Abstract
This review focuses on the new and emerging large-molecule bioactive agents delivered from stent surfaces in drug-eluting stents (DES) to inhibit vascular restenosis in the context of interventional cardiology. New therapeutic agents representing proteins, nucleic acids (small interfering RNAs and large DNA plasmids), viral delivery vectors and even engineered cell therapies require specific delivery designs distinct from traditional smaller molecule approaches on DES. While small molecules are currently the clinical standard for coronary stenting, extension of the DES to other lesion types, peripheral vasculature and non-vasculature therapies will seek to deliver an increasingly sophisticated armada of drug types. This review describes many of the larger molecule and biopharmaceutical approaches reported recently for stent-based delivery with the challenges associated with formulating and delivering these drug classes compared to the current small molecule drugs. It also includes perspectives on possible future applications that may improve safety and efficacy and facilitate diversification of the DES to other clinical applications.
Keywords: cardiovascular stent, restenosis, drug delivery, vascular, smooth muscle cells, therapy, biopharmaceutical, nucleic acid, gene, PTCA, protein delivery, cell therapy
Introduction
Stenting – the surgical placement of an intravascular hollow cylindrical device into vascular lesion sites following percutaneous transluminal coronary angioplasty (PTCA) – is a common procedure to inhibit vessel restenosis, particularly in the coronary vasculature. However, in-stent restenosis is observed at the stented site in 15–20% of PTCA patients following bare metal stent implantation.1–4 Clinical trials using systemic drug administration (i.e., parenterally administered anti-restenotic agents) have failed. This is attributed to insufficient drug doses actually reaching the vascular target site, and adverse systemic side effects.1, 5–7 This emphasizes the importance of local delivery directly from the stent surface to influence local cell populations in the vascular wall. To reduce doses required and the incidence of systemic complications, and ensure appropriate drug dosing directly at the target site, drugs can be directly and continuously delivered to the tissue of interest. Catheter-based delivery devices have been developed to locally deliver drugs to vascular sites as well to perform PTCA.1, 7 However, beyond simple drug delivery, controlled release of therapeutic agents to specific targets, primarily anti-restenotic agents at stented sites, is often required. Drug-eluting stents (DES) provide anti-restenotic drug therapy with low-dose local drug delivery from the coated stent surface to the vessel tissue bed has rapidly become a clinically routine therapeutic approach to inhibit in-stent restenosis to less than 5% in millions of cases annually.8 The DES comprises a dose of drug typically embedded within a carrier coating (generally polymer, but also inorganic phases) applied to the wire mesh comprising the stent.3, 9
Implantation of bare metal stents, while common, in principle presents a highly reactive metal surface known to activate coagulation cascades, produce thrombosis and inflammatory reactions, and release toxic metal ions (i.e., chromium, nickel, molybdenum).10 Surface modification had been known for over four decades to improve metal-blood compatibility. Hence, stent coating is popular both to produce a local drug release matrix as well as limit intrinsic thrombogenicity and foreign body responses. Carbon coating is used without drug loading to produce a passivating surface with claims to improved biocompatibility in vitro and in preclinical testing.11–14 However, as these carbon coatings have been extended to human stent trials, performance in vivo has been non-distinguishing, reducing the enthusiasm for carbon-coated stents in human implants.15–17 Synthetic polymers, hydrogels and polysaccharide coatings provide a reasonable coating matrix with reduced pro-coagulant activity compared to bare metal stents. Bioactive agents (drug dose ~ micrograms/stent) loaded into these coatings are intended for local release to affect specific cell types in the vascular wall following stent intravascular deployment. To produce desired pharmacological effects while avoiding side effects, the DES must be designed to limit drug release prior to deployment, and release the proper dosing at the desired kinetics after deployment. Additional important DES design criteria are (1) minimal thrombogenic DES procoagulant or complement activation in the blood stream, (2) acceptable stent and coating stability in the vascular wall upon placement, (3) solubility compatibility between polymer, solvent and drug during the coating formulation process, (4) and coating stability and integrity during stent deployment where shape-memory or luminal expansion effects produce mechanical stress and strain.
Many previous reviews have extensively documented the current US Food and Drug Administration (FDA)-approved clinical methodologies and pharmacologies, and expected related innovations, all emphasizing smaller molecule drug release.1, 6, 18–20 Nevertheless, newer emerging DES designs are using increasingly sophisticated bioactive agents, including new macromolecular drugs (e.g., proteins, nucleic acids, viral vectors) with unique pharmacologies but also specific delivery requirements. Device-based delivery approaches that have shown clinical efficacy to date with small drugs do not necessarily translate to efficacy with biopharmaceuticals. It is likely therefore that many aspects of controlled release DES formulations used in coronary stenting will require re-design to both accommodate dosing and delivery issues for large molecule drugs and distinct pathology and pharmacology in many other cardiovascular stenting sites.
1. Overview of small molecule therapeutics in DES designs
Several clinical trials have already shown that rapamycin (e.g., CYPHER, RAVEL, SIRUS) and paclitaxel (e.g., ASPECT, ELUTES, TAXUS) eluted from polymer-coated stents exhibit superior performance in preventing vessel restenosis compared to bare metal stents.9, 18, 19, 21–26 Within cells, sirolimus inactivates mammalian target of rapamycin (mTOR) by forming a complex with FK506-binding protein 12 (FKBP12). As a result, the down-regulation of kinase p27 is prohibited, inhibiting passage of the cell cycle from G1 to S phase, and preventing cell proliferation.9 By contrast, paclitaxel binds to beta-tubulin, resulting in inhibition of microtubule depolymerization in a dose-dependent fashion. At high concentrations, this inhibits smooth muscle cell (SMC) proliferation and migration by disturbing the cell’s M phase.25 As shown in this review, there may be many other strategies now evolving to produce the same targeting of proliferating, mobilized cell types in the vascular bed more specifically, with improved efficacy and safety for DES deployment.
Since approval of the sirolimus-eluting stent by FDA, various sirolimus analogues including zotarolimus (ABT-578), biolimus-A9 and everolimus have also been shown to be effective against restenosis (e.g., ENDEAVOR, FUTURE and other trials) in DES formats.19, 20, 27–29 Sirolimus-eluting DES may also elicit adverse effects as rapamycin pharmacology is not specific to cell type but more generally toward all proliferating phenotypes. In particular, recent studies suggested that DES implantation adversely affects local endothelium regeneration.30–33 While the coronary arterial stent represents substantial clinical impact, other more difficult stenting sites are current targets for new DES techniques, seeking to demonstrate efficacy in challenging diabetic patients34–36 and more complex disease sites, e.g., small vessel22, 37 and bifurcated lesions38–40, and peripheral vasculature including venous sites.41–43 Thus, new stent designs will likely be combined with new therapeutic drug mixtures. For example, stent coatings releasing dexamethasone (STRIDE) and actinomycin D (ACTION) are shown to inhibit SMC proliferation.44, 45 Nitric oxide (NO) with its potent modulation of significant several vascular events including SMC proliferation,46–48 and select bioactive peptides with specific binding activities (cyclic Arg-Gly-Asp (RGD)-containing peptides, angiotensin, and angiopeptin)49–51 might also be candidates for DES-released anti-restenotic agents. Recently, anti-restenotic effects of NO donor-loaded stents have been investigated.52, 53 Angiopeptin blockade of growth factor-induced SMC proliferation51 and of platelet receptors that inhibit platelet aggregation by RGD-containing peptides,49 relevant peptide-eluting stents might demonstrate efficacy in reducing neointimal hyperplasia.54, 55 Recently, the delivery of microfilament inhibitor, cytochalasin D, produced significant inhibition of platelet aggregation and SMC proliferation in pig coronary arteries, compared with that of paclitaxel.56 In summary, many different bio-active agents have been or are being screened to address in-stent restenosis, targeting many different cellular mechanisms in the vascular bed.
Drug therapeutic efficacy in stent-based delivery depends upon many factors, including:
local physiology (i.e., venous vs. arterial sites, vessel lesion pathology)
drug pharmacology (specificity, potency) and toxicity
stent-based drug formulation (dose, release rate, bioavailability, duration)
Effective drug dosing and dose release from stent coatings depends on many factors including potency, local pharmacology and toxicity, clearance, formulating constraints on-stent, and drug physicochemical properties (molecular weight, stability, chemistry, solubility). To date, most drugs loaded onto and released from stents are relatively low molecular weight compounds (e.g., 400–900 Da). Current small molecule therapeutics in this context have been well-documented and often reviewed1, 6, 18–20 and are summarized in Table 1. Small molecule drugs provide several strategic advantages in dosing and formulation: smaller drugs tend to be more robust than large biological drugs (i.e., proteins or nucleic acids), and amounts of drug (i.e., moles) are larger per unit mass formulated. Nevertheless, at present, a diverse array of candidate drugs is required to address various targets against restenosis, (i.e., inhibition of neointimal hyperplasia), thrombosis, blockade of inflammatory responses, and promotion of re-endothelialization.2, 4, 5 Use of new, relative high molecular weight molecules expands therapeutic possibilities of drugs and their targets for stent-based delivery systems. Currently, innovative new biopolymer-based therapeutics (RNAi, DNA transgenes, proteins and polysaccharide drugs) are being investigated for stent-based delivery against numerous targets. While primarily focused at anti-restenotic effects, these drug candidates could provide appropriate treatments diverse bio-active actions beyond this treatment regimen in the future, depending respective lesions and stent formulations.
Table 1.
Small molecular weight anti-restenotic DES-based drugs
| drug | MW | ref |
|---|---|---|
| sirolimus | 914.1 | (9, 21) |
| zotarolimus | 966.2 | (28, 29) |
| everolimus | 958.2 | (27) |
| tacrolimus | 822.0 | (57, 58) |
| paclitaxel | 853.9 | (22–25) |
| actinomycin D | 1255.4 | (45) |
| cytochalasin D | 507.6 | (56) |
| dexamethasone | 392.4 | (44, 59) |
| 17-beta-estradiol | 272.3 | (60, 61) |
| mycophenolic acid | 320.3 | (20) |
| angiopeptin | 1096.3 | (54) |
| cyclic RGD containing poptide | 948.0 | (55) |
2. Nucleotide-based drugs for stent-based delivery
2.1 DNA-based drugs
Gene therapy – the deliberate exogenous delivery of a therapeutic transgene62 – is currently being evaluated in hundreds of clinical trials for various conditions. It also offers the potential for new treatments for specific cardiovascular diseases, both systemically and from the surfaces of cardiovascular devices.63–67 DNA plasmids encoding an anti-restenotic transgene can be directly perfused or injected at an injury site using catheters. While naked DNA plasmids are commonly used, transgene vectors – various viral or non-viral polymers or lipid carriers of the DNA therapeutic gene – can be exploited to facilitate necessary gene penetration into cells and into the nucleus. However, in all cases, control of the possible systemic bio-distribution of the therapeutic gene is required. Transgene uptake into vascular tissue sites takes time, so to prevent systemic distribution of the catheter-based gene therapy dose from vascular flow, direct perfusion methods often temporarily halt blood flow in the vasculature surrounding the lesion, infuse the dose via catheter, incubate the transgene dose under no flow conditions, rinse with saline, then restore normal vascular flow. To prevent hypoxia or necrosis, intravascular perfusion times can not exceed minutes, limiting dose penetration to the desired site and increasing prospects for systemized exposure. In contrast, stent-based gene delivery puts a smaller transgene dose on the stent surface, places the delivery surface directly into contact with the vascular bed to better deliver a reliable and local dose without systemic issues or kinetic delivery limitations. Tissue penetration from locally delivered doses is conceivably a much more attractive method to both control adverse systemic side effects while attempting to inhibit restenosis.
Biodegradable polymer coatings with a strong medical device track record, FDA approval in a number of therapeutic contexts with accepted biocompatibility and drug reservoir capacities, Degradable FDA-approved polymer coatings comprising poly-L-lactic acid (PLLA), poly-D,L-lactic acid (PDLA), poly(caprolactones) (PCL) and polyglycolic acid (PGA) have been often used for stent coatings as well as completely degradable stents.68, 69 Bioresorbable polymers show high tensile strength, controlled degradation rates and reliable fatigue properties.70 Importantly, these biodegradable polymers also have been used as non-viral gene vectors for over a decade.71, 72 For example, plasmid DNA is loaded into polylactic-polyglycolic acid (PLGA) on a bare metal stent by stent immersion into a PLGA emulsified organic-aqueous solution containing plasmid DNA (e.g., model green fluorescent protein (GFP) plasmids, 0.9–1.1 mg of DNA loaded into 8–9 mg of PLGA emulsion on a Crown™ stent, Figure 1), producing a coating.73 As shown in Figure 2, plasmid DNA releases from the stent coating and transfects cells locally within the vascular wall. Additionally, non-degradable polymer coatings might better be used to deliver plasmid DNA release from stents74 while also limiting associated thrombosis issues after stent implantation using polymer coatings comprising biomedical polyurethanes, heparin sulfates and other glycosaminoglycans, hyaluronic acids, and other hydrophilic polymers (e.g., hydrogels). To date, polymer coatings have been used to demonstrate the possibility for local gene delivery from gene-eluting stents. However, stent-based gene delivery would likely require a transfection system (e.g., vector) for efficacious gene expression. Vectors are either viral (e.g., gene-loaded adenovirus or adeno-associated virus (AAV)) or non-viral (cationic synthetic polymeric or colloidal electrostatically complexed transgenes).62, 72, 75, 76 Muhs et al. performed gene delivery by catheter injection of plasmid lipoplexes with cationic lipids into pig coronary arteries in vivo,77 demonstrating that these complexes of plasmid DNA encoding nitric oxide synthase (NOS) and lipids could produce gene expression sufficient to inhibit SMC proliferation and migration, attributed to the anti-restenotic activity of expressed NOS as an NO producer.78, 79 However, while claims to efficacy are often made for such transgene systems in vitro (e.g., in serum-free media), further improvements in transfection efficiency and duration of expression are needed to achieve significant inhibition of restenosis in vivo from non-viral vectors. Thus, while polymer-based (e.g., non-viral) gene delivery agents might effectively produce local arterial gene transfer,80 their use in gene delivery from stents has been quite limited to few studies.73, 81
Figure 1.
Balloon-expanded arterial stents without (left) and with DNA-PLGA coating (right) (with Nile Red dye for visualization). Reprinted with permission from Macmillan Publishers Ltd: Nature Biotechnology (ref 73), copyright 2000.
Figure 2.
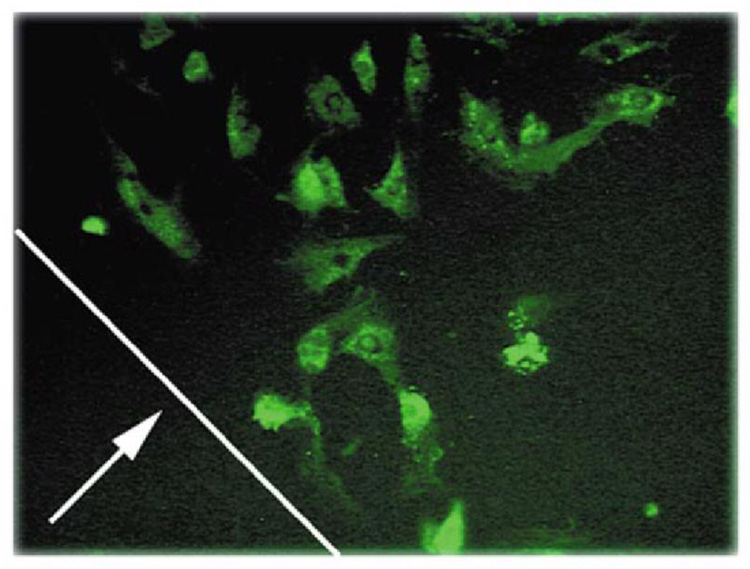
Cell transfection with GFP plasmid DNA into A10 cells using a DNA-PLGA coated stent wire. Line indicated by arrows the original location of the coated steel rod’s edge, at perimeter of GFP-positive cells (200×). Reprinted with permission from Macmillan Publishers Ltd: Nature Biotechnology (ref 73), copyright 2000.
Because of their higher intrinsic transfection efficiency, viral vectors have realized significant reduction in neointimal formation using therapeutic genes.82, 83 Adenoviral vectors in particular have been studied for therapeutic effects on hyperplasia and restenosis.63, 64, 79, 84, 85 For example, adenovirus encoding PTEN, an intracellular protein regulator inhibiting neointimal hyperplasia, was injected into ligated rat carotid artery under no-flow conditions, reducing neointimal hyperplasia.85 Ye et al. reported stent-based delivery of adenovirus encoding β-galactosidase using bioresorbable microporous stents comprising a polylactide/polycaprolactone blend for the stent coating.86
At present, stent-based drug loading is realized primarily by direct application of polymer solutions containing the drug of choice to stent surfaces (dip coating or spray coating). Many polymers of interest are not readily water-soluble, producing problems for the stability of many attractive biologically derived drugs in organic media. As an alternative, collagen can be used as a base coating capable of drug physical incorporation, direct collagen bioconjugation and drug surface coupling. Recently, stent spray coating using collagen solutions mixed with drug was investigated.87 Collagen coating also provides significant biocompatibility, biodegradability and tensile strength to stenting.88–90 Combined use of adenoviral vectors and collagen using antiviral antibodies covalently conjugated to pre-coated collagen and subsequent gene-loaded viral binding to the antibody has been reported.91–93 Stainless steel stents were coated with bovine type I collagen by immersion into collagen solutions. Anti-knob (Fab)’2 antiviral antibodies were conjugated with the collagen activated with standard thiol coupling reagents (SPDP) and then viral particles loaded with transgenes were bound by simple association. Adenovirus encoding GFP loaded on-stent produces GFP local expression in cultured SMCs around this collagen-coated stent. A surface density of 2.5 × 1010 viral particles per mg of collagen was achieved by this technique, and adenovirus was successfully delivered into coronary arteries upon deployment in vivo as shown by GFP expression in a stented coronary artery.91 Denatured collagen (gelatin) was used to deliver naked plasmid DNA encoding GFP without adenoviral vectors.94 Enhanced gene expression was proposed to be enhanced by specific interaction of denatured collagen with the SMC αvβ3 integrin.95, 96 In this system, 500 µg of plasmid DNA produced 10.4 ± 1.23% neointimal cells expressing GFP in a pig coronary artery.
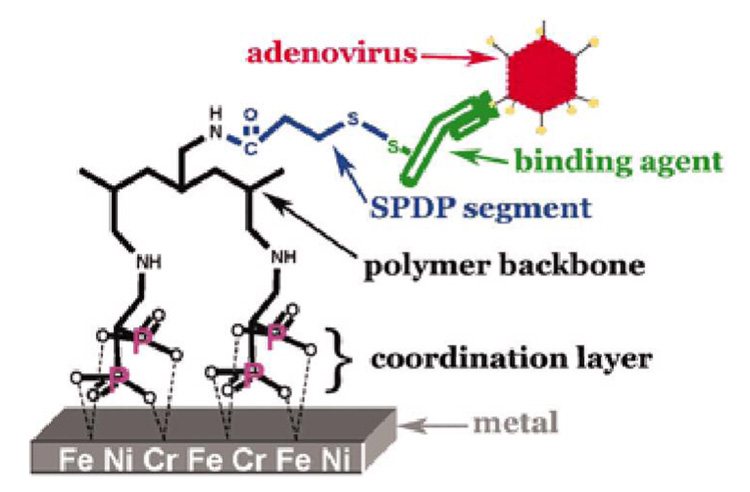
Recently, stent-based polymer coatings have been correlated with late thrombosis, inflammation, and restenosis,97–102 prompting some approaches to deliver bio-active agents from stent surfaces without coatings. Sirolimus-eluting stents have been reported without polymer coating.103, 104 Rapamycin is loaded onto stainless steel microporous stents by spray coating with rapamycin solutions. This drug-loaded stent produced significant inhibition of neointimal formation in a coronary artery stent model. Fishbein et al. reported adenovirus loaded directly onto metal stents without polymer coating.105 Because bisalkylphosphonates exhibit high-affinity binding activity to certain metallic oxide surfaces though phosphonate-metal coordination,106, 107 adenovirus vectors have been loaded on metal stents using polyallylamine grafted with bisphosphonate and modified with anti-adenovirus antibodies as shown in Figure 3. Local delivery of adenovirus encoding inducible NOS from the stent showed significant therapeutic effects following rat carotid stent implantation with inhibition of restenosis compared with bare metal stents.
Figure 3.
A schematic illustration of adenoviral vector conjugation to a bisphosphonate-modified metal surface for direct gene delivery upon deployment. Reprinted with permission from ref 105. Copyright 2006 National Academy of Sciences, U.S.A.
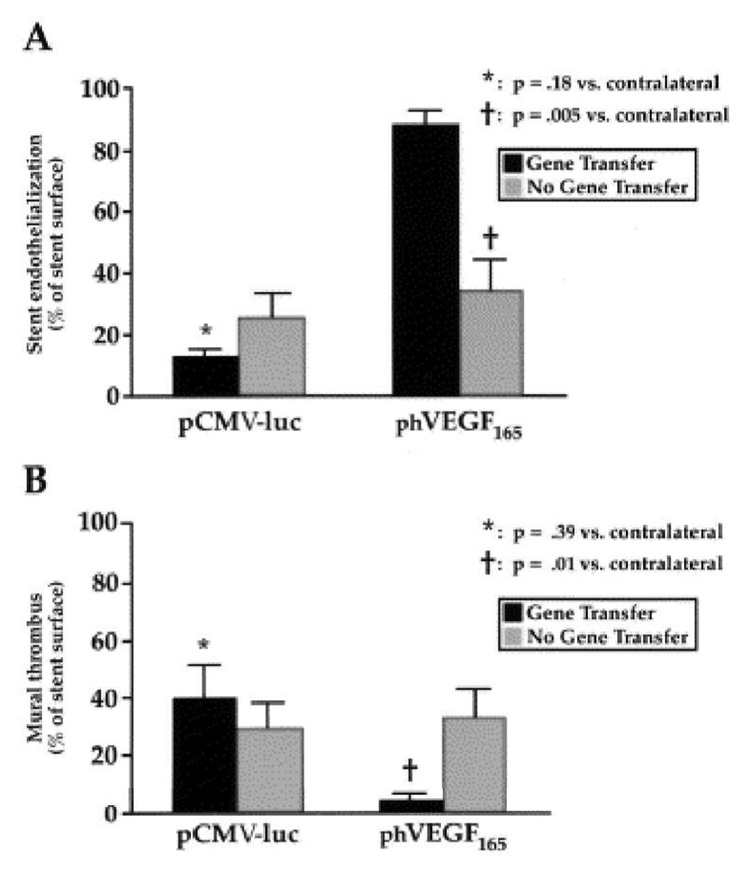
Phosphorylcholine (PC)-based co-polymer coatings have been reported to enhance stent blood- and bio-compatibility.108–110 Large numbers of PC-analog polymer coatings have been developed for DES as well as biodegradable polymer coatings,28, 29, 44, 54, 60, 61, 111, 112 including that currently commercialized by Abbott Labs’ DES (ENDEAVOR trial).20 Walter et al. reported local delivery of plasmid DNA encoding vascular endothelial growth factor-2 (VEGF-2) from PC-coated stents.113 Because of the acceleration of re-endothelialization,2, 4, 5 local delivery of VEGF is useful to reduce neointimal formation114, 115 as shown using catheters to deliver the VEGF gene at balloon-injured sites (see Figure 4).116, 117 In stent-based delivery, PC coating enabled loading of ~200 µg VEGF-2 plasmid, achieving a reduction of SMC proliferation in rabbit iliac arteries. Tissue inhibitor of metalloproteinase (TIMP) is also a potentially therapeutic gene against restenosis because it limits matrix metalloproteinase (MMP) activity and has direct effects on cell growth. In this regard, gene expression of TIMP in SMCs was reported to inhibit neointimal formation.118, 119 In particular, the study demonstrated that TIMP-3 was superior to TIMP-2 for inhibition of neointimal formation in pig vein grafts.120, 121 A follow-on study demonstrated that local delivery of adenovirus encoding TIMP-3 from PC-coated stents successfully reduced neointimal formation in a pig coronary model.122
Figure 4.
Effects of local phVEGF delivery on endothelialization (A) and mural thrombus (B) at stented sites in iliac arteries at 7 days after catheter injection. Reprinted with permission from ref 116 (as published in E. van Belle et al., “Passivation of metallic stents after arterial gene transfer of phVEGF165 inhibits thrombus formation and intimal thickening” in J. Am. Coll. Cardiol., 29, 1371–1379, Copyright: American College of Cardiology 1997).
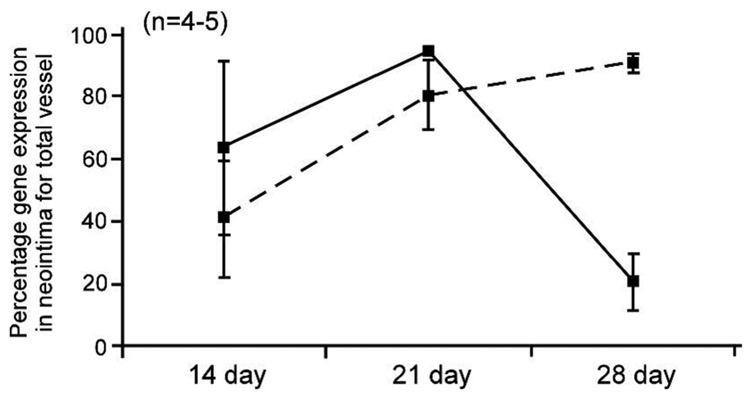
While adenoviral vectors have produced high gene expression in many studies using gene-eluting stents, more recently AAV has become a next-generation focus because of its high safety profile and preference in clinical trials to disease.83 Because AAV serotype 2 (AAV2) has been particularly investigated, Sharif et al. loaded AAV2 encoding model β-galactosidase onto PC-coated stents.123 Though no difference in transfection activity between AVV2-eluting and adenovirus-eluting stents was observed, release kinetics for up to 28 days distinguished the former from the latter approach (see Figure 5). Importantly, different AVV serotypes are expected to show different transfection activity depending on targeted cell and tissue types.76, 124 Hence, further study of AAV-eluting stents should better clarify specific effects of AAV on the potential inhibition of restenosis and other stent-associated issues.
Figure 5.
Difference in gene expression profiles between adenoviral and adeno-associated viral (AAV) delivery from their respective gene-eluting stents. Relative expression of the lacZ reporter gene released from adenovirus (dashed line, 109 PFU) or AAV-eluting stents (solid line, 109 drp) into the neointima of rabbit external iliac arteries at 14, 21 and 28 days following stent deployment. Adapted from ref 123.
Stent-based delivery allows lower doses of therapeutic genes compared with systematic gene delivery or even local catheter-based intravascular infusion. Control of the transgene dose and distribution is essential to improve and optimize the effect of gene delivery on restenosis. Local dose tissue processing, pharmacokinetics and expression duration of most delivered genes are virtually unknown. Systemic fate and viral vector distribution analysis is limited by method detection limits. As described above, stent coatings of biodegradable synthetic polymers, collagen and PC are primary techniques for gene-eluting stents to date. The polyelectrolyte layer-by-layer (LBL) technique is also becoming more useful for controlled local release of DNA to tissues and cells.125, 126 Polymer electrostatically based multilayer coating formation enables control over DNA loading and possibly transfection efficiency. Additionally, it is expected to protect plasmid DNA loaded on-stent from ubiquitous nuclease digestion.127, 128 A recent study demonstrated gene delivery from a stent coated with polyelectrolyte multilayers.81 Plasmid DNA encoding GFP was delivered from a multilayered film of the degradable cationic polymer, poly(β-amino ester) and DNA to cultured COS-7 cells in vitro, producing GFP expression. In other studies, Nakayama et al. developed a gelatin-based photocuring coating system triggered by visible laser.129 Brief laser irradiation on a stent immersed in gelatin solution produced a hydrogel coating on the stent by photopolymerization of styrene-grafted gelatin.130 Adenoviral vector encoding model β-galactosidase was delivered from the photocurable gelatin-coated stent to rabbit carotid arteries. Drug release rate from the photocurable gelatin was controlled by the crosslinking density and gel density.131 Thus, these stent coating strategies could be useful to achieve significant loading and controlled release of anti-restenotic genes from stents.
2.2 Antisense oligonucleotides
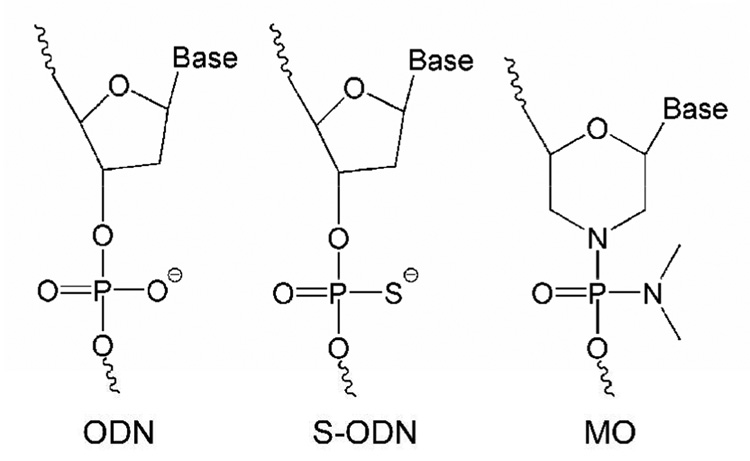
Antisense oligonucleotide (ODN) delivery can effectively modulate gene expression within cells, with therapeutic potential.132, 133 Antisense ODN interference in critical steps in the SMC growth cycle has been investigated using cdc2 kinase, proliferating-cell nuclear antigen,134 the ERK family of mitogen activated protein kinases (AMK1),135 early growth response factor-1 (Egr-1)136 and other targets. As noted for other antisense therapies, chemical modifications of antisense ODN influence antisense strategies for treatment of restenosis.133 Gunn et al. reported that because of their nuclease stability and relative ease of synthesis, phosphorothioate-ODN (S-ODN) (shown in Figure 6) may effectively reduce neointimal formation after PTCA, compared to unmodified ODNs.137 For example, S-ODN against Midkine successfully inhibited neointimal formation following stent implantation.138 To obtain high transfection activity, the antisense ODN was injected with LipofectAmine™ reagent (Invitrogen) directly to the injured area. However, it was concluded that Midkine antisense ODN effects were not sufficient to significantly suppress neointimal formation. That is, selection of the anti-restenosis therapeutic target is as important as ODN delivery efficiency to the target site. A very recent study reported that stent-based delivery of S-ODN targeted to platelet-derived growth factor (PDGF) A-chain significantly decreased in-stent restenosis in porcine coronary artery.139 Immersion of hydrogel-coated stents into a S-ODN solution containing polyethylenimine was used to produce antisense ODN-coated stents. Importantly, differences in endothelialization between antisense ODN-targeted PDGF A-chain and sirolimus-eluting stents were shown in vivo. In contrast to sirolimus that prevents not only SMC proliferation but also vascular re-endothelialization, resulting in late thrombosis, these data suggested that vascular delivery of PDGF A-chain did not interfere with endothelialization.
Figure 6.
Schematic representations of three common oligonucleotide analog chemistries used in therapeutics.
C-myc, a short-lived sequence-specific DNA-binding nuclear phosphoprotein and regulatory transcription factor, is the most effective target to date in antisense therapy for restenosis. Shi et al. reported that local delivery of antisense S-ODN against c-myc showed potential to reduce neointimal formation in a coronary artery balloon injury model.140 To achieve stent-based delivery of c-myc targeting S-ODN, the S-ODN (550 µg) was loaded into gelatin-coated platinum-iridium stents, and released into a rabbit carotid artery model.141 While local delivery of antisense S-ODNs against c-myc effectively inhibited SMC proliferation, Kutryk et al. reported that injection of 10 mg S-ODN directly into the stented site did not significantly reduce neointimal formation;142 one set of conclusions is that either S-ODN is not the most effective chemically modified ODN for inhibition of restenosis, or that soluble S-ODN injection is not as effective in producing a local effect versus coating-based delivery of the ODN-based therapeutic.
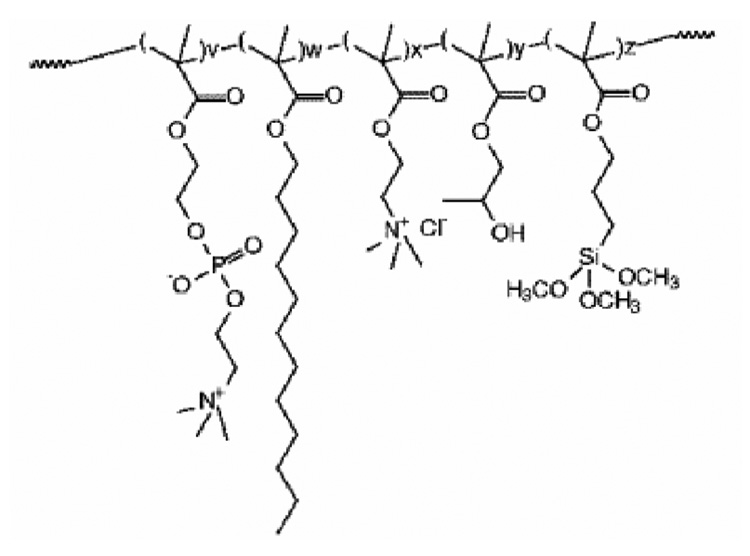
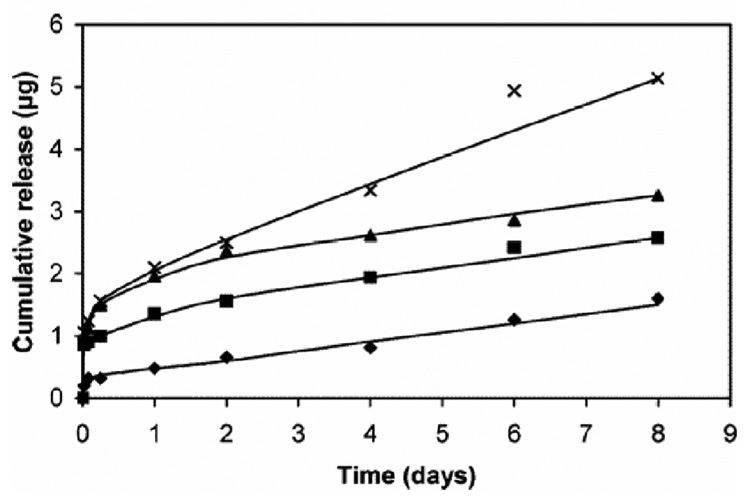
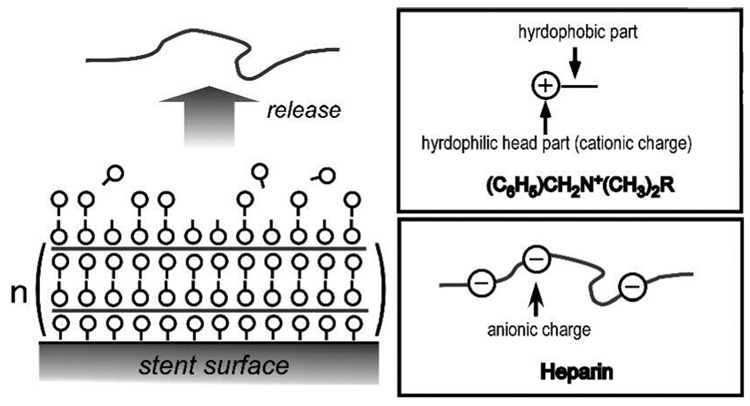
Morpholino-backbone antisense ODN against c-myc is also a potent alternative candidate therapeutic drug against restenosis.143–145 As neutrally charged nucleic acid analogue oligomers, morpholino nucleotides (MOs) represent an unusual DNA chemistry with a nucleobase tethered to a six-membered morpholine ring and uncharged phosphorodiamidate linkage (compare structures in Figure 6).133, 146 As MOs have high nuclease resistance and antisense activity against mRNA, inhibiting translation, Summerton reported that MO antisense activity often achieved equal or better efficacy compared with S-ODNs.146 Kipshidze and co-workers used antisense MOs against c-myc for inhibition of neointimal formation in the porcine coronary model, confirming that catheter-based local delivery of antisense MOs significantly reduced neointimal formation.143, 144 Moreover, they performed stent-based delivery of antisense MOs by immersing a polymer-coated stent into 30 mg/mL MO solution for 5min, yielding 81.5 ± 14.4 µg of antisense MOs on a PC-coated stent.145 MO charge neutrality protected these antisense ODNs from interactions with charged biological species (proteins, lipids) perhaps providing more efficient interactions with target mRNA to enhance cellular uptake of c-myc antisense ODNs. Recent studies, in contrast, used conventional antisense S-ODNs with a cationic PC copolymer to control ODN loading and release147, 148 using a previously reported PC copolymer.149, 150 Cationic charge density was controlled by co-polymerizing cationic co-monomers with choline methacrylate as shown in Figure 7. The loading amount and the release kinetics of S-ODNs were controllable by the combined use of S-ODN and cationic PC coating as shown in Figure 8. In contrast to the MO utility, considering that drug loading is a key factor for drug-eluting stent efficacy, the S-ODN anionic charge density could be useful to optimize stent-based delivery.
Figure 7.
Molecular structure of synthetic cationic phosphorylcholine (PC)-based copolymers used for stent-based release coatings. Reprinted with permission from ref 147. Copyright 2006 American Chemical Society.
Figure 8.
Controlled release of antisense S-ODN from different PC copolymer films (choline methacrylate monomer content: 0% ◆; 5% ■; 10% ▲; 20% ×) into PBS buffer at pH 7.4. Reprinted with permission from ref 147. Copyright 2006 American Chemical Society.
2.3 Decoy ODNs
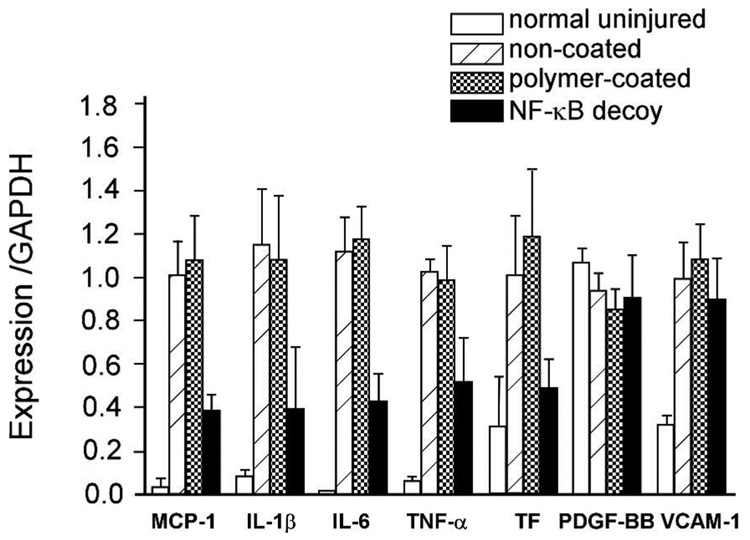
While several antisense strategies have been investigated to inhibit restenosis, therapy using decoy ODNs also provides efficient cell cycle modulation for treating certain cardiovascular diseases.151 Nuclear factor-kappa B (NF-kB) is a most popular target against restenosis in the ODN decoy strategy.152–154 NF-kB plays a critical role in coordinated gene trans-activation of cytokines and cell adhesion molecules. Kitamoto et al. reported that transfection of NF-kB decoy ODNs into coronary arteries after coronary angioplasty inhibited SMC proliferation.152 Clinical application of the NF-kB decoy in reducing restenosis after PTCA was until now investigated in only two patients.154 In experimental studies, Kalinowski et al. reported that NF-kB decoy delivery using a balloon catheter did not significantly reduce neointimal formation in rabbit iliac arteries,155 and Radke et al. loaded decoy NF-kB ODNs onto stents coated with a cationic PC copolymer for better control of loading and release evidenced by successful ex vivo ODN deposition in the vessel wall.156 As for antisense ODNs against c-myc,147, 148 ODN loading amount was controlled by cationic charge density in the polymer coating and the ODN concentration. As a result, a PC copolymer coating containing 20% cationic co-monomer was maximally loaded with 41 ± 6 µg of decoy ODNs (3 × 1012 ODN molecules) on-stent. This decoy ODN-eluting stent provided significant antisense transfer into vessel walls in ex vivo experiments. However, this did not result in significant reduction of SMC proliferation after stent implantation in vivo. These data suggest that rapid intravascular release of ODNs prior to or concomitant with stent implantation, and penetration through other potential physiological barriers to antisense transfer (e.g., lesions, thrombus, cholesterol plaques) must be improved to obtain a significant effect on restenosis. On the other hand, Ohtani et al. observed that stent-based local delivery of NF-kB decoy reduced in-stent neointimal formation in iliac arteries of hypercholesterolemic rabbits.157 This study reported that rapid release (<7 days) of NF-kB decoy from polyurethane-coated stents inhibited in-stent neointimal formation. Their results, shown in Figure 9, indicated that stent-based delivery of NF-kB decoy reduced NF-kB-dependent gene expression (e.g. monocyte chemoattractant protein-1 (MCP-1), interleukin-1, interleukin-6) but not for NF-kB-independent genes (e.g., PDGF).
Figure 9.
Effects of NF-kB decoy-eluting stents on vascular wall mRNA levels of various pro-inflammatory factors and tissue factor (TF) at 10 days after stenting (MCP: monocyte chemoattractant protein, IL: interleukin, TNF: tumor necrosis factor, PDGF: platelet-derived growth factor, VCAM: vascular cell adhesion molecule). Adapted from ref 157.
Currently, many potent new targets against restenosis have been discovered and advocated. This means that various types of therapeutic approaches and molecules now available must be matched with specific drug-eluting techniques required to produce optimal efficacy in vivo. DES therapeutic value will strongly depend on the balance of loaded drug pharmacology, release kinetics, loading method (coating polymer), site of deployment, basic stent platform, and patient variables. Stent-based delivery of plasmid DNA encoding NOS, antisense ODN against c-myc and NF-kB decoys have been investigated in pre-clinical trials, but with mixed, equivocal results. Data to date do not clearly suggest a rational route to improve these results, but progress with these biological drugs will allow extension of the techniques to other therapeutic agents.
3. Protein therapeutics
3.1 Therapeutic antibodies and affinity protein ligands
Over 20% of new drug applications are antibody-based therapeutics. It is likely that this highly successful drug class will find application in DES. Accumulation of activated inflammatory cells and release of chemotactic inflammatory mitogenic cytokines upon acute injury from stent implantation are important factors inducing SMC mobilization, proliferation and migration. Thus, blockade of local inflammatory responses is a common technique to attempt to inhibit neointimal formation.4, 7 For example, the inflammatory cytokine, tumor necrosis factor-α (TNF-α) is expressed by SMCs in balloon-injured sites, activating SMC migration.158 Blockade of TNF-α with a soluble TNF-α receptor efficiently inhibited coronary neointimal formation.159 A monoclonal anti-TNF-α antibody exhibited high anti-proliferative activity, resulting in significant reduction of SMC proliferation by neutralization of TNF-α.160 Antibody loading was achieved by immersion of a cellulose polymer-coated stent in anti-TNF-α antibody solution.161 About 0.25 µg of antibody per mg of stent achieved marked reduction of SMC proliferation in human saphenous vein organ cultures. Specific neutralization of SMC TNF-α by the antibody might avoid unwanted side effects in other cells.160 As expensive, large (~160kDa globular proteins) glycosylated therapeutics, antibodies require new delivery strategies for specialized local delivery;162 stent-based delivery of therapeutic antibodies has a large unexplored potential against numerous targets.
Anti-platelet therapy potentially reduces intimal formation induced by platelet aggregation. Platelet glycoprotein receptor GP II b/III a has an important role in platelet activation and remains a clinical target to inhibit thrombosis.163 Monoclonal antibodies against the human GP II b/III a receptor provide significant inhibition of platelet aggregation when systemically infused in human therapies (abciximab, c7E3, ReoPro™, Centocor Inc.). An anti-rabbit GP II b/III a receptor antibody, AZ1, has been used to inhibit platelet function at vascular injury sites.164 Aggarwal et al. reported local delivery of AZ1 from cellulose polymer-coated stents loaded by direct immersion of cellulose-coated stents into AZ1 coating in solution.165 After 24 h incubation in 1 mg/mL of AZ1 coating solution, antibody density of 104.3 ± 1.3 ng/10-mm stent wire segment with more than 14 days of continuous release was achieved. However, this stent-based delivery system did not significantly inhibit neointimal formation in vivo.
Blockade of the αvβ3 integrin (vitronectin receptor) on SMCs efficiently inhibits SMC proliferation and migration.163 Cyclic RGD peptide binds to αvβ3 integrin, reducing neointimal formation where delivered.49 Cyclic RGD-eluting stent implantation effectively inhibits neointimal hyperplasia.55 The human monoclonal antibody against human platelet GP II b/III a, abciximab (c7E3, ReoPro™), also has ability to bind cell vitronectin receptors including αvβ3 integrin because of its relatively non-specific binding property.163, 166 Thus, abciximab with intrinsic high anti-platelet activity administered by intravenous dose has been used in stent implantation.167 However, the delivery strategy must be considered to optimize significant benefits.168 Considering that the rabbit AZ1 anti-platelet antibody has no measurable affinity against the cell vitronectin receptor165 and produced no anti-restenotic activity in vivo, selective receptor targeting abilities to produce effective therapy could be very subtle for the success of anti-platelet agents: binding activity to SMC vitronectin receptor may be clinically advantageous to inhibit both platelet aggregation and neointimal hyperplasia in situ. Abciximab-loaded polymer-coated GR II stents (Cook Inc, USA) produced slow antibody release from the stent (approximate 50% stent release over 12 days) by simple immersive loading.169 Abciximab has also been covalently attached to stent surfaces using plasma polymerization.170, 171 The loading produced 90 µg/stent of slow antibody release, likely by hydrolysis. In vivo antibody bioactivity remains unknown. While antibody doses and optimal release kinetics are virtually unknown, stent-based delivery allows much lower antibody dosing locally (µg) compared with systemic doses (>>mg) but, due to the fragility of these globular protein therapeutics, specific assays of antibody bioactivity post-release must be carefully assessed.162
3.2 Release of vascular endothelial growth factor (VEGF)
Rapid vessel re-endothelialization is an attractive strategy to inhibit SMC proliferation.4, 7 Both host endogenous circulating endothelial cells (ECs) and endothelial progenitor cells (EPCs) are actively recruited to the stented site for re-endothelialization.172–174 Chemotactic mitogenic cytokines such as basic fibroblast growth factor (bFGF) and VEGF can act in this capacity as potent mitogenic agents of ECs and EPCs.89, 175 Local delivery of VEGF114, 115 and plasmid DNA encoding VEGF113, 116, 117 was shown to prevent restenosis by acceleration of neovascularization and re-endothelialization at the injured site. Stent-based VEGF delivery was also performed by immersion of polymer-coated stents into VEGF aqueous protein solutions loading 18.5 ± 4.1 µg of VEGF on a 3 × 20 mm stent after 2 h immersion.176 Slow VEGF release from the stent increased growth of cultured human umbilical vein endothelial cells (HUVECs) in vitro.
In most stent-based delivery systems, drug loading (dose) is a critical factor due to the limited on-stent drug loading capacity (i.e., wire coating). Dosing relationships and kinetics of release necessary to produce long-term efficacy are often thought to be dose-limited. In addition, most protein drugs are low stability, large molecular weight, large dosage-amount agents with difficult formulation requirements.177, 178 This has produced a shift from direct delivery of the therapeutic protein to delivery of the transgene coding that protein, with expected longer duration of protein dose at the locally transfected site. However, compared with delivery of plasmid DNA encoding VEGF, VEGF protein delivery has the advantage of asserting the effect of the known dose of delivered VEGF. In vitro efficacy of VEGF-eluting stents in promoting endothelial cell growth does not correlate with insignificant effects of the VEGF-eluting stent system in vivo using a rabbit iliac artery model.176 Recently, use of a thermo-responsive polymer as a VEGF release vehicle was reported to improve dosing and efficacy.179 Additionally, use of truncated, lower molecular weight recombinant protein fragments with the desired potency and improved stability (e.g., VEGF121, VEGF165)115, 180 could improve stent-based delivery approaches.
3.3 Protein C delivery from stent
Protein C and protein S manifest important roles in anti-coagulation and normal hemostatic balance.181 Thus, their local delivery at vascular injury sites is attractive for the inhibition of thrombosis. Protein C is activated by thrombin-thrombomodulin complexes on intact endothelial cell surfaces, prompting anti-thrombotic activity. Foo et al. performed stent-based delivery of protein C in a balloon injury animal model using a stent coated with cellulose polymer and then immersed in buffer solution containing protein C for 60 min.161 Local delivery of protein C produced significant inhibition of thrombosis in a rabbit iliac artery model.
4. Polysaccharide agents
Due partly to its extensive history in coating blood-contacting devices, the natural sulfated glycosaminoglycan anticoagulant, heparin, has been especially attractive as a stent coating, attempting to exploit its intrinsically high anti-coagulant activity through neutralization of thrombin by interaction with antithrombin III182, 183 even in clinical trials with mixed results.183–186 In a great deal of such studies, heparin coating is achieved by covalent coupling of aldehyde groups created on heparin by mild oxidation (or under saccharide aldol equilibria) to amino groups attached on stents.182, 183, 185, 187 Such covalent coupling produces a surface density and interaction between immobilized heparin and circulating antithrombin III. Heparin blending with other polymers as a coating produced a reduction of stent thrombosis.89, 188 Recently, combined use of heparin coating and injection of abciximab showed more effective activity against coagulation than a heparin-coated stent alone or abciximab alone.189
However, it is likely that heparin delivery could have other cell-specific pharmacological effects. Heparin specifically inhibits a protein kinase C-dependent cell cycle pathway in SMCs.190, 191 Moreover, it interacts with cell receptors, growth factors, adhesion molecules, resulting in prevention of SMC migration and proliferation.191 As many of these activities require soluble heparin to bind cell receptors or penetrate intra-cellularly, binding of heparin to stents is unfavorable to exert any anti-proliferative activity. Thus, heparin’s anti-proliferative activity was evaluated by local delivery to the injury site,192 and compared with that of rapamycin.193, 194 To achieve stent-based delivery of heparin, Matsumoto et al. created multilayers of heparin and cationic molecules via layered electrostatic interactions, depicted in Figure 10.195 Following in vivo stent implantation, immobilized heparin successfully released from the stent: multilayer coating enabled not only slow heparin release but also by control of heparin loading.
Figure 10.
Schematic illustrations of multiple coated layers of releasable heparin complexes on implantable stents. Adapted from ref 195.
Because of its longer half-life, higher dosing possible, and extended bioavailability compared with unfractionated heparin (approximately 15 kDa), low molecular weight heparin (LMWH, ~ 2–9 kDa) is expected to provide more effective inhibition of restenosis than unfractionated heparin.196–198 Koromila et al. recently approached local LMWH delivery from a stent using liposomes to achieve significant LMWH delivery from a polyethylene terephthlate (PET)-coated stent by stent immersion into liposome solutions.199 LMWH release from the stent was controllable, depending on liposome lipid composition, and liposome type (dehydrated-rehydrated vesicles versus multi-lamellar vesicles). Combinations of LMWH and liposome encapsulation could be useful as a stent-based delivery system to prevent restenosis, avoiding known systemic LMWH systemic problems (thrombocytopenia) with minimal, local doses.
Other types of polysaccharide could also be used for stent coating. Local delivery of hyaluronan (hyaluronic acid; HA) from a stent or coating on stent inhibited platelet thrombus formation.200–202 Dextran is another polysaccharide that could act as an anti-platelet agent,203, 204 and also after chemical modification inhibit SMC proliferation as evidenced by direct intralumenal injection at a stented site in rabbit iliac arteries.205 Fucoidan, a sulfated polysaccharide extracted from brown seaweed, also shows promise as a potent therapeutic agent.206 Some studies suggest differences in anti-proliferative activities between fucoidan and heparin (e.g., effects on mitogenesis induced by fetal calf serum and platelet-derived growth factor BB homodimer in human vascular SMCs). Thus, fucoidan in various molecular weight forms might prove to be a more anti-proliferative agent than heparin.207–209 Deux et al. reported inhibition of neointimal hyperplasia by intralumenal injection of LMW fucoidan to injury sites in iliac arteries.210 This evidence supports the use of local delivery of LMW fucoidan from stents as a useful strategy against restenosis.
5. Cell-based therapies
Cell-based therapeutics represent a rapidly advancing technique to treat cardiovascular diseases.211 Nonetheless, cell sourcing and functional reliability remain issues that could delay the transition of cell therapy from an attractive experimental tool to a comprehensive therapeutic application. Ex vivo gene therapy is a common method to treat autologous cells with a transgene and then implant the transfected cells back into the host. This approach was used to produce a transfected SMC-coated stent with stable gene expression.212 In this study, plasmid DNA encoding GFP was transfected into SMCs, and the SMCs were then seeded onto a mesh-stent. Using fibronectin for stent coating, more than 20-fold increase in cell seeding was realized on the stent, compared to an uncoated stent. No evidence of SMC migration from the stent was detected, and stable gene expression was shown for 1 month after stent implantation.
As EPCs have an important role in endothelial repair and enhance re-endothelialization after vessel trauma in PTCA,172–174 EPCs were seeded onto a stent to accelerate re-endothelialization at the stented site by their migration and proliferation.213, 214 An EPC-collagen hybrid material was formed by incubation of EPCs with collagen gel for one week. By insertion of a metal stent into this mixture, cell loading (6.3 ± 2.5 ×105 cells on 2-cm of stent) was achieved.213 As shown in Figure 11, EPCs covered the stent surface. EPCs delivered from a gelatin-coated stent migrated from these seeded stents, forming an endothelialized luminal surface.214 While EPC seeding on a stent promotes re-endothelialization, Aoki et al. immobilized anti-CD34 antibody specific to EPC cell surface markers onto a stent.215 Captured cells from host circulation covered more than 90% of the stent surface 1 h after stent implantation, suggesting that EPC capture provides significant clinical feasibility against restenosis. Recently, to exert maximal potential, new materials were used to improve EC seeding on stent-based templates.216 Cell-based approaches provide the benefits of autologous cell seeding or endogenous recruitment, combination therapies with stent-based drug delivery systems to suppress side effects and possible alternative cell sourcing for stent coverage in the future. However, while EPCs comprise a significant fraction of circulating cells in animal models, EPCs represent a very small fraction of circulating cells in humans (e.g., less than 0.1%)217, 218 and further development of this idea is required to demonstrate clinical potential.
Figure 11.
Endothelial progenitor cell (EPC)-seeded stents attached to a balloon catheter (A, B). Fluorescence images of an EPC-seeded stent (C, D) observed by nuclear staining of EPCs with Picogreen™ dye. Original magnification: ×40 (C), ×20 (D). Bar = 1 mm. Reprinted with permission from ref. 214 (as published in T. Shirota et al., “Fabrication of endothelial progenitor cell (EPC)-seeded intravascular stent devices and in vitro endothelialization on hybrid vascular tissue” in Biomaterials, 24, 2295–2302, Copyright: Elsevier 2003).
6. Nanotechnology approaches
Biodegradable nano- and micro-spheres enable controlled release of therapeutic drugs219, 220 and can be combined within DES coatings with combinations of other drug forms. Potent anti-restenotic agents, tyrphostin compounds (AG-1295, AGL-2043) encapsulated in PLA nanospheres were released in a size-dependent manner.221, 222 In this regard, stent-based controlled release of anti-restenotic drugs such as angiostatin223 and NO donors52 formulated within PLGA microspheres was performed from DES. Intraluminal delivery of AGL-2043 in PLA nanospheres decreased neointimal formation in rat carotid arteries in comparison to systemically administrated free AGL-2043.222 Kolodgie et al. used nanospheres to stabilize the paclitaxel bioactivity in systemic parenteral dosing.224 Stabilization of protein drugs is particularly important in their controlled release because proteins easily lose bioactivities when formulated. Biodegradable nano- and micro-spheres are known to stabilize encapsulated proteins.177, 178 Degradable poly(DL-lactide-co-glycolide) (PDLGA) microspheres containing albumin (66kDa) as a model protein were loaded onto a PLLA stent by dipping in microsphere powders and release was shown to depend on microsphere structure.225 Thus, nano- and micro-spheres may improve anti-restenotic effects of proteins and other biopharmaceutical drugs delivered locally from stents.
Nanoparticle surfaces can also be modified with various functional molecules, providing specific targeted, labeled and long-circulating nanoparticles.226 For instance, tissue factor (TF)-targeted nanoparticles modified with anti-TF antibody specifically bind to SMC membranes in vivo. TF-modified perfluorocarbon nanoparticles containing paclitaxel delivered to cultured SMCs decreased cell proliferation compared to non-targeted nanoparticles.227 Iron oxide particles were used to magnetically localize ECs to stents.228 Endothelial outgrowth cells (EOCs) labeled with magnetic particles within their cytoplasm interacted with a magnetized stent and captured on the stent surface. Following the stent implantation in pig coronary arteries, the EOCs labeled with the microspheres localized on the stented site.
7. Conclusions
A wide array of new, complex drug forms, primarily biopharmaceuticals comprising proteins, nucleic acids and cell therapies, are emerging as attractive new cardiovascular therapeutic candidates with new, specific stent-based delivery requirements for DES applications. Many of these new drugs are substantially larger in size (molecular weights ~ 103 – 106 Da) than current drugs typically used on DES systems (<103 Da). This means that large drug dosing within the DES coating or on surfaces will be even more limited than for current drugs because of these unusual drug sizes, physical chemistry, and stent loading restrictions. DES delivery of these new molecules will require new designs and extensive new stability and pharmacological analyses. Because of the unique targets and bioactivities of such biopharmaceuticals, improvements in desired anti-proliferative, anti-coagulant, pro-endothelialization, bio- and blood compatibility and/or biodegradability are expected. Increased efficacy could also result from increased specificity and limited side effects, improving local pharmacology compared to current FDA-approved small molecular weight agents. However, it is likely that drugs alone will not solve all clinical stenting issues. Further DES developments will likely combine other emerging innovations in advanced therapeutics involving related new drug classes and cell therapies relevant to future DES delivery, as well as implement new biomaterials, surgical and stent design improvements. To bring these agents forward toward clinical use, creative approaches to formulating, testing and understanding the mechanisms of action of these agents in the DES context is required. Randomized, blinded trials that assess stent clinical efficacy without bias in study designs will be required to fully assess performance enhancements.229, 230
Acknowledgements
This work was supported by a Research Fellowship from the Japan Society for Promotion of Science (JSPS) for Young Scientists (to H.T) and NIH grant EB00894 (to D.W.G.).
References
- 1.Hehrlein C, Arab A, Bode C. Basic Res. Cardiol. 2002;97:417–423. doi: 10.1007/s00395-002-0379-2. [DOI] [PubMed] [Google Scholar]
- 2.Fattori R, Piva T. Lancet. 2003;361:247–249. doi: 10.1016/S0140-6736(03)12275-1. [DOI] [PubMed] [Google Scholar]
- 3.Burt HM, Hunter WL. Adv. Drug Deliv. Rev. 2006;58:350–357. doi: 10.1016/j.addr.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 4.Scott NA. Adv. Drug Deliv. Rev. 2006;58:358–376. doi: 10.1016/j.addr.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 5.Bennett MR, O'Sullivan M. Pharmacol. Ther. 2001;91:149–166. doi: 10.1016/s0163-7258(01)00153-x. [DOI] [PubMed] [Google Scholar]
- 6.Sousa JE, Serruys PW, Costa MA. Circulation. 2003;107:2274–2279. doi: 10.1161/01.CIR.0000069330.41022.90. [DOI] [PubMed] [Google Scholar]
- 7.Kavanagh CA, Rochev YA, Gallagher WA, Dawson KA, Keenan AK. Pharmacol. Ther. 2004;102:1–15. doi: 10.1016/j.pharmthera.2003.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Faxon DP. Circulation. 2002;106:2296–2298. doi: 10.1161/01.cir.0000038412.38399.d5. [DOI] [PubMed] [Google Scholar]
- 9.Windecker S, Roffi M, Meier B. Curr. Pharm. Design. 2003;9:1077–1094. doi: 10.2174/1381612033455107. [DOI] [PubMed] [Google Scholar]
- 10.Koster R, Vieluf D, Kiehn M, Sommerauer M, Kahler J, Baldus S, Meinertz T, Hamm CW. Lancet. 2000;356:1895–1897. doi: 10.1016/S0140-6736(00)03262-1. [DOI] [PubMed] [Google Scholar]
- 11.De Scheerder I, Szilard M, Yanming H, Ping XB, Verbeken E, Neerinck D, Demeyere E, Coppens W, Van de Werf F. J. Invasive Cardiol. 2000;12:389–394. [PubMed] [Google Scholar]
- 12.Gutensohn K, Beythien C, Bau J, Fenner T, Grewe P, Koester R, Padmanaban K, Kuehnl P. Thromb. Res. 2000;99:577–585. doi: 10.1016/s0049-3848(00)00295-4. [DOI] [PubMed] [Google Scholar]
- 13.Linder S, Pinkowski W, Aepfelbacher M. Biomaterials. 2002;23:767–773. doi: 10.1016/s0142-9612(01)00182-x. [DOI] [PubMed] [Google Scholar]
- 14.Monnink SH, van Boven AJ, Peels HO, Tigchelaar I, de Kam PJ, Crijns HJ, van Oeveren W. J. Investig. Med. 1999;47:304–310. [PubMed] [Google Scholar]
- 15.Haase J, Storger H, Hofmann M, Schwarz CE, Reinemer H, Schwarz F. J Invasive Cardiol. 2003;15:562–565. [PubMed] [Google Scholar]
- 16.Airoldi F, Colombo A, Tavano D, Stankovic G, Klugmann S, Paolillo V, Bonizzoni E, Briguori C, Carlino M, Montorfano M, Liistro F, Castelli A, Ferrari A, Sgura F, Di Mario C. Am. J. Cardiol. 2004;93:474–477. doi: 10.1016/j.amjcard.2003.10.048. [DOI] [PubMed] [Google Scholar]
- 17.Colombo A, Airoldi F. J. Invasive Cardiol. 2003;15:566–567. [PubMed] [Google Scholar]
- 18.Hiatt BL, Ikeno F, Yeung AC, Carter AJ. Catheter. Cardiovasc. Interv. 2002;55:409–417. doi: 10.1002/ccd.10161. [DOI] [PubMed] [Google Scholar]
- 19.Sousa JE, Serruys PW, Costa MA. Circulation. 2003;107:2383–2389. doi: 10.1161/01.CIR.0000069331.67148.2F. [DOI] [PubMed] [Google Scholar]
- 20.Carter AJ. Catheter. Cardiovasc. Interv. 2005;66:496–498. doi: 10.1002/ccd.20490. [DOI] [PubMed] [Google Scholar]
- 21.Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O'Shaughnessy C, Caputo RP, Kereiakes DJ, Williams DO, Teirstein PS, Jaeger JL, Kuntz RE. N. Engl. J. Med. 2003;349:1315–1323. doi: 10.1056/NEJMoa035071. [DOI] [PubMed] [Google Scholar]
- 22.Stone GW, Ellis SG, Cannon L, Mann JT, Greenberg JD, Spriggs D, O'Shaughnessy CD, DeMaio S, Hall P, Popma JJ, Koglin J, Russell ME. Jama. 2005;294:1215–1223. doi: 10.1001/jama.294.10.1215. [DOI] [PubMed] [Google Scholar]
- 23.Hong MK, Mintz GS, Lee CW, Song JM, Han KH, Kang DH, Song JK, Kim JJ, Weissman NJ, Fearnot NE, Park SW, Park SJ. Circulation. 2003;107:517–520. doi: 10.1161/01.cir.0000054163.42072.d4. [DOI] [PubMed] [Google Scholar]
- 24.Gershlick A, De Scheerder I, Chevalier B, Stephens-Lloyd A, Camenzind E, Vrints C, Reifart N, Missault L, Goy JJ, Brinker JA, Raizner AE, Urban P, Heldman AW. Circulation. 2004;109:487–493. doi: 10.1161/01.CIR.0000109694.58299.A0. [DOI] [PubMed] [Google Scholar]
- 25.Halkin A, Stone GW. J. Interv. Cardiol. 2004;17:271–282. doi: 10.1111/j.1540-8183.2004.04040.x. [DOI] [PubMed] [Google Scholar]
- 26.Wessely R, Schomig A, Kastrati A. J. Am. Coll. Cardiol. 2006;47:708–714. doi: 10.1016/j.jacc.2005.09.047. [DOI] [PubMed] [Google Scholar]
- 27.Costa RA, Lansky AJ, Mintz GS, Mehran R, Tsuchiya Y, Negoita M, Gilutz Y, Nikolsky E, Fahy M, Pop R, Cristea E, Carlier S, Dangas G, Stone GW, Leon MB, Muller R, Techen G, Grube E. Am. J. Cardiol. 2005;95:113–116. doi: 10.1016/j.amjcard.2004.08.074. [DOI] [PubMed] [Google Scholar]
- 28.Fajadet J, Wijns W, Laarman GJ, Kuck KH, Ormiston J, Munzel T, Popma JJ, Fitzgerald PJ, Bonan R, Kuntz RE. Circulation. 2006;114:798–806. doi: 10.1161/CIRCULATIONAHA.105.591206. [DOI] [PubMed] [Google Scholar]
- 29.Kandzari DE, Leon MB. J. Interv. Cardiol. 2006;19:405–413. doi: 10.1111/j.1540-8183.2006.00184.x. [DOI] [PubMed] [Google Scholar]
- 30.Hofma SH, van der Giessen WJ, van Dalen BM, Lemos PA, McFadden EP, Sianos G, Ligthart JM, van Essen D, de Feyter PJ, Serruys PW. Eur. Heart J. 2006;27:166–170. doi: 10.1093/eurheartj/ehi571. [DOI] [PubMed] [Google Scholar]
- 31.Oyabu J, Ueda Y, Ogasawara N, Okada K, Hirayama A, Kodama K. Am. Heart J. 2006;152:1168–1174. doi: 10.1016/j.ahj.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 32.Lang NN, Newby DE. Arterioscler. Thromb. Vasc. Biol. 2007;27:261–262. doi: 10.1161/01.ATV.0000255308.41576.ae. [DOI] [PubMed] [Google Scholar]
- 33.Muldowney JA, 3rd, Stringham JR, Levy SE, Gleaves LA, Eren M, Piana RN, Vaughan DE. Arterioscler. Thromb. Vasc. Biol. 2007;27:400–406. doi: 10.1161/01.ATV.0000254677.12861.b8. [DOI] [PubMed] [Google Scholar]
- 34.Moussa I, Leon MB, Baim DS, O'Neill WW, Popma JJ, Buchbinder M, Midwall J, Simonton CA, Keim E, Wang P, Kuntz RE, Moses JW. Circulation. 2004;109:2273–2278. doi: 10.1161/01.CIR.0000129767.45513.71. [DOI] [PubMed] [Google Scholar]
- 35.de Araujo Goncalves P, Seabra-Gomes R, Teles R, Almeida M, Aguiar C, Raposo L, Ferreira J, Pereira Machado F. Heart. 2006;92:1155–1156. doi: 10.1136/hrt.2005.079780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jimenez-Quevedo P, Sabate M, Angiolillo DJ, Costa MA, Alfonso F, Gomez-Hospital JA, Hernandez-Antolin R, Banuelos C, Goicolea J, Fernandez-Aviles F, Bass T, Escaned J, Moreno R, Fernandez C, Macaya C. J. Am. Coll. Cardiol. 2006;47:2172–2179. doi: 10.1016/j.jacc.2006.01.063. [DOI] [PubMed] [Google Scholar]
- 37.Agostoni P, Biondi-Zoccai GG, Gasparini GL, Anselmi M, Morando G, Turri M, Abbate A, McFadden EP, Vassanelli C, Zardini P, Colombo A, Serruys PW. Eur. Heart J. 2005;26:881–889. doi: 10.1093/eurheartj/ehi116. [DOI] [PubMed] [Google Scholar]
- 38.Colombo A, Moses JW, Morice MC, Ludwig J, Holmes DR, Jr, Spanos V, Louvard Y, Desmedt B, Di Mario C, Leon MB. Circulation. 2004;109:1244–1249. doi: 10.1161/01.CIR.0000118474.71662.E3. [DOI] [PubMed] [Google Scholar]
- 39.Sharma SK. Catheter. Cardiovasc. Interv. 2005;65:10–16. doi: 10.1002/ccd.20363. [DOI] [PubMed] [Google Scholar]
- 40.Ge L, Tsagalou E, Iakovou I, Sangiorgi GM, Corvaja N, Airoldi F, Chieffo A, Montorfano M, Michev I, Colombo A. Am. J. Cardiol. 2005;95:757–760. doi: 10.1016/j.amjcard.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 41.Schainfeld RM. Catheter. Cardiovasc. Interv. 2002;56:421–431. doi: 10.1002/ccd.10211. [DOI] [PubMed] [Google Scholar]
- 42.Krueger KD, Mitra AK, DelCore MG, Hunter WJ, 3rd, Agrawal DK. J. Clin. Pathol. 2006;59:575–579. doi: 10.1136/jcp.2004.025643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Machan L. Adv. Drug Deliv. Rev. 2006;58:447–462. doi: 10.1016/j.addr.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 44.Liu X, Huang Y, Hanet C, Vandormael M, Legrand V, Dens J, Vandenbossche JL, Missault L, Vrints C, De Scheerder I. Catheter. Cardiovasc. Interv. 2003;60:172–178. doi: 10.1002/ccd.10636. [DOI] [PubMed] [Google Scholar]
- 45.Serruys PW, Ormiston JA, Sianos G, Sousa JE, Grube E, den Heijer P, de Feyter P, Buszman P, Schomig A, Marco J, Polonski L, Thuesen L, Zeiher AM, Bett JH, Suttorp MJ, Glogar HD, Pitney M, Wilkins GT, Whitbourn R, Veldhof S, Miquel K, Johnson R, Coleman L, Virmani R. J. Am. Coll. Cardiol. 2004;44:1363–1367. doi: 10.1016/j.jacc.2004.03.084. [DOI] [PubMed] [Google Scholar]
- 46.Sarkar R, Meinberg EG, Stanley JC, Gordon D, Webb RC. Circ. Res. 1996;78:225–230. doi: 10.1161/01.res.78.2.225. [DOI] [PubMed] [Google Scholar]
- 47.Rizvi MA, Myers PR. J. Mol. Cell. Cardiol. 1997;29:1779–1789. doi: 10.1006/jmcc.1996.0480. [DOI] [PubMed] [Google Scholar]
- 48.Guo JP, Milhoan KA, Tuan RS, Lefer AM. Circ. Res. 1994;75:77–84. doi: 10.1161/01.res.75.1.77. [DOI] [PubMed] [Google Scholar]
- 49.Matsuno H, Stassen JM, Vermylen J, Deckmyn H. Circulation. 1994;90:2203–2206. doi: 10.1161/01.cir.90.5.2203. [DOI] [PubMed] [Google Scholar]
- 50.Langeveld B, van Gilst WH, Tio RA, Zijlstra F, Roks AJ. Hypertension. 2005;45:138–141. doi: 10.1161/01.HYP.0000149382.83973.c2. [DOI] [PubMed] [Google Scholar]
- 51.Grant MB, Wargovich TJ, Ellis EA, Caballero S, Mansour M, Pepine CJ. Circulation. 1994;89:1511–1517. doi: 10.1161/01.cir.89.4.1511. [DOI] [PubMed] [Google Scholar]
- 52.Do YS, Kao EY, Ganaha F, Minamiguchi H, Sugimoto K, Lee J, Elkins CJ, Amabile PG, Kuo MD, Wang DS, Waugh JM, Dake MD. Radiology. 2004;230:377–382. doi: 10.1148/radiol.2302020417. [DOI] [PubMed] [Google Scholar]
- 53.Masters KSB, Lipke EA, Rice EEH, Liel MS, Myler HA, Zygourakis C, Tulis DA, West JL. J. Biomater. Sci.-Polym. Ed. 2005;16:659–672. doi: 10.1163/1568562053783722. [DOI] [PubMed] [Google Scholar]
- 54.Kwok OH, Chow WH, Law TC, Chiu A, Ng W, Lam WF, Hong MK, Popma JJ. Catheter. Cardiovasc. Interv. 2005;66:541–546. doi: 10.1002/ccd.20558. [DOI] [PubMed] [Google Scholar]
- 55.Blindt R, Vogt F, Astafieva I, Fach C, Hristov M, Krott N, Seitz B, Kapurniotu V, Kwok C, Dewor M, Bosserhoff AK, Bernhagen J, Hanrath P, Hoffmann R, Weber C. J. Am. Coll. Cardiol. 2006;47:1786–1795. doi: 10.1016/j.jacc.2005.11.081. [DOI] [PubMed] [Google Scholar]
- 56.Salu KJ, Bosmans JM, Huang Y, Hendriks M, Verhoeven M, Levels A, Cooper S, De Scheerder IK, Vrints CJ, Bult H. Cardiovasc. Res. 2006;69:536–544. doi: 10.1016/j.cardiores.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 57.Wieneke H, Dirsch O, Sawitowski T, Gu YL, Brauer H, Dahmen U, Fischer A, Wnendt S, Erbel R. Catheter. Cardiovasc. Interv. 2003;60:399–407. doi: 10.1002/ccd.10664. [DOI] [PubMed] [Google Scholar]
- 58.Kollum M, Farb A, Schreiber R, Terfera K, Arab A, Geist A, Haberstroh J, Wnendt S, Virmani R, Hehrlein C. Catheter. Cardiovasc. Interv. 2005;64:85–90. doi: 10.1002/ccd.20213. [DOI] [PubMed] [Google Scholar]
- 59.Patti G, Chello M, Pasceri V, Colonna D, Carminati P, Covino E, Di Germano S. Clin. Ther. 2005;27:1411–1419. doi: 10.1016/j.clinthera.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 60.Abizaid A, Albertal M, Costa MA, Abizaid AS, Staico R, Feres F, Mattos LA, Sousa AG, Moses J, Kipshidize N, Roubin GS, Mehran R, New G, Leon MB, Sousa JE. J. Am. Coll. Cardiol. 2004;43:1118–1121. doi: 10.1016/j.jacc.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 61.Airoldi F, Di Mario C, Ribichini F, Presbitero P, Sganzerla P, Ferrero V, Vassanelli C, Briguori C, Carlino M, Montorfano M, Biondi-Zoccai GG, Chieffo A, Ferrari A, Colombo A. Am. J. Cardiol. 2005;96:664–667. doi: 10.1016/j.amjcard.2005.04.040. [DOI] [PubMed] [Google Scholar]
- 62.Mahato RI, Kim SW. Pharmaceutical perspectives of nucleic acid-based therapeutics. London: Taylor & Francis; 2002. [Google Scholar]
- 63.Sharif F, Daly K, Crowley J, O'Brien T. Cardiovasc. Res. 2004;64:208–216. doi: 10.1016/j.cardiores.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 64.Gruchala M, Roy H, Bhardwaj S, Yla-Herttuala S. Curr. Pharm. Design. 2004;10:407–423. doi: 10.2174/1381612043453379. [DOI] [PubMed] [Google Scholar]
- 65.Labhasetwar V, Chen BR, Muller DWM, Bonadio J, Ciftci K, March K, Levy RJ. Adv. Drug Deliv. Rev. 1997;24:109–120. [Google Scholar]
- 66.Kornowski R, Fuchs S. Int. J. Cardiovasc. Intervent. 2000;3:67–70. doi: 10.1080/14628840050516145. [DOI] [PubMed] [Google Scholar]
- 67.Brewster LP, Brey EM, Greisler HP. Adv. Drug. Deliv. Rev. 2006;58:604–629. doi: 10.1016/j.addr.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peng T, Gibula P, Yao KD, Goosen MFA. Biomaterials. 1996;17:685–694. doi: 10.1016/0142-9612(96)86738-x. [DOI] [PubMed] [Google Scholar]
- 69.Eberhart RC, Su SH, Nguyen KT, Zilberman M, Tang LP, Nelson KD, Frenkel P. J. Biomater. Sci.-Polym. Ed. 2003;14:299–312. doi: 10.1163/156856203321478838. [DOI] [PubMed] [Google Scholar]
- 70.Zilberman M, Nelson KD, Eberhart RC. J. Biomed. Mater. Res. B Appl. Biomater. 2005;74:792–799. doi: 10.1002/jbm.b.30319. [DOI] [PubMed] [Google Scholar]
- 71.Luo D, Woodrow-Mumford K, Belcheva N, Saltzman WM. Pharm. Res. 1999;16:1300–1308. doi: 10.1023/a:1014870102295. [DOI] [PubMed] [Google Scholar]
- 72.Park TG, Jeong JH, Kim SW. Adv. Drug Deliv. Rev. 2006;58:467–486. doi: 10.1016/j.addr.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 73.Klugherz BD, Jones PL, Cui XM, Chen WL, Meneveau NF, DeFelice S, Connolly J, Wilensky RL, Levy RJ. Nat. Biotechnol. 2000;18:1181–1184. doi: 10.1038/81176. [DOI] [PubMed] [Google Scholar]
- 74.Takahashi A, Palmer-Opolski M, Smith RC, Walsh K. Gene Ther. 2003;10:1471–1478. doi: 10.1038/sj.gt.3302010. [DOI] [PubMed] [Google Scholar]
- 75.Niidome T, Huang L. Gene Ther. 2002;9:1647–1652. doi: 10.1038/sj.gt.3301923. [DOI] [PubMed] [Google Scholar]
- 76.Verma IM, Weitzman MD. Annu. Rev. Biochem. 2005;74:711–738. doi: 10.1146/annurev.biochem.74.050304.091637. [DOI] [PubMed] [Google Scholar]
- 77.Muhs A, Heublein B, Schletter J, Herrmann A, Rudiger M, Sturm M, Grust A, Malms J, Schrader J, Von der Leyen HE. Hum. Gene Ther. 2003;14:375–383. doi: 10.1089/104303403321208970. [DOI] [PubMed] [Google Scholar]
- 78.Vonderleyen HE, Gibbons GH, Morishita R, Lewis NP, Zhang L, Nakajima M, Kaneda Y, Cooke JP, Dzau VJ. Proc. Natl. Acad. Sci. U. S. A. 1995;92:1137–1141. doi: 10.1073/pnas.92.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang K, Kessler PD, Zhou ZM, Penn MS, Forudi F, Zhou XR, Tarakji K, Kibbe M, Kovesdi I, Brough DE, Topol EJ, Lincoff AM. Mol. Ther. 2003;7:597–603. doi: 10.1016/s1525-0016(03)00061-3. [DOI] [PubMed] [Google Scholar]
- 80.Turunen MP, Hiltunen MO, Ruponen M, Virkamaki L, Szoka FC, Jr, Urtti A, Yla-Herttuala S. Gene Ther. 1999;6:6–11. doi: 10.1038/sj.gt.3300800. [DOI] [PubMed] [Google Scholar]
- 81.Jewell CM, Zhang JT, Fredin NJ, Wolff MR, Hacker TA, Lynn DM. Biomacromolecules. 2006;7:2483–2491. doi: 10.1021/bm0604808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kay MA, Glorioso JC, Naldini L. Nat. Med. 2001;7:33–40. doi: 10.1038/83324. [DOI] [PubMed] [Google Scholar]
- 83.Carter BJ. Hum. Gene Ther. 2005;16:541–550. doi: 10.1089/hum.2005.16.541. [DOI] [PubMed] [Google Scholar]
- 84.Mano T, Luo ZY, Suhara T, Smith RC, Esser S, Walsh K. Hum. Gene Ther. 2000;11:1625–1635. doi: 10.1089/10430340050111287. [DOI] [PubMed] [Google Scholar]
- 85.Huang J, Niu XL, Pippen AM, Annex BH, Kontos CD. Arterioscler. Thromb. Vasc. Biol. 2005;25:354–358. doi: 10.1161/01.ATV.0000151619.54108.a5. [DOI] [PubMed] [Google Scholar]
- 86.Ye YW, Landau C, Willard JE, Rajasubramanian G, Moskowitz A, Aziz S, Meidell RS, Eberhart RC. Ann. Biomed. Eng. 1998;26:398–408. doi: 10.1114/1.62. [DOI] [PubMed] [Google Scholar]
- 87.Chen MC, Liang HF, Chiu YL, Chang Y, Wei HJ, Sung HW. J. Control. Release. 2005;108:178–189. doi: 10.1016/j.jconrel.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 88.Ho HO, Lin LH, Sheu MT. J. Control. Release. 1997;44:103–112. [Google Scholar]
- 89.Wissink MJB, Beernink R, Scharenborg NM, Poot AA, Engbers GHM, Beugeling T, van Aken WG, Feijen J. J. Control. Release. 2000;67:141–155. doi: 10.1016/s0168-3659(00)00202-9. [DOI] [PubMed] [Google Scholar]
- 90.Cloft HJ, Kallmes DF, Lin HB, Li ST, Marx WF, Hudson SB, Helm GA, Lopes MB, McGraw JK, Dion JE, Jensen ME. Radiology. 2000;214:557–562. doi: 10.1148/radiology.214.2.r00fe21557. [DOI] [PubMed] [Google Scholar]
- 91.Klugherz BD, Song CX, Defelice S, Cui XM, Lu ZB, Connolly J, Hinson JT, Wilensky RL, Levy RJ. Hum. Gene Ther. 2002;13:443–454. doi: 10.1089/10430340252792576. [DOI] [PubMed] [Google Scholar]
- 92.Abrahams JM, Song CX, DeFelice S, Grady MS, Diamond SL, Levy RJ. Stroke. 2002;33:1376–1382. doi: 10.1161/01.str.0000014327.03964.c0. [DOI] [PubMed] [Google Scholar]
- 93.Fishbein I, Stachelek SJ, Connolly JM, Wilensky RL, Alferiev I, Levy RJ. J. Control. Release. 2005;109:37–48. doi: 10.1016/j.jconrel.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 94.Perlstein I, Connolly JM, Cui X, Song C, Li Q, Jones PL, Lu Z, DeFelice S, Klugherz B, Wilensky R, Levy RJ. Gene Ther. 2003;10:1420–1428. doi: 10.1038/sj.gt.3302043. [DOI] [PubMed] [Google Scholar]
- 95.Yamamoto M, Yamato M, Aoyagi M, Yamamoto K. Exp. Cell Res. 1995;219:249–256. doi: 10.1006/excr.1995.1225. [DOI] [PubMed] [Google Scholar]
- 96.Jones PL, Jones FS, Zhou B, Rabinovitch M. J. Cell Sci. 1999;112:435–445. doi: 10.1242/jcs.112.4.435. [DOI] [PubMed] [Google Scholar]
- 97.vanderGiessen WJ, Lincoff AM, Schwartz RS, vanBeusekom HMM, Serruys PW, Holmes DR, Ellis SG, Topol EJ. Circulation. 1996;94:1690–1697. doi: 10.1161/01.cir.94.7.1690. [DOI] [PubMed] [Google Scholar]
- 98.Carter AJ, Aggarwal M, Kopia GA, Tio F, Tsao PS, Kolata R, Yeung AC, Llanos G, Dooley L, Falotico R. Cardiovasc. Res. 2004;63:617–624. doi: 10.1016/j.cardiores.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 99.Virmani R, Guagliumi G, Farb A, Musumeci G, Grieco N, Motta T, Mihalcsik L, Tespili M, Valsecchi O, Kolodgie FD. Circulation. 2004;109:701–705. doi: 10.1161/01.CIR.0000116202.41966.D4. [DOI] [PubMed] [Google Scholar]
- 100.McFadden EP, Stabile E, Regar E, Cheneau E, Ong AT, Kinnaird T, Suddath WO, Weissman NJ, Torguson R, Kent KM, Pichard AD, Satler LF, Waksman R, Serruys PW. Lancet. 2004;364:1519–1521. doi: 10.1016/S0140-6736(04)17275-9. [DOI] [PubMed] [Google Scholar]
- 101.Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, Kutys R, Skorija K, Gold HK, Virmani R. J. Am. Coll. Cardiol. 2006;48:193–202. doi: 10.1016/j.jacc.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 102. [last accessed 5/6/2007];FDA statement. ( http://www.fda.gov/cdrh/news/091406.html)
- 103.Wessely R, Hausleiter J, Michaelis C, Jaschke B, Vogeser M, Milz S, Behnisch B, Schratzenstaller T, Renke-Gluszko M, Stover M, Wintermantel E, Kastrati A, Schomig A. Arterioscler. Thromb. Vasc. Biol. 2005;25:748–753. doi: 10.1161/01.ATV.0000157579.52566.ee. [DOI] [PubMed] [Google Scholar]
- 104.Hausleiter J, Kastrati A, Wessely R, Dibra A, Mehilli J, Schratzenstaller T, Graf I, Renke-Gluszko M, Behnisch B, Dirschinger J, Wintermantel E, Schomig A. Eur. Heart J. 2005;26:1475–1481. doi: 10.1093/eurheartj/ehi405. [DOI] [PubMed] [Google Scholar]
- 105.Fishbein I, Alferiev IS, Nyanguile O, Gaster R, Vohs JM, Wong GS, Felderman H, Chen IW, Choi H, Wilensky RL, Levy RJ. Proc. Natl. Acad. Sci. U. S. A. 2006;103:159–164. doi: 10.1073/pnas.0502945102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Van Alsten JG. Langmuir. 1999;15:7605–7614. [Google Scholar]
- 107.Man SP, Motevalli M, Gardiner S, Sullivan A, Wilson J. Polyhedron. 2006;25:1017–1032. [Google Scholar]
- 108.Lewis AL. Colloids Surf. B-Biointerfaces. 2000;18:261–275. doi: 10.1016/s0927-7765(99)00152-6. [DOI] [PubMed] [Google Scholar]
- 109.Lewis AL, Tolhurst LA, Stratford PW. Biomaterials. 2002;23:1697–1706. doi: 10.1016/s0142-9612(01)00297-6. [DOI] [PubMed] [Google Scholar]
- 110.Whelan DM, van der Giessen WJ, Krabbendam SC, van Vliet EA, Verdouw PD, Serruys PW, van Beusekom HMM. Heart. 2000;83:338–345. doi: 10.1136/heart.83.3.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lewis AL, Vick TA, Collias ACM, Hughes LG, Palmer RR, Leppard SW, Furze JD, Taylor AS, Stratford PW. J. Mater. Sci.-Mater. Med. 2001;12:865–870. doi: 10.1023/a:1012803503667. [DOI] [PubMed] [Google Scholar]
- 112.Anis RR, Karsch KR. Heart. 2006;92:585–588. doi: 10.1136/hrt.2005.068288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Walter DH, Cejna M, Diaz-Sandoval L, Willis S, Kirkwood L, Stratford PW, Tietz AB, Kirchmair R, Silver M, Curry C, Wecker A, Yoon YS, Heidenreich R, Hanley A, Kearney M, Tio FO, Kuenzler P, Isner JM, Losordo DW. Circulation. 2004;110:36–45. doi: 10.1161/01.CIR.0000133324.38115.0A. [DOI] [PubMed] [Google Scholar]
- 114.Asahara T, Bauters C, Pastore C, Kearney M, Rossow S, Bunting S, Ferrara N, Symes JF, Isner JM. Circulation. 1995;91:2793–2801. doi: 10.1161/01.cir.91.11.2793. [DOI] [PubMed] [Google Scholar]
- 115.VanBelle E, Maillard L, Tio FO, Isner JM. Biochem. Biophys. Res. Commun. 1997;235:311–316. doi: 10.1006/bbrc.1997.6772. [DOI] [PubMed] [Google Scholar]
- 116.VanBelle E, Tio FO, Chen DH, Maillard L, Chen DF, Kearney M, Isner JM. J. Am. Coll. Cardiol. 1997;29:1371–1379. doi: 10.1016/s0735-1097(97)00049-1. [DOI] [PubMed] [Google Scholar]
- 117.Laitinen M, Hartikainen J, Hiltunen MO, Eranen J, Kiviniemi M, Narvanen O, Makinen K, Manninen H, Syvanne M, Martin JF, Laakso M, Yla-Herttuala S. Hum. Gene Ther. 2000;11:263–270. doi: 10.1089/10430340050016003. [DOI] [PubMed] [Google Scholar]
- 118.Forough R, Koyama N, Hasenstab D, Lea H, Clowes M, Nikkari ST, Clowes AW. Circ. Res. 1996;79:812–820. doi: 10.1161/01.res.79.4.812. [DOI] [PubMed] [Google Scholar]
- 119.Dollery CM, Humphries SE, McClelland A, Latchman DS, McEwan JR. Circulation. 1999;99:3199–3205. doi: 10.1161/01.cir.99.24.3199. [DOI] [PubMed] [Google Scholar]
- 120.Baker AH, Zaltsman AB, George SJ, Newby AC. J. Clin. Invest. 1998;101:1478–1487. doi: 10.1172/JCI1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.George SJ, Lloyd CT, Angelini GD, Newby AC, Baker AH. Circulation. 2000;101:296–304. doi: 10.1161/01.cir.101.3.296. [DOI] [PubMed] [Google Scholar]
- 122.Johnson TW, Wu YX, Herdeg C, Baumbach A, Newby AC, Karsch KR, Oberhoff M. Arterioscler. Thromb. Vasc. Biol. 2005;25:754–759. doi: 10.1161/01.ATV.0000157582.33180.a9. [DOI] [PubMed] [Google Scholar]
- 123.Sharif F, Hynes SO, McMahon J, Cooney R, Conroy S, Dockery P, Duffy G, Daly K, Crowley J, Bartlett JS, O'Brien T. Hum. Gene Ther. 2006;17:741–750. doi: 10.1089/hum.2006.17.741. [DOI] [PubMed] [Google Scholar]
- 124.Davidson BL, Stein CS, Heth JA, Martins I, Kotin RM, Derksen TA, Zabner J, Ghodsi A, Chiorini JA. Proc. Natl. Acad. Sci. U. S. A. 2000;97:3428–3432. doi: 10.1073/pnas.050581197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yamauchi F, Koyamatsu Y, Kato K, Iwata H. Biomaterials. 2006;27:3497–3504. doi: 10.1016/j.biomaterials.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 126.Zhang J, Chua LS, Lynn DM. Langmuir. 2004;20:8015–8021. doi: 10.1021/la048888i. [DOI] [PubMed] [Google Scholar]
- 127.Ren KF, Ji J, Shen JC. Bioconjugate Chem. 2006;17:77–83. doi: 10.1021/bc050264g. [DOI] [PubMed] [Google Scholar]
- 128.Ren KF, Ji J, Shen JC. Biomaterials. 2006;27:1152–1159. doi: 10.1016/j.biomaterials.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 129.Nakayama Y, Matsuda T. J. Biomed. Mater. Res. 1999;48:511–521. doi: 10.1002/(sici)1097-4636(1999)48:4<511::aid-jbm17>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 130.Nakayama Y, Ji-Youn K, Nishi S, Ueno H, Matsuda T. J. Biomed. Mater. Res. 2001;57:559–566. doi: 10.1002/1097-4636(20011215)57:4<559::aid-jbm1202>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 131.Okino H, Nakayama Y, Tanaka M, Matsuda T. J. Biomed. Mater. Res. 2002;59:233–245. doi: 10.1002/jbm.1237. [DOI] [PubMed] [Google Scholar]
- 132.Akhtar S, Hughes MD, Khan A, Bibby M, Hussain M, Nawaz Q, Double J, Sayyed P. Adv. Drug Deliv. Rev. 2000;44:3–21. doi: 10.1016/s0169-409x(00)00080-6. [DOI] [PubMed] [Google Scholar]
- 133.Pan WH, Clawson GA. J. Cell. Biochem. 2006;98:14–35. doi: 10.1002/jcb.20790. [DOI] [PubMed] [Google Scholar]
- 134.Morishita R, Gibbons GH, Ellison KE, Nakajima M, Zhang L, Kaneda Y, Ogihara T, Dzau VJ. Proc. Natl. Acad. Sci. U. S. A. 1993;90:8474–8478. doi: 10.1073/pnas.90.18.8474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fisher M, Liu B, Glennon PE, Southgate KM, Sale EM, Sale GJ, Lewis MJ, Groves PH. Atherosclerosis. 2001;156:289–295. doi: 10.1016/s0021-9150(00)00656-0. [DOI] [PubMed] [Google Scholar]
- 136.Lowe HC, Fahmy RG, Kavurma MM, Baker A, Chesterman CN, Khachigian LM. Circ. Res. 2001;89:670–677. doi: 10.1161/hh2001.097867. [DOI] [PubMed] [Google Scholar]
- 137.Gunn J, Holt CM, Francis SE, Shepherd L, Grohmann M, Newman CM, Crossman DC, Cumberland DC. Circ. Res. 1997;80:520–531. doi: 10.1161/01.res.80.4.520. [DOI] [PubMed] [Google Scholar]
- 138.Hayashi K, Banno H, Kadomatsu K, Takei Y, Komori K, Muramatsu T. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H2203–H2209. doi: 10.1152/ajpheart.00555.2004. [DOI] [PubMed] [Google Scholar]
- 139.Li Y, Fukuda N, Kunimoto S, Yokoyama S, Hagikura K, Kawano T, Takayama T, Honye J, Kobayashi N, Mugishima H, Saito S, Serie K. J. Cardiovasc. Pharmacol. 2006;48:184–190. doi: 10.1097/01.fjc.0000246940.91191.1f. [DOI] [PubMed] [Google Scholar]
- 140.Shi Y, Fard A, Galeo A, Hutchinson HG, Vermani P, Dodge GR, Hall DJ, Shaheen F, Zalewski A. Circulation. 1994;90:944–951. doi: 10.1161/01.cir.90.2.944. [DOI] [PubMed] [Google Scholar]
- 141.Zhang XX, Cui CC, Xu XG, Hu XS, Fang WH, Kuang BJ. Chin. Med. J. (Engl) 2004;117:258–263. [PubMed] [Google Scholar]
- 142.Kutryk MJ, Foley DP, van den Brand M, Hamburger JN, van der Giessen WJ, deFeyter PJ, Bruining N, Sabate M, Serruys PW. J. Am. Coll. Cardiol. 2002;39:281–287. doi: 10.1016/s0735-1097(01)01741-7. [DOI] [PubMed] [Google Scholar]
- 143.Kipshidze N, Keane E, Stein D, Chawla P, Skrinska V, Shankar LR, Khanna A, Komorowski R, Haudenschild C, Iversen P, Leon MB, Keelan MH, Moses J. Catheter. Cardiovasc. Interv. 2001;54:247–256. doi: 10.1002/ccd.1277. [DOI] [PubMed] [Google Scholar]
- 144.Kipshidze NN, Kim HS, Iversen P, Yazdi HA, Bhargava B, New G, Mehran R, Tio F, Haudenschild C, Dangas G, Stone GW, Iyer S, Roubin GS, Leon MB, Moses JW. J. Am. Coll. Cardiol. 2002;39:1686–1691. doi: 10.1016/s0735-1097(02)01830-2. [DOI] [PubMed] [Google Scholar]
- 145.Kipshidze NN, Iversen P, Kim HS, Yiazdi H, Dangas G, Seaborn R, New G, Tio F, Waksman R, Mehran R, Tsapenko M, Stone GW, Roubin GS, Iyer S, Leon MB, Moses JW. Catheter. Cardiovasc. Interv. 2004;61:518–527. doi: 10.1002/ccd.20007. [DOI] [PubMed] [Google Scholar]
- 146.Summerton J. Biochim. Biophys. Acta-Gene Struc. Expression. 1999;1489:141–158. doi: 10.1016/s0167-4781(99)00150-5. [DOI] [PubMed] [Google Scholar]
- 147.Zhang ZQ, Cao XC, Zhao XB, Withers SB, Holt CM, Lewis AL, Lu JR. Biomacromolecules. 2006;7:784–791. doi: 10.1021/bm050840b. [DOI] [PubMed] [Google Scholar]
- 148.Chan KH, Armstrong J, Withers S, Malik N, Cumberland DC, Gunn J, Holt CM. Biomaterials. 2007;28:1218–1224. doi: 10.1016/j.biomaterials.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 149.Lewis AL, Cumming ZL, Goreish HH, Kirkwood LC, Tolhurst LA, Stratford PW. Biomaterials. 2001;22:99–111. doi: 10.1016/s0142-9612(00)00083-1. [DOI] [PubMed] [Google Scholar]
- 150.Lewis AL, Berwick J, Davies MC, Roberts CJ, Wang JH, Small S, Dunn A, O'Byrne V, Redman RP, Jones SA. Biomaterials. 2004;25:3099–3108. doi: 10.1016/j.biomaterials.2003.09.082. [DOI] [PubMed] [Google Scholar]
- 151.Tomita N, Azuma H, Kaneda Y, Ogihara T, Morishita R. Curr. Drug Targets. 2003;4:339–346. doi: 10.2174/1389450033491055. [DOI] [PubMed] [Google Scholar]
- 152.Kitamoto S, Egashira K, Kataoka C, Koyanagi M, Katoh M, Shimokawa H, Morishita R, Kaneda Y, Sueishi K, Takeshita A. Circulation. 2000;102:806–812. doi: 10.1161/01.cir.102.7.806. [DOI] [PubMed] [Google Scholar]
- 153.Yoshimura S, Morishita R, Hayashi K, Yamamoto K, Nakagami H, Kaneda Y, Sakai N, Ogihara T. Gene Ther. 2001;8:1635–1642. doi: 10.1038/sj.gt.3301566. [DOI] [PubMed] [Google Scholar]
- 154.Suzuki J, Ito H, Gotoh R, Morishita R, Egashira K, Isobe M. Circ. J. 2004;68:270–271. [Google Scholar]
- 155.Kalinowski M, Viehofer K, Hamann C, Barry JJ, Kleb B, Klose KJ, Wagner HJ, Alfke H. Cardiovasc. Intervent. Radiol. 2005;28:331–337. doi: 10.1007/s00270-003-0239-y. [DOI] [PubMed] [Google Scholar]
- 156.Radke PW, Griesenbach U, Kivela A, Vick T, Judd D, Munkonge F, Willis S, Geddes DM, Yla-Herttuala S, Alton EW. Hum. Gene Ther. 2005;16:734–740. doi: 10.1089/hum.2005.16.734. [DOI] [PubMed] [Google Scholar]
- 157.Ohtani K, Egashira K, Nakano K, Zhao G, Funakoshi K, Ihara Y, Kimura S, Tominaga R, Morishita R, Sunagawa K. Circulation. 2006;114:2773–2779. doi: 10.1161/CIRCULATIONAHA.105.582254. [DOI] [PubMed] [Google Scholar]
- 158.Jovinge S, Hultgardh-Nilsson A, Regnstrom J, Nilsson J. Arterioscler. Thromb. Vasc. Biol. 1997;17:490–497. doi: 10.1161/01.atv.17.3.490. [DOI] [PubMed] [Google Scholar]
- 159.Clausell N, Molossi S, Sett S, Rabinovitch M. Circulation. 1994;89:2768–2779. doi: 10.1161/01.cir.89.6.2768. [DOI] [PubMed] [Google Scholar]
- 160.Javed Q, Swanson N, Vohra H, Thurston H, Gershlick AH. Exp. Mol. Pathol. 2002;73:104–111. doi: 10.1006/exmp.2002.2450. [DOI] [PubMed] [Google Scholar]
- 161.Foo RS, Gershlick AH, Hogrefe K, Baron JH, Johnston TW, Hussey AJ, Garner I, de Bono DP. Thromb. Haemost. 2000;83:496–502. [PubMed] [Google Scholar]
- 162.Grainger DW. Expert Opin Biol Ther. 2004;4:1029–1044. doi: 10.1517/14712598.4.7.1029. [DOI] [PubMed] [Google Scholar]
- 163.Lefkovits J, Plow EF, Topol EJ. N. Engl. J. Med. 1995;332:1553–1559. doi: 10.1056/NEJM199506083322306. [DOI] [PubMed] [Google Scholar]
- 164.Azrin MA, Ling FS, Chen Q, Pawashe A, Migliaccio F, Homer R, Todd M, Ezekowitz MD. Circ. Res. 1994;75:268–277. doi: 10.1161/01.res.75.2.268. [DOI] [PubMed] [Google Scholar]
- 165.Aggarwal RK, Ireland DC, Azrin MA, Ezekowitz MD, de Bono DP, Gershlick AH. Circulation. 1996;94:3311–3317. doi: 10.1161/01.cir.94.12.3311. [DOI] [PubMed] [Google Scholar]
- 166.Tam SH, Sassoli PM, Jordan RE, Nakada MT. Circulation. 1998;98:1085–1091. doi: 10.1161/01.cir.98.11.1085. [DOI] [PubMed] [Google Scholar]
- 167.Valgimigli M, Percoco G, Malagutti P, Campo G, Ferrari F, Barbieri D, Cicchitelli G, McFadden EP, Merlini F, Ansani L, Guardigli G, Bettini A, Parrinello G, Boersma E, Ferrari R. Jama. 2005;293:2109–2117. doi: 10.1001/jama.293.17.2109. [DOI] [PubMed] [Google Scholar]
- 168.The ERASER Investigators. Circulation. 1999;100:799–806. doi: 10.1161/01.cir.100.8.799. [DOI] [PubMed] [Google Scholar]
- 169.Baron JH, Gershlick AH, Hogrefe K, Armstrong J, Holt CM, Aggarwal RK, Azrin M, Ezekowitz M, de Bono DP. Cardiovasc. Res. 2000;46:585–594. doi: 10.1016/s0008-6363(00)00042-0. [DOI] [PubMed] [Google Scholar]
- 170.Hong YJ, Jeong MH, Kim W, Lim SY, Lee SH, Hong SN, Kim JH, Ahn YK, Cho JG, Park JC, Cho DL, Kim H, Kang JC. Am. J. Cardiol. 2004;94:1050–1054. doi: 10.1016/j.amjcard.2004.06.066. [DOI] [PubMed] [Google Scholar]
- 171.Kim W, Jeong MH, Kim KH, Sohn IS, Hong YJ, Park HW, Kim JH, Ahn YK, Cho JG, Park JC, Cho DL, Kang JC. J. Am. Coll. Cardiol. 2006;47:933–938. doi: 10.1016/j.jacc.2005.10.054. [DOI] [PubMed] [Google Scholar]
- 172.Melo LG, Gnecchi M, Pachori AS, Kong D, Wang K, Liu X, Pratt RE, Dzau VJ. Arterioscler. Thromb. Vasc. Biol. 2004;24:1761–1774. doi: 10.1161/01.ATV.0000142363.15113.88. [DOI] [PubMed] [Google Scholar]
- 173.Kong D, Melo LG, Mangi AA, Zhang L, Lopez-Ilasaca M, Perrella MA, Liew CC, Pratt RE, Dzau VJ. Circulation. 2004;109:1769–1775. doi: 10.1161/01.CIR.0000121732.85572.6F. [DOI] [PubMed] [Google Scholar]
- 174.Werner N, Nickenig G. J. Cell. Mol. Med. 2006;10:318–332. doi: 10.1111/j.1582-4934.2006.tb00402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Conklin BS, Wu H, Lin PH, Lumsden AB, Chen C. Artif. Organs. 2004;28:668–675. doi: 10.1111/j.1525-1594.2004.00062.x. [DOI] [PubMed] [Google Scholar]
- 176.Swanson N, Hogrefe K, Javed Q, Gershlick AH. Int. J. Cardiol. 2003;92:265–269. doi: 10.1016/s0167-5273(03)00102-5. [DOI] [PubMed] [Google Scholar]
- 177.Zhu G, Mallery SR, Schwendeman SP. Nat. Biotechnol. 2000;18:52–57. doi: 10.1038/71916. [DOI] [PubMed] [Google Scholar]
- 178.Jiang W, Schwendeman SP. Pharm. Res. 2001;18:878–885. doi: 10.1023/a:1011009117586. [DOI] [PubMed] [Google Scholar]
- 179.Kavanagh CA, Gorelova TA, Selezneva, Rochev YA, Dawson KA, Gallagher WM, Gorelov AV, Keenan AK. J. Biomed. Mater. Res. A. 2005;72:25–35. doi: 10.1002/jbm.a.30192. [DOI] [PubMed] [Google Scholar]
- 180.Ehrbar M, Djonov VG, Schnell C, Tschanz SA, Martiny-Baron G, Schenk U, Wood J, Burri PH, Hubbell JA, Zisch AH. Circ. Res. 2004;94:1124–1132. doi: 10.1161/01.RES.0000126411.29641.08. [DOI] [PubMed] [Google Scholar]
- 181.Arnljots B, Dahlback B. Arterioscler. Thromb. Vasc. Biol. 1995;15:937–941. doi: 10.1161/01.atv.15.7.937. [DOI] [PubMed] [Google Scholar]
- 182.Hardhammar PA, van Beusekom HM, Emanuelsson HU, Hofma SH, Albertsson PA, Verdouw PD, Boersma E, Serruys PW, van der Giessen WJ. Circulation. 1996;93:423–430. doi: 10.1161/01.cir.93.3.423. [DOI] [PubMed] [Google Scholar]
- 183.Serruys PW, Emanuelsson H, van der Giessen W, Lunn AC, Kiemeney F, Macaya C, Rutsch W, Heyndrickx G, Suryapranata H, Legrand V, Goy JJ, Materne P, Bonnier H, Morice MC, Fajadet J, Belardi J, Colombo A, Garcia E, Ruygrok P, de Jaegere P, Morel MA. Circulation. 1996;93:412–422. doi: 10.1161/01.cir.93.3.412. [DOI] [PubMed] [Google Scholar]
- 184.Serruys PW, van Hout B, Bonnier H, Legrand V, Garcia E, Macaya C, Sousa E, van der Giessen W, Colombo A, Seabra-Gomes R, Kiemeneij F, Ruygrok P, Ormiston J, Emanuelsson H, Fajadet J, Haude M, Klugmann S, Morel MA. Lancet. 1998;352:673–681. doi: 10.1016/s0140-6736(97)11128-x. [DOI] [PubMed] [Google Scholar]
- 185.Haude M, Konorza TF, Kalnins U, Erglis A, Saunamaki K, Glogar HD, Grube E, Gil R, Serra A, Richardt HG, Sick P, Erbel R. Circulation. 2003;107:1265–1270. doi: 10.1161/01.cir.0000053442.64637.34. [DOI] [PubMed] [Google Scholar]
- 186.Wohrle J, Al-Khayer E, Grotzinger U, Schindler C, Kochs M, Hombach V, Hoher M. Eur. Heart J. 2001;22:1808–1816. doi: 10.1053/euhj.2001.2608. [DOI] [PubMed] [Google Scholar]
- 187.Lin PH, Chronos NA, Marijianowski MM, Chen C, Conklin B, Bush RL, Lumsden AB, Hanson SR. J. Surg. Res. 2003;112:84–90. doi: 10.1016/s0022-4804(03)00124-0. [DOI] [PubMed] [Google Scholar]
- 188.Hietala EM, Maasilta P, Valimaa T, Harjula AL, Tormala P, Salminen US, Lassila R. J. Biomed. Mater. Res. A. 2003;67:785–791. doi: 10.1002/jbm.a.10154. [DOI] [PubMed] [Google Scholar]
- 189.Christensen K, Larsson R, Emanuelsson H, Elgue G, Larsson A. Thromb. Res. 2005;115:245–253. doi: 10.1016/j.thromres.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 190.Castellot JJ, Jr, Pukac LA, Caleb BL, Wright TC, Jr, Karnovsky MJ. J. Cell. Biol. 1989;109:3147–3155. doi: 10.1083/jcb.109.6.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Letourneur D, Caleb BL, Castellot JJ., Jr J. Cell. Physiol. 1995;165:676–686. doi: 10.1002/jcp.1041650327. [DOI] [PubMed] [Google Scholar]
- 192.Rogers C, Karnovsky MJ, Edelman ER. Circulation. 1993;88:1215–1221. doi: 10.1161/01.cir.88.3.1215. [DOI] [PubMed] [Google Scholar]
- 193.Thomas AC, Campbell JH. Atherosclerosis. 2004;176:73–81. doi: 10.1016/j.atherosclerosis.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 194.Thomas AC, Campbell JH. J. Control. Release. 2004;100:357–377. doi: 10.1016/j.jconrel.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 195.Matsumoto Y, Shimokawa H, Morishige K, Eto Y, Takeshita A. J. Cardiovasc. Pharmacol. 2002;39:513–522. doi: 10.1097/00005344-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 196.Hanke H, Oberhoff M, Hanke S, Hassenstein S, Kamenz J, Schmid KM, Betz E, Karsch KR. Circulation. 1992;85:1548–1556. doi: 10.1161/01.cir.85.4.1548. [DOI] [PubMed] [Google Scholar]
- 197.Weitz JI. N. Engl. J. Med. 1997;337:688–698. doi: 10.1056/NEJM199709043371007. [DOI] [PubMed] [Google Scholar]
- 198.Norrby K. Apmis. 2006;114:79–102. doi: 10.1111/j.1600-0463.2006.apm_235.x. [DOI] [PubMed] [Google Scholar]
- 199.Koromila G, Michanetzis GP, Missirlis YF, Antimisiaris SG. Biomaterials. 2006;27:2525–2533. doi: 10.1016/j.biomaterials.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 200.Verheye S, Markou CP, Salame MY, Wan B, King SB, 3rd, Robinson KA, Chronos NA, Hanson SR. Arterioscler. Thromb. Vasc. Biol. 2000;20:1168–1172. doi: 10.1161/01.atv.20.4.1168. [DOI] [PubMed] [Google Scholar]
- 201.Thierry B, Winnik FM, Merhi Y, Silver J, Tabrizian M. Biomacromolecules. 2003;4:1564–1571. doi: 10.1021/bm0341834. [DOI] [PubMed] [Google Scholar]
- 202.Thierry B, Merhi Y, Silver J, Tabrizian M. J. Biomed. Mater. Res. A. 2005;75:556–566. doi: 10.1002/jbm.a.30450. [DOI] [PubMed] [Google Scholar]
- 203.Haught WH, Sokol M, Kerensky RA, Mehta JL. Am. J. Cardiol. 1995;76:314–315. doi: 10.1016/s0002-9149(99)80091-5. [DOI] [PubMed] [Google Scholar]
- 204.Gossman DE, Schneider F. Cathet. Cardiovasc. Diagn. 1996;38:56–61. doi: 10.1002/(SICI)1097-0304(199605)38:1<56::AID-CCD12>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 205.Deux JF, Prigent-Richard S, d'Angelo G, Feldman LJ, Puvion E, Logeart-Avramoglou D, Pelle A, Boudghene FP, Michel JB, Letourneur D. J. Vasc. Surg. 2002;35:973–981. doi: 10.1067/mva.2002.123093. [DOI] [PubMed] [Google Scholar]
- 206.Anastase-Ravion S, Carreno MP, Blondin C, Ravion O, Champion J, Chaubet F, Haeffner-Cavaillon N, Letourneur D. J. Biomed. Mater. Res. 2002;60:375–383. doi: 10.1002/jbm.10112. [DOI] [PubMed] [Google Scholar]
- 207.Patel MK, Mulloy B, Gallagher KL, O'Brien L, Hughes AD. Thromb. Haemost. 2002;87:149–154. [PubMed] [Google Scholar]
- 208.Chabut D, Fischer AM, Colliec-Jouault S, Laurendeau I, Matou S, Le Bonniec B, Helley D. Mol. Pharmacol. 2003;64:696–702. doi: 10.1124/mol.64.3.696. [DOI] [PubMed] [Google Scholar]
- 209.Luyt CE, Meddahi-Pelle A, Ho-Tin-Noe B, Colliec-Jouault S, Guezennec J, Louedec L, Prats H, Jacob MP, Osborne-Pellegrin M, Letourneur D, Michel JB. J. Pharmacol. Exp. Ther. 2003;305:24–30. doi: 10.1124/jpet.102.046144. [DOI] [PubMed] [Google Scholar]
- 210.Deux JF, Meddahi-Pelle A, Le Blanche AF, Feldman LJ, Colliec-Jouault S, Bree F, Boudghene F, Michel JB, Letourneur D. Arterioscler. Thromb. Vasc. Biol. 2002;22:1604–1609. doi: 10.1161/01.atv.0000032034.91020.0a. [DOI] [PubMed] [Google Scholar]
- 211.Nugent HM, Edelman ER. Circ. Res. 2003;92:1068–1078. doi: 10.1161/01.RES.0000073844.41372.38. [DOI] [PubMed] [Google Scholar]
- 212.Panetta CJ, Miyauchi K, Berry D, Simari RD, Holmes DR, Schwartz RS, Caplice NM. Hum. Gene Ther. 2002;13:433–441. doi: 10.1089/10430340252792567. [DOI] [PubMed] [Google Scholar]
- 213.Shirota T, Yasui H, Matsuda T. Tissue Eng. 2003;9:473–485. doi: 10.1089/107632703322066651. [DOI] [PubMed] [Google Scholar]
- 214.Shirota T, Yasui H, Shimokawa H, Matsuda T. Biomaterials. 2003;24:2295–2302. doi: 10.1016/s0142-9612(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 215.Aoki J, Serruys PW, van Beusekom H, Ong AT, McFadden EP, Sianos G, van der Giessen WJ, Regar E, de Feyter PJ, Davis HR, Rowland S, Kutryk MJ. J. Am. Coll. Cardiol. 2005;45:1574–1579. doi: 10.1016/j.jacc.2005.01.048. [DOI] [PubMed] [Google Scholar]
- 216.Yeh HI, Lu SK, Tian TY, Hong RC, Lee WH, Tsai CH. J. Biomed. Mater. Res. A. 2006;76:835–841. doi: 10.1002/jbm.a.30595. [DOI] [PubMed] [Google Scholar]
- 217.Lambiase PD, Edwards RJ, Anthopoulos P, Rahman S, Meng YG, Bucknall CA, Redwood SR, Pearson JD, Marber MS. Circulation. 2004;109:2986–2992. doi: 10.1161/01.CIR.0000130639.97284.EC. [DOI] [PubMed] [Google Scholar]
- 218.Schmidt-Lucke C, Rossig L, Fichtlscherer S, Vasa M, Britten M, Kamper U, Dimmeler S, Zeiher AM. Circulation. 2005;111:2981–2987. doi: 10.1161/CIRCULATIONAHA.104.504340. [DOI] [PubMed] [Google Scholar]
- 219.Labhasetwar V, Song C, Humphrey W, Shebuski R, Levy RJ. J. Pharm. Sci. 1998;87:1229–1234. doi: 10.1021/js980021f. [DOI] [PubMed] [Google Scholar]
- 220.Panyam J, Labhasetwar V. Adv. Drug Deliv. Rev. 2003;55:329–347. doi: 10.1016/s0169-409x(02)00228-4. [DOI] [PubMed] [Google Scholar]
- 221.Chorny M, Fishbein I, Danenberg HD, Golomb G. J. Control. Release. 2002;83:389–400. doi: 10.1016/s0168-3659(02)00211-0. [DOI] [PubMed] [Google Scholar]
- 222.Banai S, Chorny M, Gertz SD, Fishbein I, Gao J, Perez L, Lazarovichi G, Gazit A, Levitzki A, Golomb G. Biomaterials. 2005;26:451–461. doi: 10.1016/j.biomaterials.2004.02.040. [DOI] [PubMed] [Google Scholar]
- 223.Ganaha F, Kao EY, Wong H, Elkins CJ, Lee J, Modanlou S, Rhee C, Kuo MD, Yuksel E, Cifra PN, Waugh JM, Dake MD. J. Vasc. Interv. Radiol. 2004;15:601–608. doi: 10.1097/01.rvi.0000127888.70058.93. [DOI] [PubMed] [Google Scholar]
- 224.Kolodgie FD, John M, Khurana C, Farb A, Wilson PS, Acampado E, Desai N, Soon-Shiong P, Virmani R. Circulation. 2002;106:1195–1198. doi: 10.1161/01.cir.0000032141.31476.15. [DOI] [PubMed] [Google Scholar]
- 225.Zilberman M, Schwade ND, Eberhart RC. J. Biomed. Mater. Res. B Appl. Biomater. 2004;69:1–10. doi: 10.1002/jbm.b.20026. [DOI] [PubMed] [Google Scholar]
- 226.Grainger DW, Castner DG. Adv. Mater. 2007 in review. [Google Scholar]
- 227.Lanza GM, Yu X, Winter PM, Abendschein DR, Karukstis KK, Scott MJ, Chinen LK, Fuhrhop RW, Scherrer DE, Wickline SA. Circulation. 2002;106:2842–2847. doi: 10.1161/01.cir.0000044020.27990.32. [DOI] [PubMed] [Google Scholar]
- 228.Pislaru SV, Harbuzariu A, Gulati R, Witt T, Sandhu NP, Simari RD, Sandhu GS. J. Am. Coll. Cardiol. 2006;48:1839–1845. doi: 10.1016/j.jacc.2006.06.069. [DOI] [PubMed] [Google Scholar]
- 229.Edelman ER, Rogers C. Circulation. 1999;100:896–898. doi: 10.1161/01.cir.100.9.896. [DOI] [PubMed] [Google Scholar]
- 230.Rogers C, Edelman ER. Circulation. 2006;113:2262–2265. doi: 10.1161/CIRCULATIONAHA.106.623470. [DOI] [PubMed] [Google Scholar]