Abstract
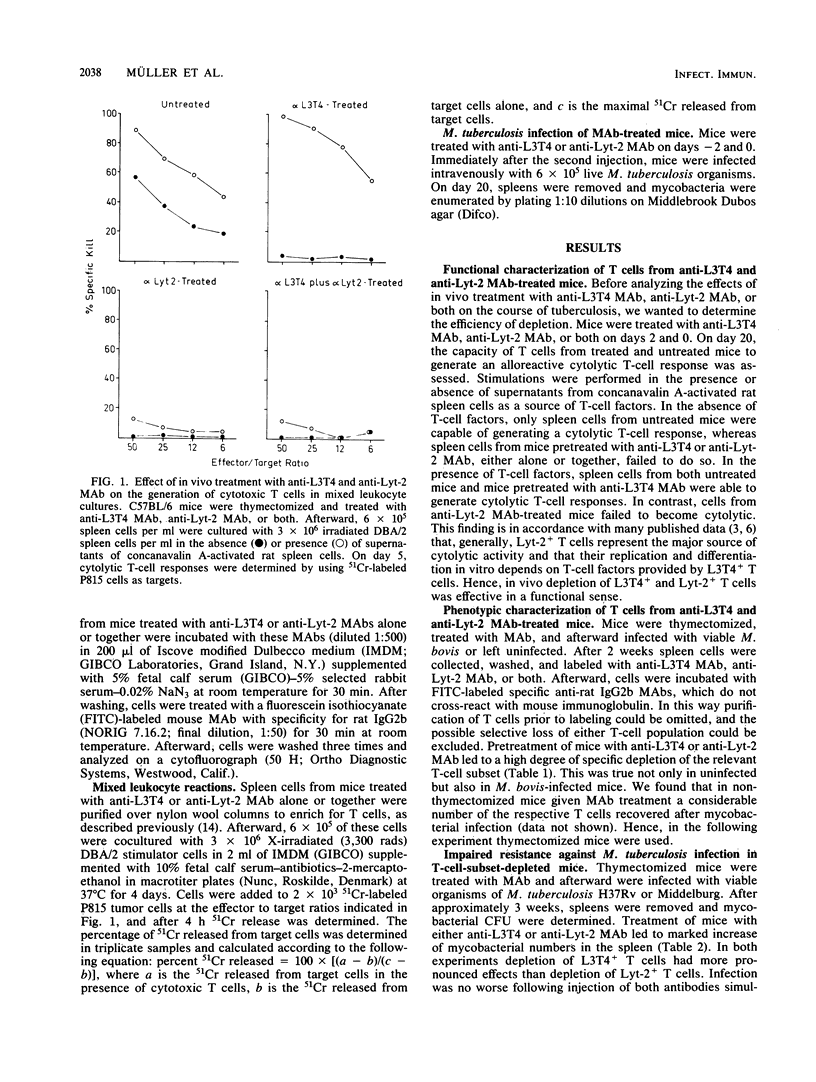
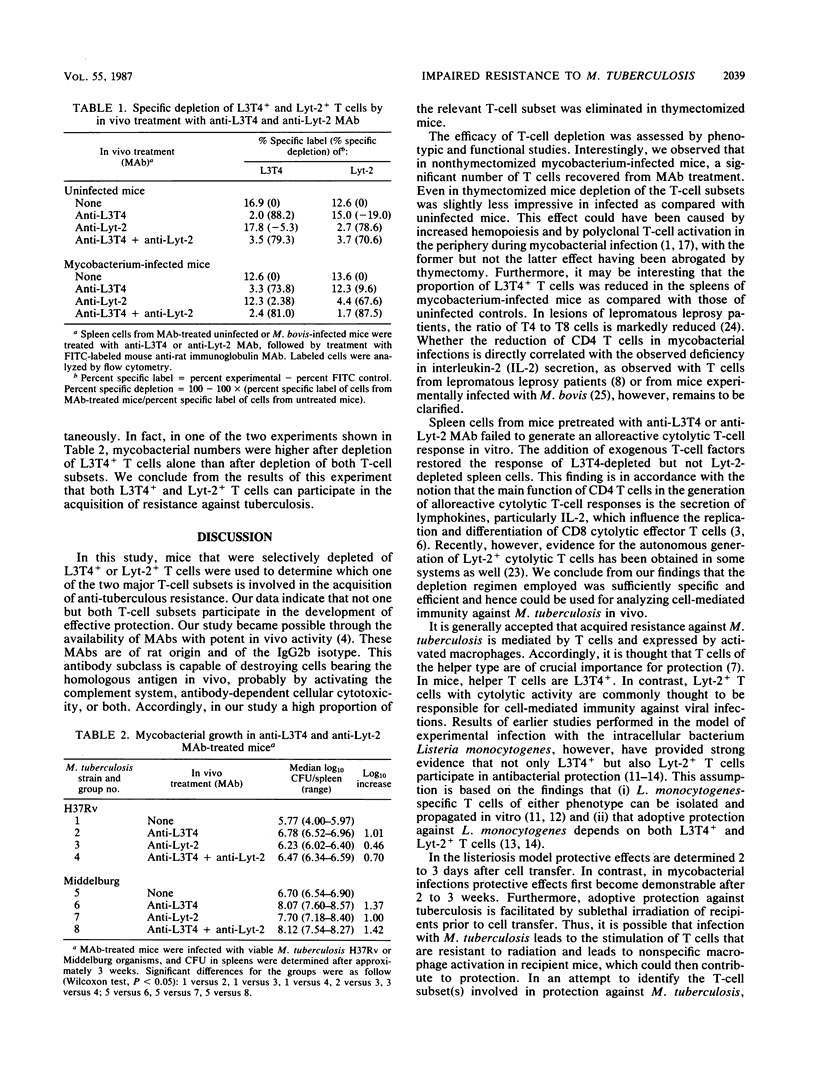
The resistance of mice against Mycobacterium tuberculosis infection after selective in vivo depletion of L3T4+ and Lyt-2+ T cells was studied. Thymectomized mice were treated with rat monoclonal antibodies against the L3T4 or Lyt-2 molecule to selectively eliminate the respective T-cell subset. In both L3T4+ and Lyt-2+ T-cell-depleted mice, resistance against subsequent infection with M. tuberculosis was markedly impaired compared with that in untreated controls, with L3T4+ T-cell-depleted mice showing more pronounced effects. Simultaneous depletion of L3T4+ and Lyt-2+ T cells did not further exacerbate infection. These findings suggest that both L3T4+ and Lyt-2+ T cells are involved in the acquisition of resistance against tuberculosis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cantor H., Boyse E. A. Lymphocytes as models for the study of mammalian cellular differentiation. Immunol Rev. 1977 Jan;33:105–124. doi: 10.1111/j.1600-065x.1977.tb00364.x. [DOI] [PubMed] [Google Scholar]
- Cobbold S. P., Jayasuriya A., Nash A., Prospero T. D., Waldmann H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature. 1984 Dec 6;312(5994):548–551. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- De Libero G., Kaufmann S. H. Antigen-specific Lyt-2+ cytolytic T lymphocytes from mice infected with the intracellular bacterium Listeria monocytogenes. J Immunol. 1986 Oct 15;137(8):2688–2694. [PubMed] [Google Scholar]
- Fitch F. W. T-cell clones and T-cell receptors. Microbiol Rev. 1986 Mar;50(1):50–69. doi: 10.1128/mr.50.1.50-69.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn H., Kaufmann S. H. The role of cell-mediated immunity in bacterial infections. Rev Infect Dis. 1981 Nov-Dec;3(6):1221–1250. doi: 10.1093/clinids/3.6.1221. [DOI] [PubMed] [Google Scholar]
- Haregewoin A., Godal T., Mustafa A. S., Belehu A., Yemaneberhan T. T-cell conditioned media reverse T-cell unresponsiveness in lepromatous leprosy. Nature. 1983 May 26;303(5915):342–344. doi: 10.1038/303342a0. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Chiplunkar S., Flesch I., De Libero G. Possible role of helper and cytolytic T cells in mycobacterial infections. Lepr Rev. 1986 Dec;57 (Suppl 2):101–111. doi: 10.5935/0305-7518.19860060. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Flesch I. Function and antigen recognition pattern of L3T4+ T-cell clones from Mycobacterium tuberculosis-immune mice. Infect Immun. 1986 Nov;54(2):291–296. doi: 10.1128/iai.54.2.291-296.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Hahn H. Biological functions of t cell lines with specificity for the intracellular bacterium Listeria monocytogenes in vitro and in vivo. J Exp Med. 1982 Jun 1;155(6):1754–1765. doi: 10.1084/jem.155.6.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Hug E., De Libero G. Listeria monocytogenes-reactive T lymphocyte clones with cytolytic activity against infected target cells. J Exp Med. 1986 Jul 1;164(1):363–368. doi: 10.1084/jem.164.1.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Hug E., Väth U., Müller I. Effective protection against Listeria monocytogenes and delayed-type hypersensitivity to listerial antigens depend on cooperation between specific L3T4+ and Lyt 2+ T cells. Infect Immun. 1985 Apr;48(1):263–266. doi: 10.1128/iai.48.1.263-266.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Simon M. M., Hahn H. Specific Lyt 123 cells are involved in protection against Listeria monocytogenes and in delayed-type hypersensitivity to listerial antigens. J Exp Med. 1979 Oct 1;150(4):1033–1038. doi: 10.1084/jem.150.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S., Weber L., Hahn H. Macrophage inhibiting activity in serum and central lymph of Listeria-immune mice. Eur J Immunol. 1975 Nov;5(11):799–800. doi: 10.1002/eji.1830051114. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Rouse R. V., Micklem H. S., Herzenberg L. A. T cell subsets defined by expression of Lyt-1,2,3 and Thy-1 antigens. Two-parameter immunofluorescence and cytotoxicity analysis with monoclonal antibodies modifies current views. J Exp Med. 1980 Aug 1;152(2):280–295. doi: 10.1084/jem.152.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchal G., Milon G. Control of hemopoiesis in mice by sensitized L3T4+ Lyt2-lymphocytes during infection with bacillus Calmette-Guérin. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3977–3981. doi: 10.1073/pnas.83.11.3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder J. W. Comparative biology of intracellular parasitism. Microbiol Rev. 1985 Sep;49(3):298–337. doi: 10.1128/mr.49.3.298-337.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller I., Maier B., Brinkmann V., Kaufmann S. H. Autoreactive T cell clones from mice infected with Mycobacterium bovis, strain Bacillus Calmette-Guérin (BCG). I. Phenotype, specificity and in vitro function. Immunobiology. 1986 Jul;171(4-5):366–380. doi: 10.1016/S0171-2985(86)80069-9. [DOI] [PubMed] [Google Scholar]
- Orme I. M., Collins F. M. Adoptive protection of the Mycobacterium tuberculosis-infected lung. Dissociation between cells that passively transfer protective immunity and those that transfer delayed-type hypersensitivity to tuberculin. Cell Immunol. 1984 Mar;84(1):113–120. doi: 10.1016/0008-8749(84)90082-0. [DOI] [PubMed] [Google Scholar]
- Orme I. M. The kinetics of emergence and loss of mediator T lymphocytes acquired in response to infection with Mycobacterium tuberculosis. J Immunol. 1987 Jan 1;138(1):293–298. [PubMed] [Google Scholar]
- Plan of action for research in the immunology of tuberculosis: memorandum from a WHO meeting. Bull World Health Organ. 1983;61(5):779–785. [PMC free article] [PubMed] [Google Scholar]
- Sprent J., Schaefer M., Lo D., Korngold R. Properties of purified T cell subsets. II. In vivo responses to class I vs. class II H-2 differences. J Exp Med. 1986 Apr 1;163(4):998–1011. doi: 10.1084/jem.163.4.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Voorhis W. C., Kaplan G., Sarno E. N., Horwitz M. A., Steinman R. M., Levis W. R., Nogueira N., Hair L. S., Gattass C. R., Arrick B. A. The cutaneous infiltrates of leprosy: cellular characteristics and the predominant T-cell phenotypes. N Engl J Med. 1982 Dec 23;307(26):1593–1597. doi: 10.1056/NEJM198212233072601. [DOI] [PubMed] [Google Scholar]
- Vismara D., Lombardi G., Piccolella E., Colizzi V. Dissociation between interleukin-1 and interleukin-2 production in proliferative response to microbial antigens: restorative effect of exogenous interleukin-2. Infect Immun. 1985 Aug;49(2):298–304. doi: 10.1128/iai.49.2.298-304.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]


