Abstract
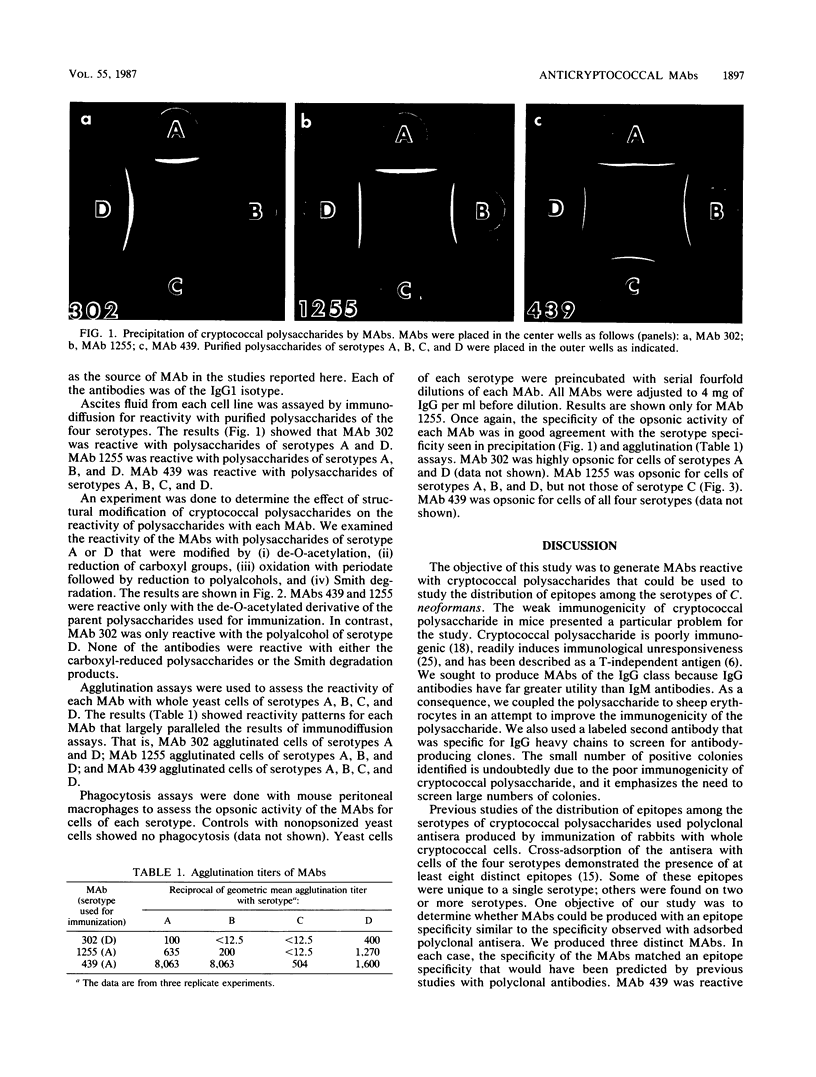
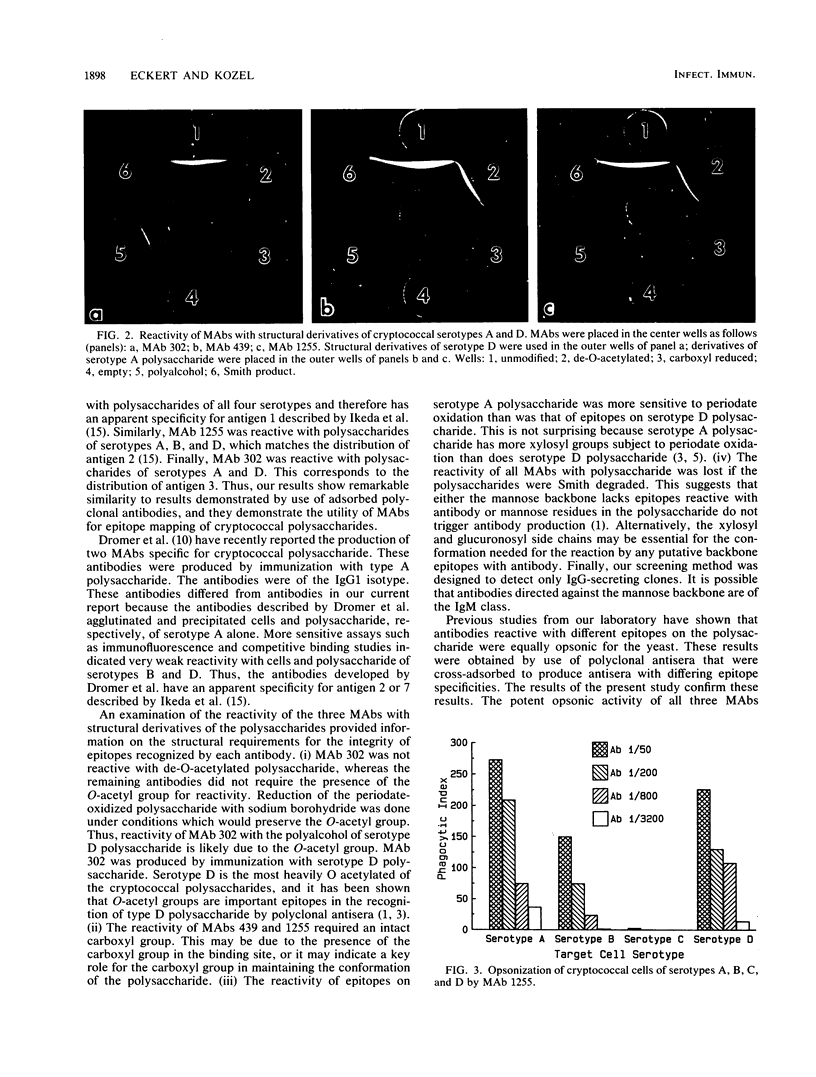
Cryptococcus neoformans is surrounded by a capsular polysaccharide. There are at least four known serotypes of the polysaccharide. The objective of this study was to produce monoclonal antibodies (MAbs) that could be used to study the distribution of epitopes among the serotypes of C. neoformans. BALB/c mice were immunized with cryptococcal polysaccharides of serotype A or D that were coupled to sheep erythrocytes. Splenocytes were isolated, and hybridomas secreting MAbs specific for cryptococcal polysaccharides were isolated. Two hybridomas, designated MAbs 439 and 1255, were produced from mice immunized with serotype A polysaccharide. One hybridoma, designated MAb 302, was produced from mice immunized with serotype D polysaccharide. All three antibodies were of the immunoglobulin G1 isotype. MAb 302 showed a specificity for serotypes A and D in Ouchterlony diffusion, agglutination, and opsonophagocytosis assays. MAb 1255 was reactive with polysaccharides and cells of serotypes A, B, and D. MAb 439 was reactive with polysaccharides and cells of serotypes A, B, C, and D. The reactivity of these MAbs closely matched the distribution of epitopes among cryptococcal polysaccharides predicted in previous studies of polyclonal antibodies reactive with cryptococcal polysaccharides. The ability to produce a MAb against an epitope shared by all four serotypes may have value for the detection of cryptococcal antigens in body fluids.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhattacharjee A. K., Bennett J. E., Glaudemans C. P. Capsular polysaccharides of Cryptococcus neoformans. Rev Infect Dis. 1984 Sep-Oct;6(5):619–624. doi: 10.1093/clinids/6.5.619. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee A. K., Kwon-Chung K. J., Glaudemans C. P. Capsular polysaccharides from a parent strain and from a possible, mutant strain of Cryptococcus neoformans serotype A. Carbohydr Res. 1981 Sep 16;95(2):237–248. doi: 10.1016/s0008-6215(00)85580-9. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee A. K., Kwon-Chung K. J., Glaudemans C. P. On the structure of the capsular polysaccharide from Cryptococcus neoformans serotype C. Immunochemistry. 1978 Sep;15(9):673–679. doi: 10.2196/40846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee A. K., Kwon-Chung K. J., Glaudemans C. P. Structural studies on the major, capsular polysaccharide from Cryptococcus bacillisporus serotype B. Carbohydr Res. 1980 Jun;82(1):103–111. doi: 10.1016/s0008-6215(00)85524-x. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee A. K., Kwon-Chung K. J., Glaudemans C. P. The structure of the capsular polysaccharide from Cryptococcus neoformans serotype D. Carbohydr Res. 1979 Aug;73:183–192. doi: 10.1016/s0008-6215(00)85488-9. [DOI] [PubMed] [Google Scholar]
- Breen J. F., Lee I. C., Vogel F. R., Friedman H. Cryptococcal capsular polysaccharide-induced modulation of murine immune responses. Infect Immun. 1982 Apr;36(1):47–51. doi: 10.1128/iai.36.1.47-51.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodeur B. R., Tsang P., Larose Y. Parameters affecting ascites tumour formation in mice and monoclonal antibody production. J Immunol Methods. 1984 Jul 6;71(2):265–272. doi: 10.1016/0022-1759(84)90073-5. [DOI] [PubMed] [Google Scholar]
- Cherniak R., Reiss E., Slodki M. E., Plattner R. D., Blumer S. O. Structure and antigenic activity of the capsular polysaccharide of Cryptococcus neoformans serotype A. Mol Immunol. 1980 Aug;17(8):1025–1032. doi: 10.1016/0161-5890(80)90096-6. [DOI] [PubMed] [Google Scholar]
- Dromer F., Salamero J., Contrepois A., Carbon C., Yeni P. Production, characterization, and antibody specificity of a mouse monoclonal antibody reactive with Cryptococcus neoformans capsular polysaccharide. Infect Immun. 1987 Mar;55(3):742–748. doi: 10.1128/iai.55.3.742-748.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasty D. L., Beachey E. H., Simpson W. A., Dale J. B. Hybridoma antibodies against protective and nonprotective antigenic determinants of a structurally defined polypeptide fragment of streptococcal M protein. J Exp Med. 1982 Apr 1;155(4):1010–1018. doi: 10.1084/jem.155.4.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda R., Nishikawa A., Shinoda T., Fukazawa Y. Chemical characterization of capsular polysaccharide from Cryptococcus neoformans serotype A-D. Microbiol Immunol. 1985;29(10):981–991. doi: 10.1111/j.1348-0421.1985.tb02962.x. [DOI] [PubMed] [Google Scholar]
- Ikeda R., Shinoda T., Fukazawa Y., Kaufman L. Antigenic characterization of Cryptococcus neoformans serotypes and its application to serotyping of clinical isolates. J Clin Microbiol. 1982 Jul;16(1):22–29. doi: 10.1128/jcm.16.1.22-29.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Cazin J., Jr Immune response to Cryptococcus neoformans soluble polysaccharide. I. Serological assay for antigen and antibody. Infect Immun. 1972 Jan;5(1):35–41. doi: 10.1128/iai.5.1.35-41.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Cazin J., Jr Induction of humoral antibody response by soluble polysaccharide of Cryptococcus neoformans. Mycopathol Mycol Appl. 1974 Oct 15;54(1):21–30. doi: 10.1007/BF02055969. [DOI] [PubMed] [Google Scholar]
- Kozel T. R., Cazin J. Nonencapsulated Variant of Cryptococcus neoformans I. Virulence Studies and Characterization of Soluble Polysaccharide. Infect Immun. 1971 Feb;3(2):287–294. doi: 10.1128/iai.3.2.287-294.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Gotschlich E. C. The capsule of cryptococcus neoformans passively inhibits phagocytosis of the yeast by macrophages. J Immunol. 1982 Oct;129(4):1675–1680. [PubMed] [Google Scholar]
- Kozel T. R., Hermerath C. A. Binding of cryptococcal polysaccharide to Cryptococcus neoformans. Infect Immun. 1984 Mar;43(3):879–886. doi: 10.1128/iai.43.3.879-886.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Mastroianni R. P. Inhibition of phagocytosis by cryptococcal polysaccharide: dissociation of the attachment and ingestion phases of phagocytosis. Infect Immun. 1976 Jul;14(1):62–67. doi: 10.1128/iai.14.1.62-67.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane R. D., Crissman R. S., Lachman M. F. Comparison of polyethylene glycols as fusogens for producing lymphocyte-myeloma hybrids. J Immunol Methods. 1984 Aug 3;72(1):71–76. doi: 10.1016/0022-1759(84)90434-4. [DOI] [PubMed] [Google Scholar]
- Leinonen M., Frasch C. E. Class-specific antibody response to group B Neisseria meningitidis capsular polysaccharide: use of polylysine precoating in an enzyme-linked immunosorbent assay. Infect Immun. 1982 Dec;38(3):1203–1207. doi: 10.1128/iai.38.3.1203-1207.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner E. A. How to make a hybridoma. Yale J Biol Med. 1981 Sep-Oct;54(5):387–402. [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W., Cozad G. C. Immunological unresponsiveness induced by cryptococcal capsular polysaccharide assayed by the hemolytic plaque technique. Infect Immun. 1972 Jun;5(6):896–901. doi: 10.1128/iai.5.6.896-901.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radbruch A., Liesegang B., Rajewsky K. Isolation of variants of mouse myeloma X63 that express changed immunoglobulin class. Proc Natl Acad Sci U S A. 1980 May;77(5):2909–2913. doi: 10.1073/pnas.77.5.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbuch M., Audran R. The isolation of IgG from mammalian sera with the aid of caprylic acid. Arch Biochem Biophys. 1969 Nov;134(2):279–284. doi: 10.1016/0003-9861(69)90285-9. [DOI] [PubMed] [Google Scholar]
- Taylor R. L., Conrad H. E. Stoichiometric depolymerization of polyuronides and glycosaminoglycuronans to monosaccharides following reduction of their carbodiimide-activated carboxyl groups. Biochemistry. 1972 Apr 11;11(8):1383–1388. doi: 10.1021/bi00758a009. [DOI] [PubMed] [Google Scholar]
- Wilson D. E., Bennett J. E., Bailey J. W. Serologic grouping of Cryptococcus neoformans. Proc Soc Exp Biol Med. 1968 Mar;127(3):820–823. doi: 10.3181/00379727-127-32812. [DOI] [PubMed] [Google Scholar]




