Abstract
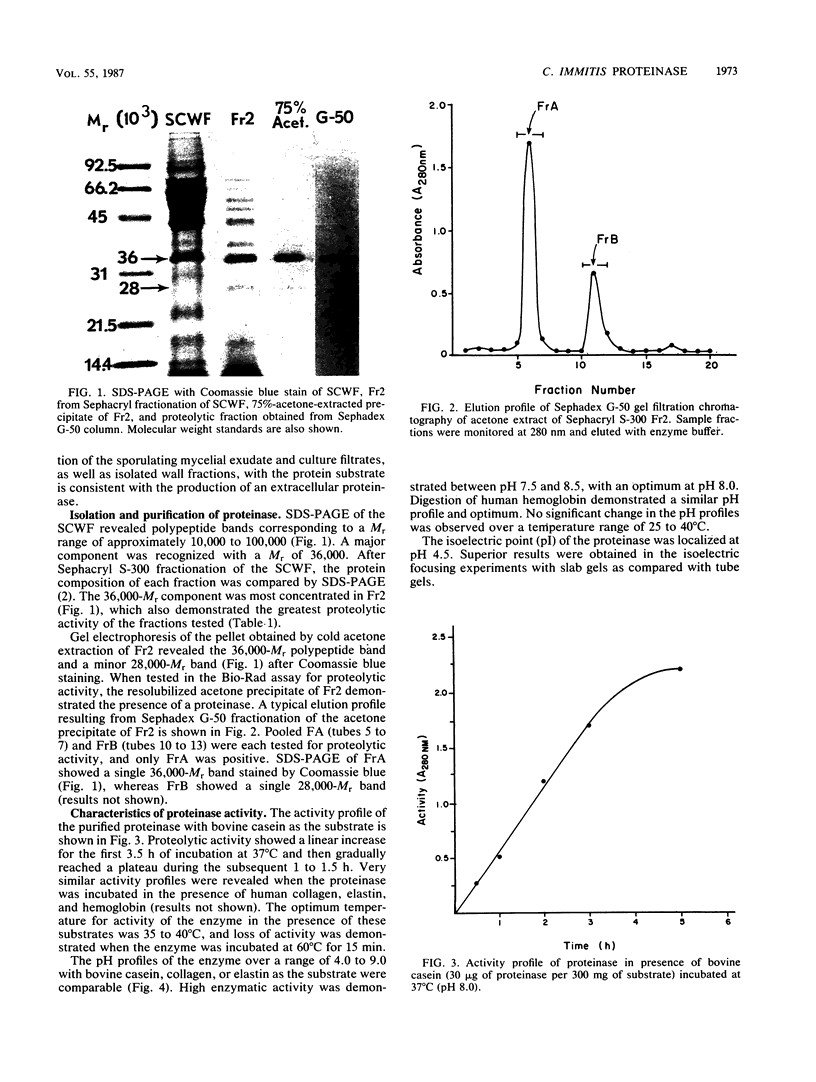
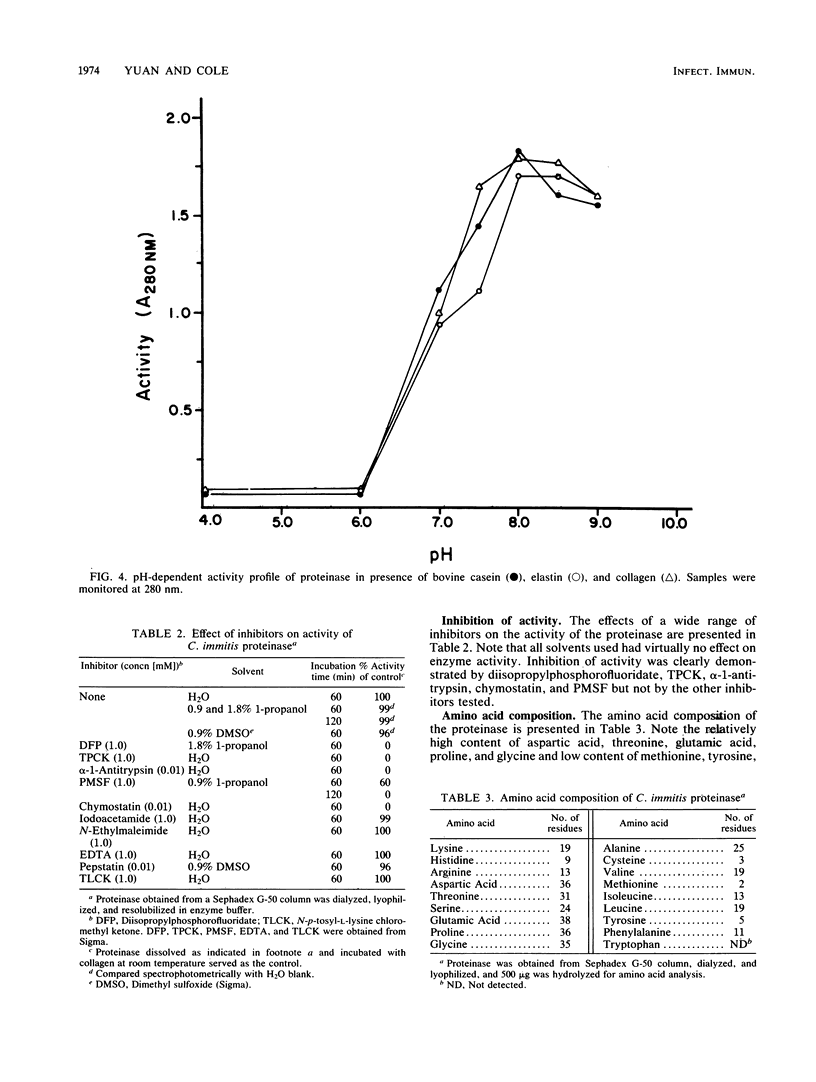
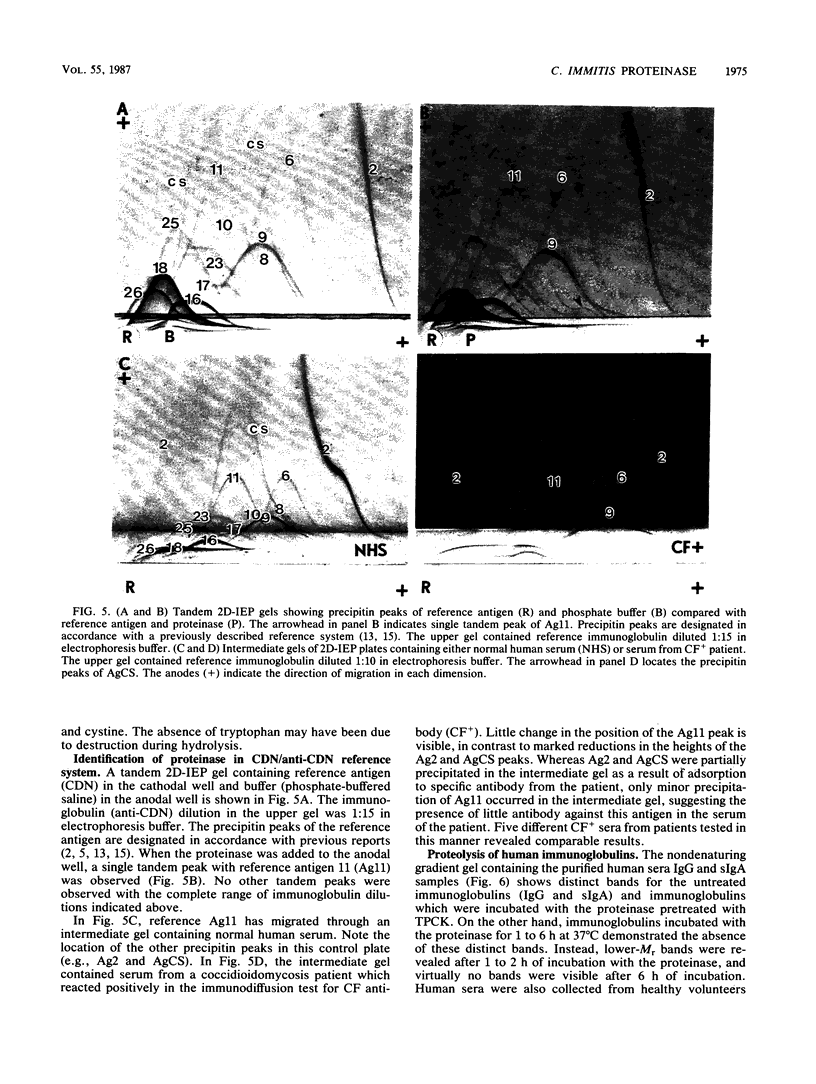
A proteinase isolated from the respiratory pathogen, Coccidioides immitis, was shown to have collagenolytic and elastinolytic activity, as well as the ability to cleave human serum immunoglobulin G and secretory immunoglobulin A. Proteolytic activity was demonstrated with a bovine casein digestion assay in conidial culture exudates, mycelial and spherule culture filtrates, conidial and spherule wall material, and Sephacryl S-300 fractions of the isolated soluble conidial wall material described previously. One of the latter fractions (fraction 2) demonstrated high proteolytic activity. The proteinase was purified from this chromatographic fraction by cold acetone extraction followed by Sephadex G-50 gel filtration and was identified as a polypeptide band of 36,000 Mr by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. By means of tandem two-dimensional immunoelectrophoresis, the proteinase was identified as antigen 11 on the basis of its reaction in the coccidioidin/anticoccidioidin reference system. The proteinase is characterized by a broad substrate specificity, optimal activity at 35 to 40 degrees C (pH 8.0) in the presence of human collagen, elastin, or hemoglobin, an isoelectric point of pH 4.5, and inhibition by organofluorides, N-tosyl-L-phenylalanine chloromethyl ketone, chymostatin, and alpha-1-antitrypsin. These features of the enzyme are comparable to those of chymotrypsinlike serine proteinases. Demonstration that the proteinase can cleave human immunoglobulins and digest ubiquitous tissue structural proteins (e.g., collagen and elastin) suggests that it may play a role in the virulence of the fungal pathogen.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cole G. T., Kirkland T. N., Sun S. H. An immunoreactive, water-soluble conidial wall fraction of Coccidioides immitis. Infect Immun. 1987 Mar;55(3):657–667. doi: 10.1128/iai.55.3.657-667.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole G. T., Sun S. H., Huppert M. Isolation and ultrastructural examination of conidial wall components of Coccidioides and Aspergillus. Scan Electron Microsc. 1982;(Pt 4):1677–1685. [PubMed] [Google Scholar]
- Drutz D. J., Huppert M. Coccidioidomycosis: factors affecting the host-parasite interaction. J Infect Dis. 1983 Mar;147(3):372–390. doi: 10.1093/infdis/147.3.372. [DOI] [PubMed] [Google Scholar]
- Huppert M., Adler J. P., Rice E. H., Sun S. H. Common antigens among systemic disease fungi analyzed by two-dimensional immunoelectrophoresis. Infect Immun. 1979 Feb;23(2):479–485. doi: 10.1128/iai.23.2.479-485.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert M., Krasnow I., Vukovich K. R., Sun S. H., Rice E. H., Kutner L. J. Comparison of coccidioidin and spherulin in complement fixation tests for coccidioidomycosis. J Clin Microbiol. 1977 Jul;6(1):33–41. doi: 10.1128/jcm.6.1.33-41.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert M., Spratt N. S., Vukovich K. R., Sun S. H., Rice E. H. Antigenic analysis of coccidioidin and spherulin determined by two-dimensional immunoelectrophoresis. Infect Immun. 1978 May;20(2):541–551. doi: 10.1128/iai.20.2.541-551.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon-Chung K. J., Lehman D., Good C., Magee P. T. Genetic evidence for role of extracellular proteinase in virulence of Candida albicans. Infect Immun. 1985 Sep;49(3):571–575. doi: 10.1128/iai.49.3.571-575.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINE H. B., COBB J. M., SMITH C. E. Immunity to coccidioi-domycosis induced in mice by purified spherule, arthrospore, and mycelial vaccines. Trans N Y Acad Sci. 1960 Apr;22:436–449. doi: 10.1111/j.2164-0947.1960.tb00711.x. [DOI] [PubMed] [Google Scholar]
- LEVINE H. B. Purification of the spherule-endospore phase of Coccidioides immitis. Sabouraudia. 1961 Jun;1:112–115. doi: 10.1080/00362176285190231. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lupan D. M., Nziramasanga P. Collagenolytic activity of Coccidioides immitis. Infect Immun. 1986 Jan;51(1):360–361. doi: 10.1128/iai.51.1.360-361.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss D. E., Fahrney D. Kinetic analysis of differences in brain acetylcholinesterase from fish or mammalian sources. Biochem Pharmacol. 1978;27(23):2693–2698. doi: 10.1016/0006-2952(78)90044-8. [DOI] [PubMed] [Google Scholar]
- Nziramasanga P., Lupan D. M. Elastase activity of Coccidioides immitis. J Med Microbiol. 1985 Feb;19(1):109–114. doi: 10.1099/00222615-19-1-109. [DOI] [PubMed] [Google Scholar]
- Plaut A. G. The IgA1 proteases of pathogenic bacteria. Annu Rev Microbiol. 1983;37:603–622. doi: 10.1146/annurev.mi.37.100183.003131. [DOI] [PubMed] [Google Scholar]
- Rüchel R., Böning B., Borg M. Characterization of a secretory proteinase of Candida parapsilosis and evidence for the absence of the enzyme during infection in vitro. Infect Immun. 1986 Aug;53(2):411–419. doi: 10.1128/iai.53.2.411-419.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüchel R. Cleavage of immunoglobulins by pathogenic yeasts of the genus Candida. Microbiol Sci. 1986 Oct;3(10):316–319. [PubMed] [Google Scholar]
- Shepherd M. G., Poulter R. T., Sullivan P. A. Candida albicans: biology, genetics, and pathogenicity. Annu Rev Microbiol. 1985;39:579–614. doi: 10.1146/annurev.mi.39.100185.003051. [DOI] [PubMed] [Google Scholar]
- Staib F. Serum-Protein-Agar-pH 4,2 und 5,0 für Sprosspilze. Zentralbl Bakteriol Orig. 1964 Dec;195(2):265–267. [PubMed] [Google Scholar]
- Sun S. H., Huppert M., Vukovich K. R. Rapid in vitro conversion and identification of Coccidioides immitis. J Clin Microbiol. 1976 Feb;3(2):186–190. doi: 10.1128/jcm.3.2.186-190.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S. H., Sekhon S. S., Huppert M. Electron microscopic studies of saprobic and parasitic forms of Coccidioides immitis. Sabouraudia. 1979 Sep;17(3):265–273. doi: 10.1080/00362177985380391. [DOI] [PubMed] [Google Scholar]
- Turini P., Kurooka S., Steer M., Corbascio A. N., Singer T. P. The action of phenylmethylsulfonyl fluoride on human acetylcholinesterase, chymotyrpsin and trypsin. J Pharmacol Exp Ther. 1969 May;167(1):98–104. [PubMed] [Google Scholar]





