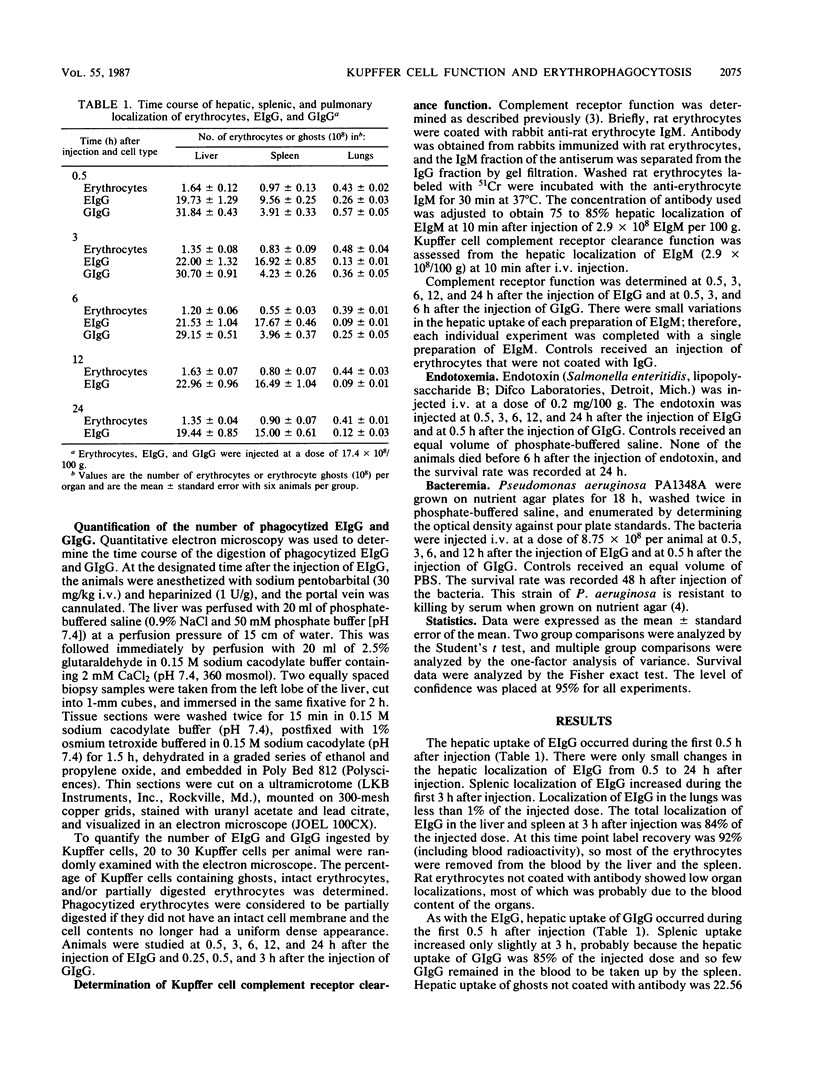
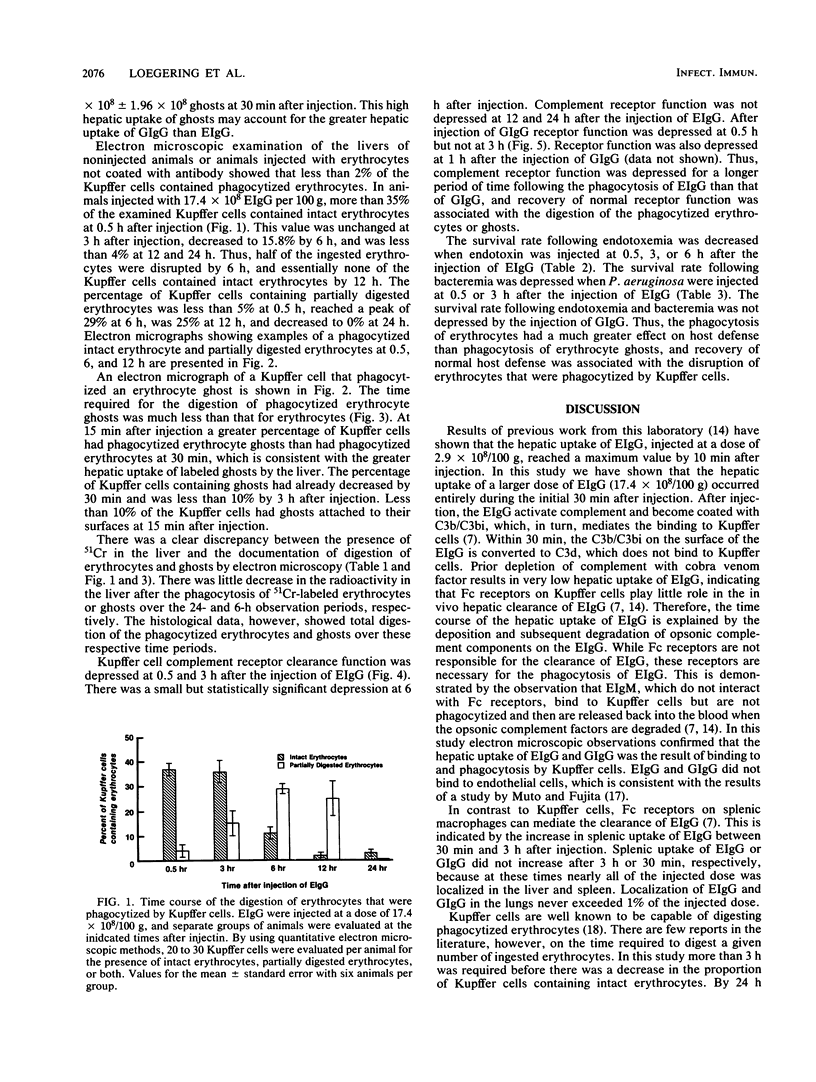
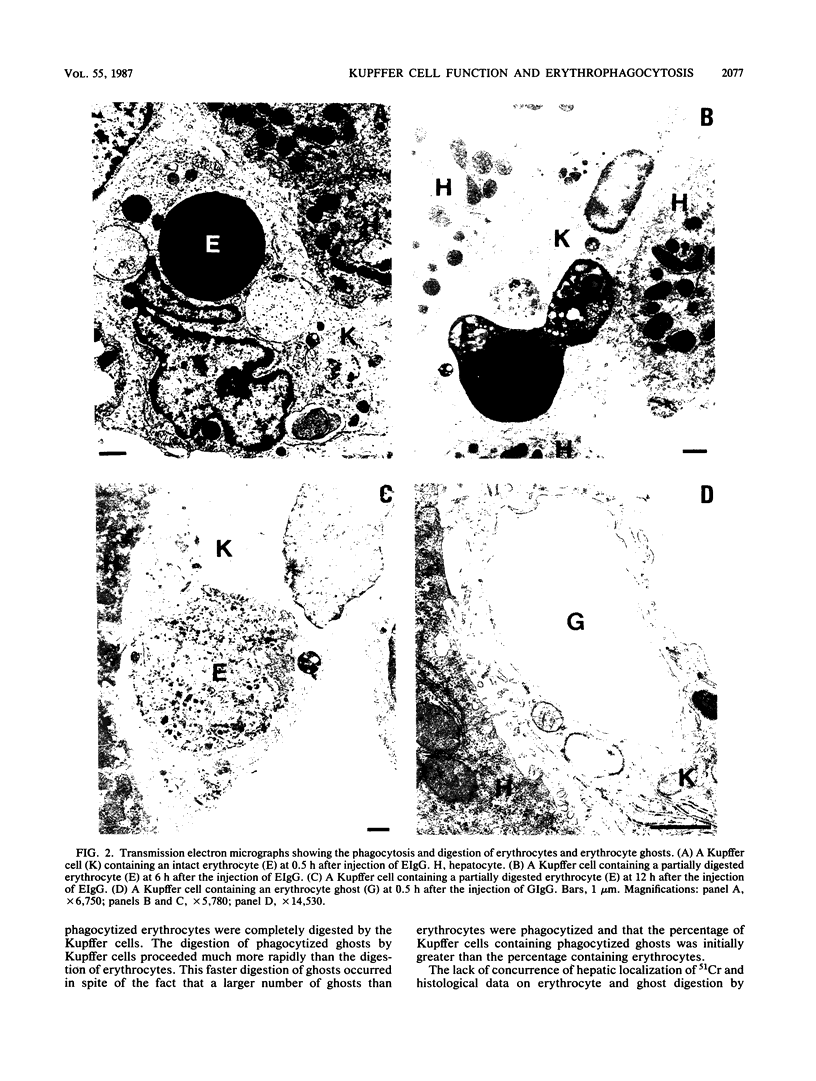
Abstract
The phagocytosis of erythrocytes by macrophages has previously been shown to depress macrophage function. In this study we compared the effect of the phagocytosis of erythrocytes and erythrocyte ghosts by Kupffer cells on the duration of the depression of complement receptor clearance function and host defense against endotoxemia and bacteremia. Phagocytosis of erythrocytes and erythrocyte ghosts was induced in rats by the injection of rat erythrocytes or erythrocyte ghosts coated with anti-rat erythrocyte immunoglobulin G (EIgG and GIgG, respectively). The hepatic uptake of EIgG and GIgG (17.4 X 10(8)/100 g) occurred during the first 30 min after injection. The digestion of phagocytized EIgG and GIgG, as assessed by electron microscopy, was complete at 24 and 3 h after injection, respectively. The depression of Kupffer cell complement receptor clearance function caused by EIgG and GIgG returned to normal by 6 h after injection of EIgG and by 3 h after injection of GIgG. Phagocytosis of EIgG depressed the survival rate after endotoxemia and bacteremia when endotoxin or bacteria were injected at 30 min after EIgG. The survival rate returned to normal when the endotoxin and bacteria were injected at 12 and 6 h after the EIgG, respectively. Phagocytosis of GIgG did not depress the survival rate after endotoxemia and bacteremia. Thus, compared with erythrocytes, erythrocyte ghosts are more rapidly digested after phagocytosis, depress complement receptor function for a shorter period of time, and cause less depression of host defense. These findings indicate that the contents of erythrocytes play an important role in the impairment of host defense caused by the phagocytosis of erythrocytes by Kupffer cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agar N. S., Sadrzadeh S. M., Hallaway P. E., Eaton J. W. Erythrocyte catalase. A somatic oxidant defense? J Clin Invest. 1986 Jan;77(1):319–321. doi: 10.1172/JCI112294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuddy B. G., Loegering D. J., Blumenstock F. A. Depression of in vivo clearance function of hepatic macrophage complement receptors following thermal injury. Proc Soc Exp Biol Med. 1984 Sep;176(4):443–451. doi: 10.3181/00379727-176-41896. [DOI] [PubMed] [Google Scholar]
- Cuddy B. G., Loegering D. J., Blumenstock F. A., Shah D. M. Hepatic macrophage complement receptor clearance function following injury. J Surg Res. 1986 Mar;40(3):216–224. doi: 10.1016/0022-4804(86)90154-x. [DOI] [PubMed] [Google Scholar]
- DeMatteo C. S., Hammer M. C., Baltch A. L., Smith R. P., Sutphen N. T., Michelsen P. B. Susceptibility of Pseudomonas aeruginosa to serum bactericidal activity. A comparison of three methods with clinical correlations. J Lab Clin Med. 1981 Oct;98(4):511–518. [PubMed] [Google Scholar]
- Eaton J. W., Boraas M., Etkin N. L. Catalase activity and red cell metabolism. Adv Exp Med Biol. 1972;28:121–131. doi: 10.1007/978-1-4684-3222-0_8. [DOI] [PubMed] [Google Scholar]
- Fischer A., Seger R., Durandy A., Grospierre B., Virelizier J. L., Le Deist F., Griscelli C., Fischer E., Kazatchkine M., Bohler M. C. Deficiency of the adhesive protein complex lymphocyte function antigen 1, complement receptor type 3, glycoprotein p150,95 in a girl with recurrent bacterial infections. Effects on phagocytic cells and lymphocyte functions. J Clin Invest. 1985 Dec;76(6):2385–2392. doi: 10.1172/JCI112251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill F. A., Kaye D., Hook E. W. The influence of erythrophagocytosis on the interaction of macrophages and salmonella in vitro. J Exp Med. 1966 Aug 1;124(2):173–183. doi: 10.1084/jem.124.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand W. L. Inhibition of cell-free oxidative bactericidal activity by erythrocytes and hemoglobin. Infect Immun. 1984 May;44(2):465–468. doi: 10.1128/iai.44.2.465-468.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand W. L., King-Thompson N. L. Effect of erythrocyte ingestion on macrophage antibacterial function. Infect Immun. 1983 Jun;40(3):917–923. doi: 10.1128/iai.40.3.917-923.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANDL J. H., JONES A. R., CASTLE W. B. The destruction of red cells by antibodies in man. I. Observations of the sequestration and lysis of red cells altered by immune mechanisms. J Clin Invest. 1957 Oct;36(10):1428–1459. doi: 10.1172/JCI103542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye D., Gill F. A., Hook E. W. Factors influencing host resistance to Salmonella infections: the effects of hemolysis and erythrophagocytosis. Am J Med Sci. 1967 Aug;254(2):205–215. doi: 10.1097/00000441-196708000-00011. [DOI] [PubMed] [Google Scholar]
- Loebl E. C., Baxter C. R., Curreri P. W. The mechanism of erythrocyte destruction in the early post-burn period. Ann Surg. 1973 Dec;178(6):681–686. doi: 10.1097/00000658-197312000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loegering D. J., Blumenstock F. A. Depressing hepatic macrophage complement receptor function causes increased susceptibility to endotoxemia and infection. Infect Immun. 1985 Mar;47(3):659–664. doi: 10.1128/iai.47.3.659-664.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loegering D. J., Grover G. J., Schneidkraut M. J. Effect of red blood cells and red blood cell ghosts on reticuloendothelial system function. Exp Mol Pathol. 1984 Aug;41(1):67–73. doi: 10.1016/0014-4800(84)90008-x. [DOI] [PubMed] [Google Scholar]
- NIH conference. Pathophysiology of immune hemolytic anemia. Ann Intern Med. 1977 Aug;87(2):210–222. doi: 10.7326/0003-4819-87-2-210. [DOI] [PubMed] [Google Scholar]
- Paglia D. E., Valentine W. N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967 Jul;70(1):158–169. [PubMed] [Google Scholar]
- Schneidkraut M. J., Loegering D. J. Effect of extravascular hemolysis on the RES depression following thermal injury. Exp Mol Pathol. 1984 Jun;40(3):271–279. doi: 10.1016/0014-4800(84)90044-3. [DOI] [PubMed] [Google Scholar]
- Schreiber R. D. The chemistry and biology of complement receptors. Springer Semin Immunopathol. 1984;7(2-3):221–249. doi: 10.1007/BF01893021. [DOI] [PubMed] [Google Scholar]
- Springer T. A., Thompson W. S., Miller L. J., Schmalstieg F. C., Anderson D. C. Inherited deficiency of the Mac-1, LFA-1, p150,95 glycoprotein family and its molecular basis. J Exp Med. 1984 Dec 1;160(6):1901–1918. doi: 10.1084/jem.160.6.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth K. M., Clifford D. P., Berger E. M., White C. W., Repine J. E. Intact human erythrocytes prevent hydrogen peroxide-mediated damage to isolated perfused rat lungs and cultured bovine pulmonary artery endothelial cells. J Clin Invest. 1984 Jul;74(1):292–295. doi: 10.1172/JCI111414. [DOI] [PMC free article] [PubMed] [Google Scholar]