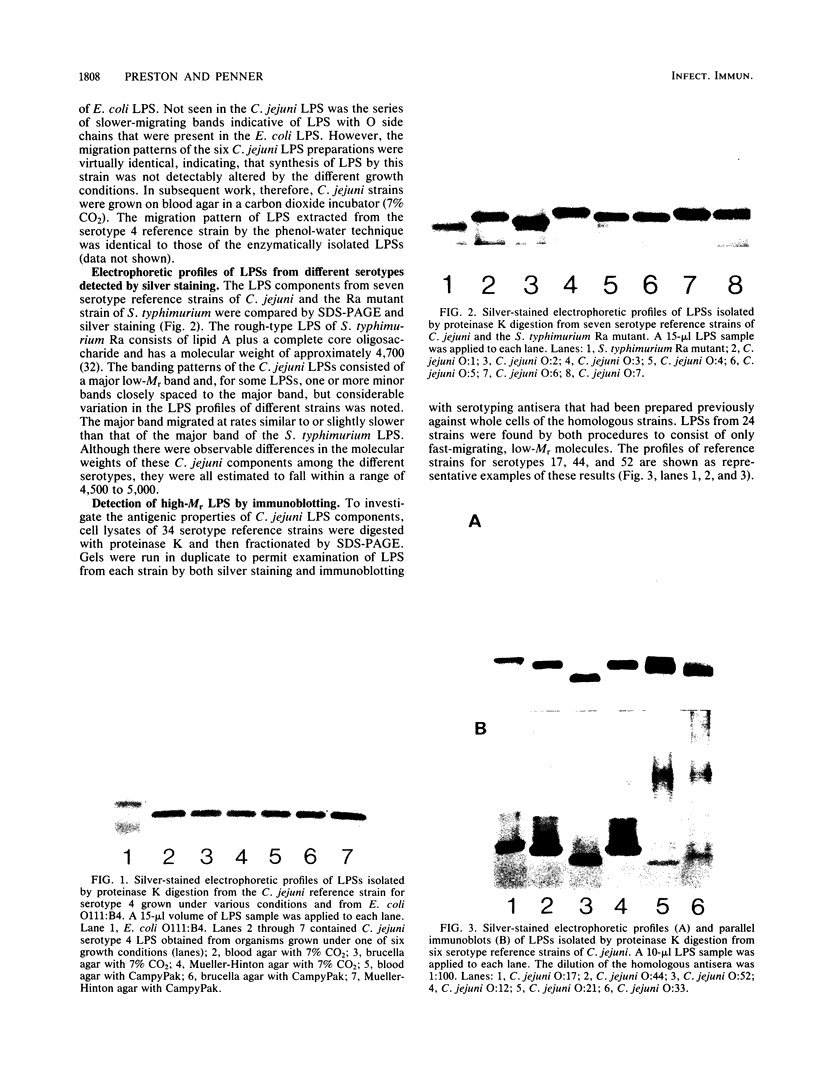
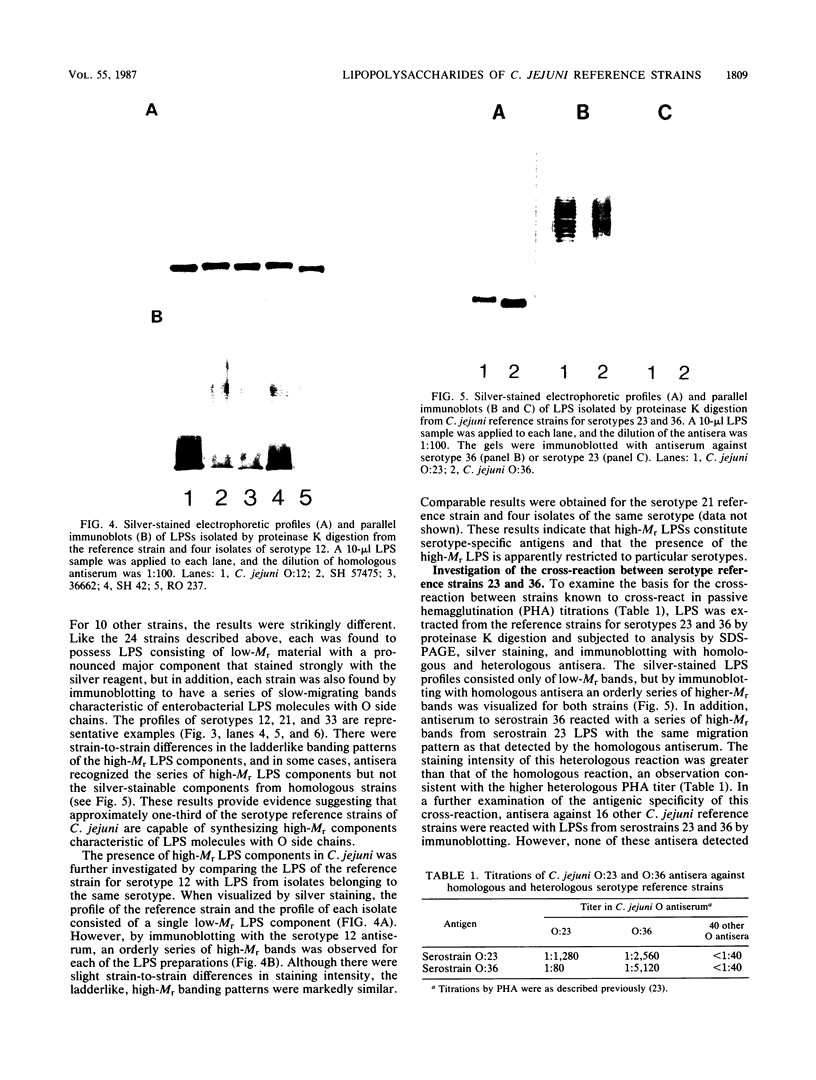
To investigate the molecular basis for heat-stable antigenic diversity in Campylobacter jejuni, lipopolysaccharides (LPSs) from serotype reference strains and serotyped isolates were examined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis coupled with silver staining and immunoblotting. By silver staining, only low-Mr components, consisting of one major band and as many as three minor bands ranging in Mr from 4,500 to 5,000, were detected. However, by immunoblotting with homologous antisera, 10 of 34 strains were shown to have a series of high-Mr LPS components characteristic of molecules with O side chains of various lengths. Isolates of the same serotype as the reference strain that had high-Mr LPS molecules were also found to have high-Mr LPS and in one case of cross-reacting strains it was found that the cross-reaction was associated with antibodies against high-Mr LPS. The reactions of LPSs with homologous and heterologous antisera indicated that both high- and low-Mr-type LPSs were strain-specific antigens, but in some cases cross-reactions were noted. Evidently, all C. jejuni strains possess low-Mr LPS that is readily detectable by silver staining, but some serotypes also possess high-Mr LPS components that can be visualized by immunoblotting.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaser M. J., Perez G. P., Smith P. F., Patton C., Tenover F. C., Lastovica A. J., Wang W. I. Extraintestinal Campylobacter jejuni and Campylobacter coli infections: host factors and strain characteristics. J Infect Dis. 1986 Mar;153(3):552–559. doi: 10.1093/infdis/153.3.552. [DOI] [PubMed] [Google Scholar]
- Butzler J. P., Skirrow M. B. Campylobacter enteritis. Clin Gastroenterol. 1979 Sep;8(3):737–765. [PubMed] [Google Scholar]
- Goldman R. C., Leive L. Heterogeneity of antigenic-side-chain length in lipopolysaccharide from Escherichia coli 0111 and Salmonella typhimurium LT2. Eur J Biochem. 1980;107(1):145–153. doi: 10.1111/j.1432-1033.1980.tb04635.x. [DOI] [PubMed] [Google Scholar]
- Gustafsson B., Rosén A., Holme T. Monoclonal antibodies against Vibrio cholerae lipopolysaccharide. Infect Immun. 1982 Nov;38(2):449–454. doi: 10.1128/iai.38.2.449-454.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackstadt T., Peacock M. G., Hitchcock P. J., Cole R. L. Lipopolysaccharide variation in Coxiella burnetti: intrastrain heterogeneity in structure and antigenicity. Infect Immun. 1985 May;48(2):359–365. doi: 10.1128/iai.48.2.359-365.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzana T. J., Seifert W. E., Jr, Williams R. P. Composition and antigenic activity of the oligosaccharide moiety of Haemophilus influenzae type b lipooligosaccharide. Infect Immun. 1985 May;48(2):324–330. doi: 10.1128/iai.48.2.324-330.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings H. J., Bhattacharjee A. K., Kenne L., Kenny C. P., Calver G. The R-type lipopolysaccharides of Neisseria meningitidis. Can J Biochem. 1980 Feb;58(2):128–136. doi: 10.1139/o80-018. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Logan S. M., Trust T. J. Structural and antigenic heterogeneity of lipopolysaccharides of Campylobacter jejuni and Campylobacter coli. Infect Immun. 1984 Jul;45(1):210–216. doi: 10.1128/iai.45.1.210-216.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrell R., Schneider H., Apicella M., Zollinger W., Rice P. A., Griffiss J. M. Antigenic and physical diversity of Neisseria gonorrhoeae lipooligosaccharides. Infect Immun. 1986 Oct;54(1):63–69. doi: 10.1128/iai.54.1.63-69.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills S. D., Bradbury W. C., Penner J. L. Basis for serological heterogeneity of thermostable antigens of Campylobacter jejuni. Infect Immun. 1985 Oct;50(1):284–291. doi: 10.1128/iai.50.1.284-291.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naess V., Hofstad T. Antigenicity of lipopolysaccharides from Campylobacter jejuni and Campylobacter coli in passive haemagglutination tests and enzyme-linked immunosorbent assays. Acta Pathol Microbiol Immunol Scand C. 1985 Apr;93(2):97–104. doi: 10.1111/j.1699-0463.1985.tb02929.x. [DOI] [PubMed] [Google Scholar]
- Naess V., Hofstad T. Chemical composition and biological activity of lipopolysaccharides prepared from type strains of Campylobacter jejuni and Campolybacter coli. Acta Pathol Microbiol Immunol Scand B. 1984 Aug;92(4):217–222. doi: 10.1111/j.1699-0463.1984.tb02824.x. [DOI] [PubMed] [Google Scholar]
- Naess V., Hofstad T. Chemical studies of partially hydrolysed lipopolysaccharides from four strains of Campylobacter jejuni and two strains of Campylobacter coli. J Gen Microbiol. 1984 Nov;130(11):2783–2789. doi: 10.1099/00221287-130-11-2783. [DOI] [PubMed] [Google Scholar]
- Orskov I., Orskov F., Jann B., Jann K. Serology, chemistry, and genetics of O and K antigens of Escherichia coli. Bacteriol Rev. 1977 Sep;41(3):667–710. doi: 10.1128/br.41.3.667-710.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner J. L., Hennessy J. N., Congi R. V. Serotyping of Campylobacter jejuni and Campylobacter coli on the basis of thermostable antigens. Eur J Clin Microbiol. 1983 Aug;2(4):378–383. doi: 10.1007/BF02019474. [DOI] [PubMed] [Google Scholar]
- Penner J. L., Hennessy J. N. Passive hemagglutination technique for serotyping Campylobacter fetus subsp. jejuni on the basis of soluble heat-stable antigens. J Clin Microbiol. 1980 Dec;12(6):732–737. doi: 10.1128/jcm.12.6.732-737.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppler M. S. Two physically and serologically distinct lipopolysaccharide profiles in strains of Bordetella pertussis and their phenotype variants. Infect Immun. 1984 Jan;43(1):224–232. doi: 10.1128/iai.43.1.224-232.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez Perez G. I., Blaser M. J. Lipopolysaccharide characteristics of pathogenic campylobacters. Infect Immun. 1985 Feb;47(2):353–359. doi: 10.1128/iai.47.2.353-359.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez G. I., Hopkins J. A., Blaser M. J. Antigenic heterogeneity of lipopolysaccharides from Campylobacter jejuni and Campylobacter fetus. Infect Immun. 1985 May;48(2):528–533. doi: 10.1128/iai.48.2.528-533.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skirrow M. B. Campylobacter enteritis: a "new" disease. Br Med J. 1977 Jul 2;2(6078):9–11. doi: 10.1136/bmj.2.6078.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. Microbial surfaces in relation to pathogenicity. Bacteriol Rev. 1977 Jun;41(2):475–500. doi: 10.1128/br.41.2.475-500.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Boykins R., Frasch C. E. Heterogeneity and variation among Neisseria meningitidis lipopolysaccharides. J Bacteriol. 1983 Aug;155(2):498–504. doi: 10.1128/jb.155.2.498-504.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]