Abstract
Appropriately timed integration of breeding into avian annual cycles is critical to both reproductive success and survival. The mechanisms by which birds regulate timing of breeding depend on environmental cue response systems that regulate both when birds do and do not breed. Despite there being multiple possible explanations for birds' abilities to time breeding appropriately in different environments, and for the distribution of different cue response system characteristics among taxa, many studies infer that adaptive specialization of cue response systems has occurred without explicitly considering the alternatives. In this paper, we make explicit three hypotheses concerning the timing of reproduction and distribution of cue response characteristics among taxa: adaptive specialization; conditional plasticity; and phylogenetic history. We emphasize in particular that although conditional plasticity built into avian cue response systems (e.g. differing rates of gonadal development and differing latencies until onset of photorefractoriness) may lead to maladaptive annual cycles in some novel circumstances, this plasticity also can lead to what appear to be adaptively specialized cue response systems if not viewed in a comparative context. We use a comparative approach to account for the distribution of one important feature of avian reproductive cue response systems, photorefractoriness. Analysis of the distribution within songbirds of one criterion for absolute photorefractoriness, the spontaneous regression of the gonads without any decline in photoperiod, reveals that a failure to display this trait probably represents an adaptive specialization to facilitate a flexible reproductive schedule. More finely resolved analysis of both criteria for absolute photorefractoriness (the second being total lack of a reproductive response even to constant light after gonadal regression has occurred) within the cardueline finches not only provides further confirmation of this interpretation, but also indicates that these two criteria for photorefractoriness can be, and have been, uncoupled in some taxa. We suggest that careful comparative studies at different phylogenetic scales will be extremely valuable for distinguishing between adaptive specialization and non-adaptive explanations, such as phylogenetic history as explanations of cue response traits in particular taxa. We also suggest that particular focus on taxa in which individuals may breed on very different photoperiods (latitudes or times of year) in different years should be particularly valuable in identifying the range of environmental conditions across which conditionally plastic cue responses can be adaptive.
Keywords: seasonal breeding, photoperiodism, photorefractoriness, environmental cues, songbird
1. Introduction: timing of breeding and ‘fit' to different environments
A basic goal in biology is to understand how organisms come to be ‘well suited' to their environments (Fretwell 1972). From a fitness standpoint, the choice of when to breed is a key indicator of being well suited to an environment, since timing of breeding affects both the likelihood of producing high-quality offspring and the probability of adult survival (Drent & Daan 1980; Farner et al. 1983; Svensson 1997; McNamara et al. 2004; Komdeur & Daan 2005; Verhulst & Nilsson 2008). In environments where conditions vary in space and time (i.e. most environments), the reproductive schedule should track changes in ultimate environmental factors (e.g. food supply, weather) that affect reproductive success (Baker 1938; Perrins 1970). Consequently, selection should favour individuals that possess mechanisms permitting them to detect and respond to proximate cues that predict impending relevant changes in the environment (Baker 1938). Processing of such cues is achieved by the neuroendocrine system, which transduces information from both external cues (e.g. changing day length, rainfall, food supply) and internal processes (e.g. the readout from circannual clocks and modulation, if any, of responsiveness to environmental cues) into neuroendocrine signals that regulate changes in reproductive physiology, morphology and behaviour (e.g. Follett 1984; Ball 1993).
Appropriate timing of reproduction in a particular environment clearly could be achieved if the neuroendocrine cue processing system is adaptively specialized to be particularly effective at tracking the environmental changes that occur in that environment. However, reproductive schedules well suited to specific environments do not necessarily require specific adaptations of the neuroendocrine cue processing system to the details of those environments. Appropriate reproductive timing also may occur if the neuroendocrine cue processing system's conditional plasticity (how it responds to different patterns of cues) facilitates effective tracking of changes in a variety of environments, including the one the animal happens to occupy (the jack of all trades; cf. Huey & Hertz 1984). Further, neuroendocrine cue processing systems also may exhibit features retained through phylogenetic history but not specifically related to timing of reproduction in the current environment. Such features may exist as neutral traits and may be potential preadaptations or may have been adaptive for ancestral species. Thus, determining whether species possess specific adaptations to facilitate appropriate timing of reproduction requires consideration of three different hypotheses:
(i) Adaptive specialization hypothesis. Species possess cue response systems with features that represent specialized adaptations to the details of the environment occupied.
(ii) Conditional plasticity hypothesis. Species possess cue response systems that can generate a range of appropriate responses depending on conditions experienced, and therefore can time reproduction appropriately in a range of environments.
(iii) Phylogenetic history hypothesis. Features of the cue response system are retained by evolutionary descent and may not be specifically adaptive in the current environment.
Decades of research on the mechanisms by which birds process environmental cues to time reproduction, beginning with the pioneering studies of Rowan (1925, 1926), provide a basis for evaluating the extent to which adaptive natural reproductive schedules in different taxa result from adaptive specializations of cue response patterns and mechanisms or, alternatively, emerge from conditionally plastic outputs of interspecifically similar cue response characteristics. This issue has been of interest for many years and is the subject of some excellent and stimulating older reviews (e.g. Lofts & Murton 1968; Farner et al. 1983; Nicholls et al. 1988) that are still highly pertinent. However, for several reasons, it is appropriate now to revisit the question of adaptive specialization of reproductive cue response systems. First, substantial new empirical data are available, some of which specifically speak to the phenomenon of local adaptation of environmental cue response systems (e.g. Lambrechts et al. 1996, 1997) and to the evolutionary mechanisms that would facilitate such adaptation (e.g. Coppack et al. 2003; Coppack & Pulido 2004). Second, the advent of quantitative comparative methods (e.g. Felsenstein 1985; Harvey & Pagel 1991) permits a more rigorous assessment of the likelihood that interspecies differences either in cue response patterns or in underlying mechanisms represent specific adaptations to different environments as opposed to being accounted for at least in part by non-adaptive explanations, such as phylogenetic history (MacDougall-Shackleton et al. 2005). Third, the current interest in how global climate change may affect different populations warrants specific consideration of how different species' cue response systems may facilitate or hinder adjustment of breeding schedules to changing environmental conditions (e.g. Coppack & Both 2002; Coppack & Pulido 2004).
The intent of this paper is not to perform either an exhaustive review of existing empirical data on avian reproductive cue response systems or an exhaustive comparative analysis of it. Instead we review briefly the history of evaluations of adaptive specialization of avian reproductive cue response systems, with particular emphasis on the regulation of reproductive development prior to breeding and of gonadal collapse at the end of breeding, and examine the three alternative hypotheses—adaptive specialization, conditional plasticity and phylogenetic history—enunciated above. We suggest that these should be considered explicitly when contemplating the adaptive significance of patterns of reproductive schedules and mechanisms among species. These hypotheses have not, to our knowledge, been applied to avian reproductive timing patterns and mechanisms; reviews of the topic to date generally assume specialized adaptations without explicitly considering any alternatives (e.g. Lofts & Murton 1968; Farner et al. 1983). In fact, it is difficult to find papers on avian cue response patterns and mechanisms that do not report support for what amounts to adaptive specialization despite lacking any discussion of possible alternative explanations. It is important to keep in mind that environmental cue response systems are also involved in the regulation of actual onset of nesting once reproductive development is complete, as well as in the coordination of the complex sequence of behavioural and physiological events that occur within nesting cycles (Wingfield 1980, 1983); we do not deal with those interesting processes here.
To illustrate the value of the approach we suggest, we will perform a limited comparison of a prominent and important feature of many birds' reproductive cue response systems, the development of a state of photorefractoriness, which has presumably evolved (perhaps multiple times; Farner et al. 1983) to terminate and then prevent breeding so as to facilitate adult survival, and minimize wasted reproductive effort on broods with little chance of survival, in seasonal environments at mid to high latitudes. Two different criteria have been used to detect photorefractoriness, spontaneous gonadal regression on long days and complete lack of reproductive responsiveness to very long days once regression has occurred (Farner et al. 1983; see below for details). We will compare the first criterion among songbirds generally and will compare both criteria within one tribe of songbirds, the cardueline finches, in more detail. We will finish the paper with a discussion of the implications of our conceptual framework and comparative findings for evolution, colonization of new areas and responses to climate change.
2. Diversity of avian cue response patterns and mechanisms
Intertaxon diversity of reproductive cue response patterns and mechanisms has interested avian endocrinologists for many years. Following Rowan's discoveries of photoresponsiveness (1925, 1926) in juncos (Junco hyemalis), data accumulated rapidly on a number of other temperate zone taxa, and broad similarities (reviewed especially by Nicholls et al. 1988) and differences (e.g. Lofts & Murton 1968; Farner et al. 1983) among taxa quickly became evident. There was, for a time, particular interest in whether tropical and temperate zone taxa might have evolved to differ fundamentally from one another (e.g. Marshall & Disney 1957; Farner & Serventy 1960). Since tropical and desert-dwelling taxa occupy environments where photoperiod either changes very little, presumably rendering seasonal changes difficult to measure, or where it is apparently uncorrelated with changes in ultimate factors dictating reproductive success, rendering it useless as a predictive cue even if changes could be measured, day length was thought to be of little use to such species (e.g. Lofts & Murton 1968). This idea directed attention to non-photic cues, such as weather, temperature and food supply, and a number of studies demonstrated effects of non-photic cues on avian reproduction in tropical and xerophilous birds, as well as in ‘typical' seasonal taxa of higher latitudes (reviewed by Marshall 1970; see also Ligon 1974; Jones & Ward 1976). Interest in how non-photic cues are integrated with long-term predictive cues like photoperiod has persisted (e.g. Wingfield 1980, 1983; Wingfield et al. 1992, 1993, 1996, 1997, 2003; Hau et al. 2000; Hau 2001; Hahn et al. 2005). Nevertheless, surprisingly strong photoperiodic responses were found early on in some tropical and desert-dwelling taxa (e.g. Vaugien 1952, 1953; Marshall & Disney 1956; see also Gwinner & Dittami 1985), and subsequent research clearly shows that tropical birds respond to sufficiently small changes in photoperiod to be able to use this cue to help time breeding (Hau et al. 1998; Misra et al. 2004; Styrsky et al. 2004; see also Rani et al. 2005b). Although this finding may seem to support the idea of adaptive specialization of cue response systems in different environments (which it may), it also illustrates well the importance of considering alternative explanations: sensitivity to small differences in photoperiod in tropical taxa does not necessarily represent a specific adaptation to facilitate the use of photoperiod at low latitudes. Many (perhaps all) higher-latitude photoperiodic species display striking sensitivity to what amounts to tiny differences in photoperiod. For instance, in some taxa (e.g. European starlings, Sturnus vulgaris; Gambel's white-crowned sparrows, Zonotrichia leucophrys gambelii), very slight increases in constant photoperiod exposure produce dramatic changes in physiological responses. On constant photoperiods just below 12 L : 12 D, gonads grow slowly and then remain active indefinitely, whereas on exactly 12 L : 12 D photoperiod, oscillating cycles of gonadal activity and inactivity occur, and on photoperiods just above 12 L : 12 D, permanent photorefractoriness follows the initial reproductive development (see Farner et al. (1983) and Nicholls et al. (1988) for reviews). Likewise, male house sparrows on natural photoperiod begin gonadal regression by the time photoperiod has declined less than 30 min after the summer solstice photoperiod of 18 L : 6 D, while others maintained at the summer solstice photoperiod delay onset of regression (Dawson 1991). Thus, even against a background of 18 hours of light per day, at least some temperate zone species are capable of detecting and responding to differences in day length of similar absolute magnitude as tropical species (Hau et al. 2000).
The fact that both low- and high-latitude birds possess exquisite sensitivity to small differences in photoperiod illustrates the problem of assuming that differences in the reproductive cue response systems of birds in different environments necessarily represent adaptations. We should be testing the hypothesis that specific features of the cue response systems represent adaptations, not simply searching for evidence that differences exist, and that they appear to be adaptive in the sense of making the birds well suited to those environments. Well suited equals neither ‘adapted for' nor ‘necessary for'(Ketterson & Nolan 1999). Thus, the intertaxon diversity in different elements of birds' reproductive cue response systems provides the raw material for evaluating whether specific adaptations exist to facilitate appropriate timing of breeding in different environments, but we should use caution when inferring adaptation.
3. Evidence of adaptation in reproductive cue response systems: historical perspective
Although a vast number of experimental studies have been performed on a wide array of avian taxa, comparisons among taxa were hampered from the start by inconsistencies in the experimental approaches used (Farner et al. 1983). Nevertheless, illuminating evaluations of the evolutionary significance of differences and similarities among taxa in their cue response systems have been made. Many general reviews discuss differences in cue response patterns and mechanisms that suggest adaptive specialization for different reproductive patterns, especially seasonality versus opportunism (Ball & Hahn 1997; Hahn et al. 1997; Dawson et al. 2001; Hau 2001; Coppack & Pulido 2004). The most thorough effort to date is still Lofts & Murton's (1968) classic review. They broke photoperiodic taxa into six categories primarily according to experimentally demonstrated differences in photoperiodic thresholds for initial photostimulation, in the degree of tolerance for prolonged exposure to very long days before development of photorefractoriness, and in the subsequent length of the photorefractory period, and discussed how these different features facilitated the different reproductive schedules and migratory patterns displayed, as well as the way that plumage moult was fit into the annual cycle.
Under Lofts & Murton's scenario, Type A species are those with short breeding seasons that begin early in spring but end before the summer solstice (because, from an ultimate standpoint, favourable food supplies and the like have disappeared by then), facilitated by low thresholds for photostimulation (i.e. below 12 L), and a tendency to develop refractoriness rapidly and maintain it for a long time. This pattern is suitable for sedentary (or only longitudinally migratory), primarily single-brooded taxa living at modest latitudes and exploiting resources that appear for a restricted period of time very seasonally. The examples cited by them include mallards (Anas platyrhynchos), European starlings (S. vulgaris) and rooks (Corvus frugilegus).
Type B species also have a low threshold for photostimulation and, therefore, the capacity to achieve reproductive competence very early in spring, but a high tolerance for long-day exposure without developing refractoriness and a shorter refractory period, leading to a long breeding season that ends well after the summer solstice. This pattern facilitates production of two or more broods, and would be suitable for species exploiting environments where favourable conditions for successful breeding persist longer than for Type A species. Examples include the non-migratory, multi-brooded Nuttall's white-crowned sparrow (Zonotrichia leucophrys nuttalli). In both Type A and B species, the fact that the birds remain at mid-latitudes after breeding requires that the refractory period carries far enough into the autumn for short days to prevent reproductive development after refractoriness dissipates, guaranteeing that gonadal recrudescence will not resume until the following winter/spring.
Type C species are those migrating to moderately high latitudes to breed and wintering near the equator where their ‘winter' day lengths remain modestly long (e.g. around or somewhat above 12 L : 12 D). A requirement for relatively long photoperiods to induce reproductive competence in spring would help guarantee that they did not achieve full breeding condition until they reached their relatively high-latitude breeding areas. The relatively rapid development of refractoriness, and the long refractory period that ensues in these species, would terminate breeding in time for moult and migration and prevent maladaptive acquisition of breeding condition on the equatorial breeding grounds. Many waders and other equatorial migrants that breed at moderately high northern latitudes were proposed to fall in this category.
Like Type C, Type D species also have a high photostimulation threshold, guaranteeing very late acquisition of breeding condition, but these migratory taxa winter well north of the equator and therefore do not require as long a refractory period as Type C because the day lengths they encounter drop below a fully photosensitive stimulation threshold relatively early in autumn (compared with Type C) and remain short until the following spring. Examples cited include high-latitude breeding subspecies of white-crowned sparrows and juncos—though it is worth noting that the natural refractory period of the taiga-and tundra-breeding Gambel's white-crowned sparrow (Z. l. gambelii) extends substantially later into the autumn than suggested would be required for this type of cycle (Farner & Mewaldt 1955).
Type E would be typical of transequatorial migrants that never, or at most briefly, encounter photoperiods less than 12 L : 12 D that prevail as they cross the equator during migration. Birds like the bobolink (Dolichonyx oryzivorus) display migratory patterns that match these conditions and possess the expected long threshold for photostimulation, long refractory period and ability to dissipate refractoriness while on relatively long days (Engels 1962).
Type F species are those lacking a refractory period and therefore capable of responding to photostimulation throughout the year. Such species would typically have reproductive schedules symmetrical about the solstices (see Nicholls et al. 1988). From the standpoint of the possibility that phylogenetic history may play a role in dictating species' characteristics, it is noteworthy that all of the taxa suggested by Lofts & Murton (1968) to fall into this category are either Columbiformes or Galliformes (mostly quail). It should also be noted, however, that subsequent experimentation with quail has demonstrated that they, in fact, do become refractory, just not absolutely refractory (Robinson & Follett 1982; Nicholls et al. 1988; Cockrem 1995; see also Ball & Hahn 1997; Hahn et al. 1997; Dawson et al. 2001).
Lofts & Murton (1968) also discuss at length the putative adaptations of taxa that presumably do not regulate breeding schedules using photoperiod. They emphasize the importance of responses to non-photic cues such as rainfall and green vegetation, and of the relatively modest temporal changes in gonad size and in spermatogenic status of males compared with photoperiodic temperate zone taxa. Of course, non-photic cues are now well known to be important in highly photoperiodic taxa as well, and are integrated with photoperiod and endogenous annual rhythms for optimal timing of gonadal development, onset of nesting and termination of reproductive competence (see Wingfield et al. 1992; Hahn et al. 1997).
One of the key points to be taken from Lofts & Murton's (1968) careful analysis is the fact that there are many elements to avian reproductive cue response systems, and these all have the potential to be modified differentially by selection. When considering features of cue responsiveness that would affect the degree to which different taxa are capable of different degrees of temporal reproductive flexibility, Hahn et al. (1997) highlighted three general traits that would be highly relevant. First, the minimum photoperiodic threshold (‘critical day-length' of Hamner 1968) required for advancement of gonadal development would dictate how early breeding could commence in winter or spring. This feature received extensive attention from Lofts & Murton (1968, see above; see also Hau 2001). Second, the nature of the refractory period (absolute, relative, or absent) would play a fundamental role in determining whether breeding is physiologically possible at different times of a year; taxa displaying only relative refractoriness (reduced, but not completely eliminated, responsiveness to reproductive stimuli) or no refractoriness would be capable of greater temporal flexibility than would be permitted by an absolute refractory period that eliminates responsiveness to all manner of cues (Ball & Hahn 1997; Hahn et al. 1997). Third, the extent to which non-photic cues such as food supply, temperature and social interactions can supersede or at least be integrated with photic cues (or circannual rhythms) in different species could strongly affect the potential for temporal flexibility of reproduction (see also Wingfield et al. 1992, 1993; Hau 2001). Hahn et al. (1997) emphasized additional details of the refractory period, in particular, that would affect capacity for reproductive flexibility: (i) timing of onset, (ii) duration, (iii) conditions permitting dissipation, and (iv) the form, absolute or relative. The first three of these were also emphasized by Lofts & Murton (1968), but the existence of relative refractoriness was only reported for the first time, as a transitional stage during dissipation of absolute refractoriness in the house finch, at the time Lofts & Murton's review appeared (Hamner 1968). It was not appreciated to be present as a terminal condition (i.e. present without then proceeding to absolute refractoriness) in Japanese quail for more than another decade (Robinson & Follett 1982). Relative refractoriness is interesting from the standpoint of species' capacity for temporal reproductive flexibility because it facilitates seasonal termination of breeding, as day length declines during summer, without precluding maintenance or resumption of reproductive activity if conditions remain particularly favourable (see Ball & Hahn 1997; Hahn et al. 1997; see also below, §5).
Many recent studies have focused especially on photoperiodic threshold as a trait that may be under selective pressure (e.g. Silverin et al. 1989, 1993; Silverin 1995; Lambrechts et al. 1996), and although this is perfectly reasonable, we want to emphasize the utility of considering a variety of features of the reproductive cue response system owing to the diverse and important effects they may have on reproductive timing. Table 1 summarizes some features that we feel deserve attention, including all those noted above as well as a few others.
Table 1.
Summary of cue response characteristics potentially susceptible to natural selection and their suggested significance to reproductive scheduling.
| cue response feature | significance |
|---|---|
| photoperiodic threshold when fully sensitive | affects possible date of earliest onset of breeding |
| long-day requirement | dictates whether photostimulation is required for gonadal development, or whether non-photic cues might be sufficient under some circumstances |
| relative sensitivity to photic and non-photic cues | dictates extent to which non-photic cues may induce gonadal development |
| presence of refractoriness of any kind | determines trade-off of reproductive flexibility and risk of breeding at inopportune times |
| form of refractoriness (absolute, relative and none) | determines whether any breeding response is possible during refractory phase |
| duration of refractory period | may determine potential for late summer and autumn breeding attempts |
| conditions permitting dissipation of refractoriness | dictates whether breeding can only resume after autumn is passed, and whether refractoriness can dissipate on long days on Southern Hemisphere wintering grounds |
| spontaneous gonadal regression without change in cues | obligate termination of breeding during favourable conditions, guarantees termination of breeding by a certain time; constrains against prolonging breeding |
| total unresponsiveness to cues during refractory period | dictates obligate hiatus in breeding, even if unusually favourable conditions indicated by non-photic cues were to appear |
| rate of development of refractoriness | dictates potential duration of breeding season at any particular photoperiod |
| plasticity of timing of onset of refractoriness depending on non-photic cues | ability to prolong breeding for additional clutches, or when unusual conditions appear late in season, even in absolutely refractory taxa |
| presence of photoperiod range where reproductive condition is possible but refractoriness does not develop | could permit protracted or continuous breeding at some lower latitudes while producing obligate seasonal breeding at higher latitudes |
| presence of juvenile refractory period | dictates whether young birds can acquire breeding condition within the same breeding season as they were born |
4. Alternative explanations: adaptive specialization, conditional plasticity and phylogenetic history
Adaptive specialization of cue response systems could find support from a variety of sources. The most common approach that has been used in the past is to consider data on features of cue response systems in light of the challenges to appropriate reproductive timing (both when to breed, and when not to breed) presented by birds'natural environments (e.g. Lofts & Murton 1968; Farner et al. 1983; Hahn et al. 1997; Hau 2001). This approach is most convincing when some attempt to consider the possible effects of phylogenetic history is made. Although past reviewers did not explicitly consider the effects of phylogenetic history vis-à-vis Felsenstein (1985), they clearly recognized the potential importance of phylogeny and not just ecology in determining the cue response traits that particular species possess. Based on differences among different groups (e.g. ploceids versus emberizines), Farner et al. (1983) argued that photorefractoriness, which is profoundly important for its effects on timing of termination of breeding and capacity for reproductive flexibility (Farner et al. 1983; Nicholls et al. 1988; Wilson & Donham 1988; Cockrem 1995; Sharp 1996; Ball & Hahn 1997; Hahn et al. 1997; Dawson et al. 2001; Hau 2001; Hahn et al. 2004; Beebe et al. 2005; MacDougall-Shackleton et al. 2005), probably has multiple evolutionary origins. Likewise, the evident ease of adaptive specialization of particular features of the photoperiodic response system was argued specifically on the strength of comparisons of very closely related taxa. For instance, whinchat, Saxicola rubetra, was categorized as type D (see above) and stonechat, Saxicola torquata, as type C. Likewise, Nuttall's white-crowned sparrow (Z. l. nuttalli) was categorized as type B and Gambel's white-crowned sparrow (Z. l. gambelii) as type D. Nonetheless, the hypothesis that phylogenetic history may dictate cue response character traits was never explicitly enunciated.
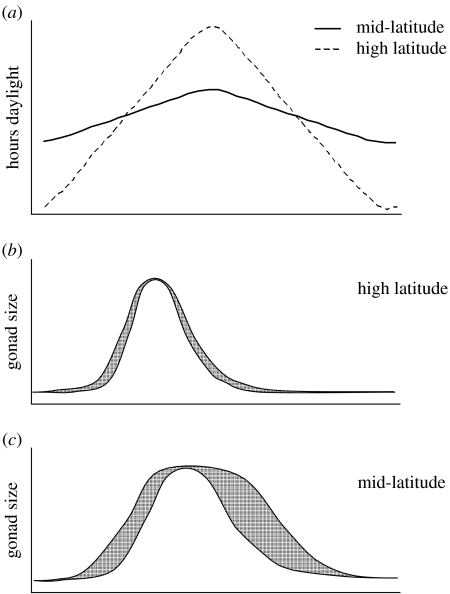
Perhaps even more significantly, the possibility that plasticity built into the cue response system might permit appropriate, or perhaps even optimal, timing of the different stages of the annual cycle in diverse environments, without benefit of specialized adaptations to those environments, has received short shrift compared with the plethora of studies reporting adaptive features of the focal species. Photoperiodic response systems are, in fact, beautifully predisposed to permit an array of appropriate responses under different environmental conditions. For instance, within a single taxon, such as Gambel's white-crowned sparrow, there is a nonlinear parametric relationship between day length and rate of gonadal growth from 12 L : 12 D up through 20 L : 4 D, beyond which the growth rate is not further enhanced by longer photoperiods (Farner & Wilson 1957; Farner et al. 1981). This suggests that ‘photoperiodic drive' on the pituitary by the hypothalamus (cf. Follett & Nicholls 1984; Nicholls et al. 1988) increases as photoperiod increases above the minimum stimulation threshold. If, as seems plausible, the total ‘reproductive drive' is actually a combination of photoperiodic drive and ‘non-photic drive' imposed by other kinds of cues (cf. Hahn et al. 1997), then as long as photoperiod is above some minimum threshold to permit other cues to have an effect on the reproductive axis at all (Ball 1993), the drive from stimulatory non-photic cues could act as accelerators (cf. Marshall 1959) to enhance the rate of gonadal development by combining with the drive from photic cues. At very long photoperiods (in this case 20 L : 4D up through constant light; Farner et al. 1981), photoperiodic drive would be maximal, thereby leaving little scope for inputs from other cues to affect gonadal condition even if the species is responsive to these types of cues under shorter photoperiods (see Hinde & Steel 1976, 1978; Morton et al. 1985; Bentley et al. 2000; Lambrechts & Perret 2000). However, on shorter long photoperiods (i.e. above threshold but well below peak photostimulation), where photoperiodic drive is not near maximal, the enhancing effects of non-photic cues could be substantial, potentially bringing individuals up to a rate of gonadal maturation similar to that induced by the longest days. Consequently, the same individual bird could adjust reproductive schedule adaptively under a range of photoperiodic conditions (illustrated in figure 1). For example, a long and relatively flexible reproductive schedule would naturally emerge if the bird lived at mid-latitudes where photoperiod never gets very long. Flexible timing of acquisition of full reproductive condition in spring would result naturally from only modest photoperiodic drive, with most rapid reproductive development only occurring if non-photic cues were sufficiently favourable to accelerate gonadal maturation. The relatively slow development of absolute photorefractoriness when long days are relatively short (Nicholls et al. 1988) inevitably would also contribute to a relatively long breeding season at lower latitudes. In contrast, the same individual bird living at higher latitudes would experience much more vigorous photoperiodic drive under the longer and more rapidly increasing photoperiods of spring, including potentially nearly stepwise increases to very long days if the birds migrate rapidly to high latitudes. This would leave relatively little room for enhancing effects of non-photic cues, which seems appropriate for high-latitude breeders in which gonadal development happens during migration when the birds are still distant from the actual breeding site, so short-term non-photic cues would not yet be relevant (Wingfield et al. 1996). Further, rapid development of refractoriness would terminate breeding relatively quickly. It is also important to keep in mind that non-photic cues, such as social interactions with mates, can specifically influence the timing of onset of photorefractoriness (e.g. Schwab & Lott 1969; Runfeldt & Wingfield 1985).
Figure 1.
Schematic illustrating conditional plasticity, whereby two birds with identical photoperiod response systems could exhibit different breeding schedules as a result of differing latitude. (a) At high latitudes, photophase increases more rapidly in spring, resulting in greater photoperiodic drive: gonads recrudesce more rapidly and refractoriness has an earlier onset. (b) Non-photic cues have reduced impact on reproductive flexibility (shaded area) because photoperiod drives the reproductive axis at near-maximal capacity. (c) At mid-latitudes photoperiodic drive is reduced: gonads recrudesce more slowly and refractoriness has a later onset. Non-photic cues can have larger impact on onset and offset of breeding.
The point to be made here is that the same individual bird living in different environments could, by virtue of this conditional plasticity built into the cue processing system, generate quite different and highly appropriate reproductive schedules in those different environments. These adaptive breeding schedules need not be the consequence of adaptive specialization to the specific photoperiodic and other cues encountered if the inherent plasticity—which may include the fine-tuning effects of non-photic cues integrated with photoperiod and endogenous rhythms—already copes effectively with the range of variation involved.
Another point of interest is the possibility that early experience programmes individuals, through phenotypic plasticity, to develop reproductive cue response characteristics that are particularly appropriate for the environment occupied. If this is the case, apparent adaptive specializations may, in fact, represent a form of individual plasticity, rather than adaptive specializations to local conditions. The amount of research on this fascinating topic is as yet small in the field of avian reproductive regulation, but there has been significant progress with respect to migratory activity (e.g. Coppack et al. 2001), and especially regarding the ‘calendar effect' of hatching date on the schedule of plumage moult (see Helm & Gwinner 1999). Several features of the first prebasic (equal to postjuvenal; see Humphrey & Parkes 1959) moult are affected by hatch date, including date of onset and completion, duration and rate (Helm & Gwinner 1999). The response of the first prebasic moult schedule to photoperiodic experience generally leads to adaptive adjustments of moult within the time constraints imposed by birds' environments, but different populations (subspecies) also differ in the degree of responsiveness of the first prebasic moult to photoperiodic influences (Gwinner et al. 1983; Helm & Gwinner 1999).
Of course, plastic responses to different environmental conditions may not be adaptive when individuals encounter novel circumstances. Indeed, maladaptive consequences appear to ensue from differences in the relative responsiveness of different elements of the annual cycle to novel photoperiodic conditions (Coppack et al. 2001). Blackcaps (Sylvia atricapilla) exposed to experimental photoperiods simulating an earlier hatching date did not delay termination of moult and onset of autumnal migratory activity to a degree that would compensate for the simulated advancement of hatching date. This would lead to individuals initiating migration substantially earlier than would be expected to be adaptive, although there is reason to suspect that selection should be able to modify this relationship between hatch date and moult/migration (Coppack et al. 2001; see below). In any case, by being born under different conditions, adjustments in responses to cues could result, some of which at least could be adaptive. This points to the cautionary note that what appears to be adaptive specializations could, in fact, be the result of developmental phenotypic plasticity.
5. Case study: absolute refractoriness in songbirds in general, and cardueline finches in particular
Although reproductive cue response systems function as integrated wholes, it is still instructive to consider particular features alone, since they can have relatively specific effects on the reproductive cycles that are possible, and may be subject to differential selection. Here we will examine one particularly familiar and well-studied phenomenon, the termination of reproductive competence despite the persistence of conditions that should be stimulatory. Changing photoperiod has several important effects on seasonally breeding birds (see Farner & Follett 1979; Farner & Gwinner 1980; Nicholls et al. 1988; Wilson & Donham 1988). Lengthening days in spring induce rapid development of the gonads to full reproductive competence. Later, the birds become unresponsive, or absolutely refractory, to the stimulatory effects of long days; the gonads regress and the birds become reproductively quiescent despite continued long days. Experimentally, absolute refractoriness has been identified by either of two criteria: (i) gonads spontaneously regress and prebasic plumage moult proceeds without any decline in photoperiod, or (ii) gonadotrophin levels and gonads are unaffected by even longer days (24 L : 0 D in the extreme) once gonads have regressed and moult is advanced (see Hamner 1968; Farner et al. 1983; Nicholls et al. 1988). During autumn, birds regain photosensitivity and can again respond to long days and other cues (see Farner et al. 1983; Nicholls et al. 1988; Wilson & Donham 1988; Ball 1993; Sharp 1996; Hahn et al. 1997; Ball & Hahn 1997; Wingfield & Farner 1993; Dawson et al. 2001). A special form of photorefractoriness, relative refractoriness (see Hamner 1968; Robinson & Follett 1982), reduces but does not eliminate photosensitivity. Species that only become relatively refractory regress gonads on declining summer photoperiod, while days still exceed the spring stimulation threshold, but maintain mature gonads indefinitely on constant long photoperiods, and are still responsive to longer days after declining day length precipitates gonadal regression (Robinson & Follett 1982). Relative photorefractoriness has been best studied in Japanese quail, Coturnix japonica, but note that some characteristics of it are present in some other species, such as song sparrows (Wingfield 1993); it may be the only form of refractoriness that crossbills display (Hahn 1995, 1998; Hahn et al. 2004). Absolute refractoriness is presumably adaptive in many environments because it completely prevents inappropriately timed late summer and autumn reproductive attempts. It may be costly, however, because it restricts when birds can breed and thus their potential reproductive flexibility. Therefore, it should only evolve, or be maintained, when the cost of missing some legitimate reproductive opportunities is outweighed by the benefit of not trying to breed at inopportune times. Relative refractoriness confers some of the same advantages as absolute refractoriness by reducing the likelihood of breeding in, for example, late summer and early autumn in largely seasonal environments, but apparently without completely eliminating the possibility of breeding, should unusually favourable conditions be encountered. It therefore seems to be an intuitively superior way to facilitate a seasonal reproductive schedule for species that occasionally encounter conditions that permit successful breeding very late in summer or early autumn (see Hahn et al. 1997 for full discussion).
The general question is whether variation in reproductive schedules (seasonal and opportunistic) reflects evolved variation in modulation of responsiveness to cues (sensitivity and refractoriness). The adaptive specialization hypothesis would predict that maximal flexibility (opportunism) requires a persistent high level of responsiveness to environmental cues to permit rapid initiation of breeding whenever unpredictable favourable conditions for breeding are encountered (cf. Farner & Serventy 1960; Ball & Hahn 1997). In contrast, strict seasonality would be facilitated by development of an absolute refractory period that prevents attempted breeding at inappropriate times. Intermediate degrees of flexibility could be facilitated by dissociation of the two absolute refractoriness criteria. Alternatives to the adaptive specialization hypothesis would be that conditional plasticity permits essentially similar cue response systems to produce different adaptive reproductive schedules under different circumstances, or that phylogenetic history explains the distribution of cue response characteristics in different taxa.
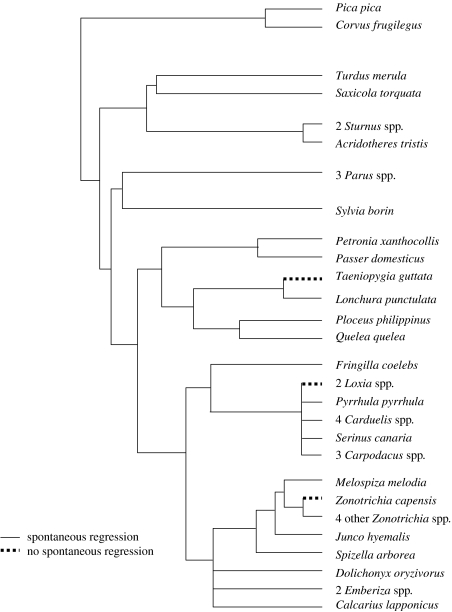
We have so far located data regarding absolute refractoriness criterion 1 (spontaneous regression of gonads without a decline in photoperiod) for 41 species of songbirds from seven families (table 2). Of these, only four species (zebra finch, red crossbill, white-winged crossbill and rufous-collared sparrow) failed to regress gonads when held for extended periods on long days (figure 2). All others either regressed spontaneously on constant long photoperiods or regressed gonads while day length was still increasing (table 2). The phylogenetic distribution of failure to meet criterion 1 for absolute refractoriness is consistent with it being a derived trait in the songbirds. Spontaneous gonadal regression definitely appears to be ancestral to the emberizines and fringillines. Among the representatives examined, only the two crossbill taxa in the fringillines and Zonotrichia capensis (specifically, the subtropical form, Z. c. hypoleuca, was studied; it will be interesting to see whether higher-latitude subspecies of this widely distributed taxon are similar to Z. c. hypoleuca) among the emberizines fail to display the trait. This distribution of the trait supports the loss of refractoriness criterion 1 representing an adaptive specialization for greater reproductive flexibility. Zebra finches (Taeniopygia guttata) and crossbills (Loxia curvirostra and Loxia leucoptera) are archetypal opportunists that have been proposed to possess features that facilitate temporal reproductive plasticity (see Hahn et al. 1997 for review; see also Perfito et al. 2006). Rufous-collared sparrow (and particularly Z. c. hypoleuca) is the sole tropical and subtropical representative of its genus (though its geographical range extends well into the south temperate zone as well), and these birds display a long flexible reproductive schedule (see Moore et al. 2004). All other taxa represented in figure 1 qualify as seasonal breeders with the exception of Quelea. It is noteworthy that although quelea do spontaneously regress their gonads when held on constant long days, they also rapidly dissipate refractoriness and reattain reproductive condition without a short-day requirement (Lofts & Murton 1968). This ability may be widespread in the Passeridae: at least some individual house sparrows (Passer domesticus) also can dissipate absolute refractoriness when continuously exposed to long days (see Farner et al. 1983; see also Hahn et al. 1997), and it may facilitate substantial reproductive plasticity in the group. Some other taxa (e.g. song sparrows, Melospiza melodia melodia; Wingfield 1993) also display this characteristic, at least in some individuals; it will be illuminating to determine how this trait is distributed among a wider array of taxa.
Table 2.
Distribution of photorefractoriness, defined by criterion 1 (spontaneous regression of gonads while on constant stimulatory long days) among songbird species. Taxonomy follows Sibley & Ahlquist (1990) and Monroe & Sibley (1993).
| common name | latin name | refractory? | conditions/comments | citation |
|---|---|---|---|---|
| family Corvidae | ||||
| black-billed magpie | Pica pica | yes | natural (gonads regress while day length is still increasing in spring) | Erpino (1969) |
| rook | Corvus frugilegus | yes | natural (gonads regress while day length is still increasing in spring) | Marshall & Coombs (1957) |
| family Muscicapidae subfamily Turdinae | ||||
| European blackbird | Turdus merula | yes | natural (gonads began regression before photoperiod declined, both sexes) | Partecke et al. (2004) |
| subfamily Muscicapinae tribe Saxicolini | ||||
| stonechat | Saxicola torquata | yes | increasing (gonads regressed while days still increasing) | Helm & Gwinner (2005) |
| family Sturnidae | ||||
| European starling | Sturnus vulgaris | yes | 15 L : 9 D | Burger (1947) |
| Brahminy myna | Sturnus pagodarum | yes | 12 L : 12 D 14 L : 10 D 16 L : 8 D both sexes tested | Kumar & Kumar (1993) |
| Common myna | Acridotheres tristis | yes | 15 L : 9 D | Chaturvedi & Thapliyal (1983) |
| family Paridae | ||||
| willow tit | Parus montanus | yes | 20 L : 4 D | Silverin & Viebke (1994) |
| black-capped chickadee | Parus (Poecile) atricapillus | yes | 15 L : 9 D | Phillmore et al. (2005) |
| great tit | Parus major | yes | increasing (testes regressed while pp still increasing) 20 L : 4 D (regressed completely) | Silverin et al. (1993); Silverin & Viebke (1994) |
| family Sylviidae | ||||
| garden warbler | Sylvia borin | yes | 15 L : 9 D | Gwinner et al. (1988) |
| family Passeridae subfamily Passerinae | ||||
| house sparrow | Passer domesticus | yes | 18 L : 6 D 16 L : 8 D | Dawson (1991); Hahn & Ball (1995) |
| chestnut-shouldered petronia | Petronia xanthocollis | yes | 15 L : 9 D | Tewary & Tripathi (1985) |
| subfamily Ploceinae | ||||
| common (Baya) weaver | Ploceus philippinus | yes | 13 L : 11 D; 14 L : 10 D but does not regress on 15 L : 9 D (held 15 months) | Thapliyal & Saxena (1964); Chandola & Chakravorty (1982); Chakravorty & Chandola-Saklani (1985) |
| red-billed quelea | Quelea quelea | yes | 12 L : 12 D; 17 L : 7 D | Lofts (1962, 1964) |
| subfamily Estrildinae | ||||
| zebra finch | Taeniopygia guttata | no | 17 L : 7 D and 9 L : 15 D for eight months, with and without water, no gonadal differences among groups…all big at end. | Sossinka (1974) |
| spotted munia | Lonchura punctulata | yes | 24 L : 0 D | Chandola-Saklani et al. (2004) |
| family Fringillidae subfamily Fringillinae tribe Fringillini | ||||
| chaffinch | Fringilla coelebs | yes | uncertain (long days) | Dolnik, (1975, 1976a,b; cited in Farner et al. (1983)) |
| tribe Carduelini | ||||
| white-winged crossbill | Loxia leucoptera | no | 20 L : 4 D gonads did regress slightly but significantly after five months, but were still breeding size | Hahn et al. (2004) |
| red crossbill | Loxia curvirostra | no | 16 L : 8 D (Groth's (1993) type 2 and type 3) | Hahn (1995), unpublished |
| common redpoll | Carduelis flammea | yes | 20 L : 4 D | Hahn et al. (2004) |
| pine siskin | Carduelis pinus | yes | 20 L : 4 D | Hahn et al. (2004) |
| American goldfinch | Carduelis tristis | yes | 18 L : 6 D both males and females | Marsh et al. (2002) |
| greenfinch | Carduelis chloris | yes | 16 L : 8 D, based on only a single individual | Damste, (Damsté 1947) |
| canary | Serinus canaria | yes | 20 L : 4 D; 16 L : 8 D. did not regress on 11 L | Storey & Nicholls (1976) |
| house finch | Carpodacus mexicanus | yes | 18 L : 6 D; 12 L : 12 D; results a bit more ambiguous on 10 L : 14 D but regression did occur | Hamner (1966) |
| Cassin's finch | Carpodacus cassinii | yes | 15 L : 9 D; based on completion of normal moult and regression of cloacal protuberances | Hahn unpublished |
| common rose-finch | Carpodacus erythrinus | yes | 15 L : 9 D; 24 L : 0 D both males and females spontaneously regress on 15 L : 9 D | Kumar & Tewary (1982); Tewary & Dixit (1983), Tewary et al. (1983) |
| eurasian bullfinch | Pyrrhula pyrrhula | yes | 16 L : 8 D | Storey & Nicholls (1982) |
| subfamily Emberizinae tribe Emberizini | ||||
| black-headed bunting | Emberiza melanocephala | yes | 11.5 L : 12.5 D; 12 L : 12 D; 12.5 L : 11.5 D; 13 L : 11 D; 15 L : 9 D all spontaneously regressed | Tewary & Kumar (1982); Misra et al. (2004) |
| red-headed bunting | Emberiza bruniceps | yes | 12 L : 12 D; 12.25 L : 11.75 D; 12.5 L : 11.5 D; 13 L : 11 D; 14 L : 10 D; 18 L : 6 D all spontaneously regressed (11.5 and 11.75 L did not) | Rani et al. (2005b) |
| Lapland longspur | Calcarius lapponicus | yes | 23 L : 1 D | Hunt & Wingfield, unpublished (personal communication) |
| song sparrow | Melospiza melodia | yes | 18 L : 6 D | Wingfield (1993) |
| rufous-collared sparrow | Zonotrichia capensis | no | 20 L : 4 D (300+days without regression) 10 L : 14 D | Lewis et al. (1974) |
| harris' sparrow | Zonotrichia querula | yes | 20 L : 4 D | Donham & Wilson (1970) |
| white-throated sparrow | Zonotrichia albicollis | yes | 13 L : 11 D; 20 L : 4 D | Harris & Turek (1982) |
| white-crowned sparrow | Zonotrichia leucophrys | yes | 16 L : 8 D; 18 L : 6 D; 20 L : 4 D; 22 L : 2 D; 23 L : 1 D; 23.5 L : 0.5 D; 24 L : 0 D | Moore et al. (1982); Farner et al. (1983) |
| golden-crowned sparrow | Zonotrichia atricapilla | yes | 20 L : 4 D | Wingfield, unpublished (2002, personal communication) |
| dark-eyed junco | Junco hyemalis | yes | 20 L : 4 D | Wolfson (1952); Wolfson (1959) |
| American tree sparrow | Spizella arborea | yes | 20 L : 4 D (both sexes tested) | Wilson & Follett (1974) |
| tribe Icterini | ||||
| Bobolink | Dolichonyx oryzivorus | yes | 14 L : 10 D | Engels (1959) |
Figure 2.
Distribution of absolute refractoriness defined by criterion 1 (spontaneous regression of gonads on constant long days). Phylogeny adapted from Sibley & Ahlquist (1990), based on DNA–DNA hybridization. Higher-order taxa, common names of taxa and references in table 2.
The Zonotrichia warrant additional comment. All four congeners of Z. capensis (Zonotrichia leucophrys, Zonotrichia atricapilla, Zonotrichia albicollis and Zonotrichia querula) winter in the north temperate zone, breed at temperate, subarctic and Arctic latitudes, and spontaneously regress gonads when held on constant long days. Z. capensis has been shown by a variety of phylogenetic methods to be the most basal lineage in the genus (Zink 1982; Zink et al. 1991; Zink & Blackwell 1996; Patten & Fugate 1998). Thus, the overall distribution of absolute refractoriness criterion 1 within Zonotrichia and among near out-groups (Melospiza, Junco and Spizella) suggests that this trait was lost as an adaptive specialization to breeding at low latitudes along the lineage leading directly to Z. capensis, but retained as an ancestral trait in the lineage leading to all other Zonotrichia. Other possible explanations (e.g. loss of absolute refractoriness criterion 1 along the lineage from other emberizines to the genus Zonotrichia, followed by reappearance of refractoriness criterion 1 at the branch point uniting Z. capensis to the rest of the genus) require more evolutionary events and are therefore less parsimonious.
Data on refractoriness criterion 1 are particularly interesting when examined in more detail within the cardueline finches. Cardueline finches are ideal for testing the relationships among reproductive schedule and cue responsiveness modulation (refractoriness, according to both criterion 1, spontaneous termination of reproductive competence without a decline in day length, and criterion 2, complete unresponsiveness to long days). They are a diverse tribe (Carduelini) in the family Fringillidae (Sibley & Monroe 1990), and they range from seasonal to opportunistic breeders. Reproductive schedules of many are restricted to late spring and early summer (e.g. Hahn 1996; MacDougall-Shackleton et al. 2000), but crossbills (opportunists) regularly breed January–September and occasionally in any month if the conifer seeds on which they specialize are sufficiently abundant (Benkman 1990, 1992; Adkisson 1996). We have sufficient data within carduelines to perform informative analyses of the distribution of both refractoriness criteria 1 and 2. House finches (Carpodacus mexicanus), Cassin's finches (Carpodacus cassinii), scarlet rose-finches (Carpodacus erythrinus) and grey-crowned rosy-finches (Leucosticte tephrocotis) are all seasonal breeders. Wild populations of canaries (Serinus canaria) have a flexible breeding schedule (Leitner et al. 2003). American goldfinches (Carduelis tristis), pine siskins (C. pinus), common redpolls (Carpodacus flammea), red crossbills (L. curvirostra) and white-winged crossbills (L. leucoptera) represent a speciose group of recently evolved close relatives (Marten & Johnson 1986; Arnaiz-Villena et al. 2001). Redpolls and goldfinches breed seasonally. Siskins are flexible, breeding from early spring through late summer if food is sufficiently abundant (Hahn, unpublished observations). Although fundamentally seasonal (Dawson 1997), siskins are capable of arresting plumage moult and resuming breeding in mid-summer, after the normal spring breeding has ended (Hahn 1992, unpublished data). Bullfinches (Pyrrhula pyrrhula) resemble pine siskins in displaying a flexible seasonal breeding schedule that appears to be capable of substantial extension in late summer if preferred foods are particularly abundant (Newton 1973). Crossbills are the most flexible, breeding at least occasionally in any month, on any naturally occurring mid-latitude photoperiod if food (conifer seeds) is abundant (Benkman 1990, 1992; Hahn 1995, 1998; Adkisson 1996). Many field-caught crossbills show clear evidence of arrested moult (indicative of reversal of reproductive regression after spring breeding; Hahn et al. 1992) when breeding in late summer (Hahn 1998 and unpublished).
Within carduelines, refractoriness criterion 1 data are, so far, clear-cut (table 2, figure 2). Both crossbill species fail to regress gonads on constant long days. All other carduelines tested, including flexibly seasonal taxa such as canaries, pine siskins and bullfinches (Newton 1973; Storey & Nicholls 1976; Bentley et al. 2003; Hahn et al. 2004), do spontaneously regress gonads on constant long days. It is worth noting that this regression may take an extended period of time in these flexible breeders—up to 50 weeks in bullfinches (Storey & Nicholls 1982). However, all seasonally breeding carduelines tested thus far, whether flexible or strictly seasonal, display refractoriness as defined by criterion 1. Conditional plasticity may account for apparent differences in the time taken by some flexible taxa take to become refractory: the bullfinches were tested on 16 L : 8 D photoperiod (Storey & Nicholls 1982) while the pine siskins were tested on 20 L : 4 D photoperiod, which is well known to drive birds through to refractoriness sooner than shorter long days (Nicholls et al. 1988). Thus, within carduelines, loss of spontaneous gonadal regression appears to represent a recent, and intuitively sensible, adaptive specialization unique to the opportunistic crossbills, facilitating continued late-season breeding if food supply permits.
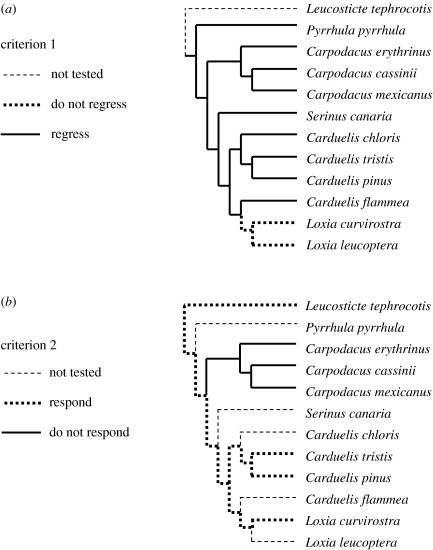
Fewer cardueline taxa have been tested for absolute refractoriness criterion 2 (lack of response to 24 L after gonadal regression). Different character states for this trait could be complete lack of response when challenged, delayed response or immediate and sustained response. Immediate and sustained response would facilitate maximum flexibility (opportunism), but a delayed response (e.g. if gonadotrophin-releasing hormone production needed to be reinitiated first; see Ball & Hahn 1997; Hahn et al. 1997) would permit flexibility. Of the seven cardueline taxa that have been tested for this trait, only the three Carpodacus (house finch, Cassin's finch and scarlet rose-finch) show no response (MacDougall-Shackleton et al. 2005). This sandwiches total unresponsiveness between responsiveness in the more basally derived rosy-finch (Leucosticte) and that in the rest of the species. Had the rosy-finches displayed unresponsiveness, like the Carpodacus, this would have supported the interpretation that total unresponsiveness evolved after Carpodacus diverged from the main cardueline lineage. All of the cardueline genera that evolved after the Carpodacus branch contain at least some highly flexible species, so this result would have supported adaptive specialization through reappearance of responsiveness after gonadal regression. However, the fact that rosy-finches are responsive to 24 L after gonadal collapse suggests the alternative possibility that responsiveness appeared (having been absent in taxa ancestral to the carduelines) early in the cardueline lineage and has been retained in many carduelines through phylogenetic descent, only having been lost in Carpodacus, perhaps as an adaptive specialization for seasonal breeding, which may be universal in that largely Old World genus. Under this scenario, response to 24 L challenge after gonadal regression (i.e. lack of real absolute refractoriness; see Hamner 1968) would exist as an ancestral character that predisposed many recently evolved carduelines to be capable of flexible reproductive schedules, but it would not represent a derived adaptive specialization for that function in those taxa. Thus, with respect to refractoriness criterion 2, there is currently provisional support for both adaptive specialization (Carpodacus re-evolving unresponsiveness to facilitate seasonal breeding) and phylogenetic history (all other taxa having inherited from an early cardueline ancestor an ability to respond to long days, and possibly other cues, after gonads regress). Thorough study of rosy-finches (Leucosticte) and other more basally derived taxa (pine grosbeak, Pinicola enucleator, and evening grosbeak, Coccothraustes vespertinus, would be good choices) is required before this interpretation can be confirmed.
Overall, the clearest support for adaptive specialization comes from the crossbills. Of all the cardueline taxa tested this far, they alone maintain active gonads indefinitely on constant long days and also remain responsive to 24 L even after the gonads regress. Interestingly, they also differ from most other songbirds studied to date in failing to show seasonal downregulation of the hypothalamic gonadotrophin-releasing hormone (GnRH) system that regulates activity of the pituitary and gonads (MacDougall-Shackleton et al. 2001; Pereyra et al. 2005; for general discussions of the functional significance of seasonal downregulation of the GnRH system, see Ball & Hahn 1997; Hahn et al. 1997; Dawson et al. 2001; MacDougall-Shackleton et al. 2005). They are also the only carduelines known to be able to breed on any naturally occurring temperate zone photoperiod. The fact that the crossbills' closest relatives, the redpolls, are seasonal breeders that spontaneously regress the gonads on constant long days (and downregulate the GnRH system, Pereyra et al. 2005) strongly reinforces the interpretation that crossbills have evolved specialized cue response system adaptations which facilitate temporal reproductive flexibility, rather than that crossbills' traits are simply inherited from ancestors (phylogenetic history) or that all carduelines are basically similar and capable of similar degrees of reproductive flexibility depending on local photic and non-photic conditions (conditional plasticity).
6. Implications for evolution, invasion of new habitats and effects of climate change
The recent excellent review by Coppack & Pulido (2004) regarding the implications of cue response (particularly photoperiodic) patterns and mechanisms for evolution, invasion of novel habitats or geographical areas and effects of climate change on bird populations obviates our going into great detail on the topic here. Consequently, we will be brief and focus mostly on ways in which the ideas we present here augment or, in some cases, deviate from their thoughts.
It is evident from the prevalence of references to adaptive significance of the features of cue response mechanisms observed in different studies (in practically every paper we have read on physiological responses to reproductive cues) that it is generally assumed that cue response systems are responsive to selection. The belief in adaptive adjustment of cue response systems is apparent in Farner et al.'s (1983) suggestion that photorefractoriness has multiple evolutionary origins within birds. Lofts & Murton (1968) are very explicit that they think cue response systems must evolve relatively easily owing to apparent adaptive specializations in close relatives, e.g. within the genera Saxicola and Zonotrichia. They also describe what they consider to be the relative ease with which the photoperiodic system of a tropical species like Quelea quelea could be modified by selection to match that of a temperate zone species like Passer domesticus. It is clear, however, that some key elements of the natural house sparrow reproductive pattern, such as accelerated termination of breeding on very long days, would emerge naturally from the conditional plasticity already present in Quelea (Lofts & Murton 1968). Common-garden experiments with different populations of blue tits (Parus caeruleus) and great tits (P. major) have now shown that local adaptation of the photoperiodic response systems of tits has occurred not only in populations at dramatically different latitudes (cf. Silverin et al. 1989, 1993; Silverin 1995), but is evident in geographically very close populations that normally experience similar photoperiods but occupy areas where timing of changes in food supply favour different reproductive schedules. Differential responses to non-photic environmental cues, such as also exist in different tit species (Silverin & Viebke 1994), are not required to explain the observed differences in egg-laying dates under experimental conditions (Lambrechts et al. 1996, 1997). This finding is of particular interest because it demonstrates an evolved adjustment of photoperiodic response patterns in a situation that would have been historically suspected to involve only responses to non-photic cues, i.e. fine-tuning of the photically controlled schedule (see Wingfield et al. 1992; Lambrechts et al. 1996).
Although adaptation may occur readily, the most recent research on this topic reveals that it does not necessarily do so. Under apparent selection for earlier onset of breeding as climate warms and peak food abundance advances, at least one population of great tits (Parus major) in the Netherlands has failed to show any adaptive advancement of laying date, apparently owing to a mismatch between the cues that the tits have evolved to use (primarily photoperiod) and the pattern of appearance of the ultimate factor under a changing climate (Visser et al. 1998, 2004). Most pertinent to our own discussion above is the idea that photoperiodic response patterns may either constrain or enhance adaptation to novel environments, depending on whether the inherent plasticity in the response system leads to adaptive or maladaptive suites of changes in the annual cycle in the short term. If the plastic (within individuals) responses are adaptive, then facilitation or reinforcement of further (genetically based) adaptation to the new conditions may occur (Coppack & Pulido 2004). In this context, it becomes particularly important to consider how novel environmental conditions might affect a suite of characteristics of the cue response system, such as those presented in table 1, rather than just one. Consideration of multiple elements of the environmental cue response system may, like consideration of different elements of the annual cycle (e.g. breeding, moult, migration), help to reveal whether failure to show an adaptive response in one component is constrained by obligations dictated by another element. In the comparative analysis we present here, we consider only one (songbirds generally) or two (carduelines) elements of the cue response system. The potential for constraints of one component on others owing to the entire syndrome of responses, and the question of whether effects of one element on others can be decoupled under selection, is clearly important to both the potential for adaptation to new environments and the response to global climate changes. In this regard, it is noteworthy that the two criteria that are generally used to identify absolute photorefractoriness apparently have been decoupled in several cardueline taxa (figure 3; see MacDougall-Shackleton et al. 2005). Clearly these should not be considered equivalent, but rather should be treated as separate elements of cue response systems.
Figure 3.
Distribution of two criteria for absolute photorefractoriness among cardueline finches. (a) Criterion 1 is spontaneous gonadal regression while on constant long days. (b) Criterion 2 is unresponsiveness to long days (24 hour light in the extreme) during photorefractoriness. Common names of taxa and references in table 2. Phylogeny adapted from Marten & Johnson (1986), Badyaev (1997) and Arnaiz-Villena et al. (2001).
When considering the interplay between adaptive specialization and conditional plasticity, it becomes critical to perform studies that facilitate characterization of reaction norms. The ideal approach of measuring the responses of individuals to a range of conditions would clearly be challenging, perhaps impossible, given the time it takes to perform cue response experiments as well as the possibility that exposure of an individual to one set of experimental circumstances might affect how it responds to other conditions tested subsequently. Even the powerful alternative split sibling approach (e.g. Helm & Gwinner 1999; Coppack et al. 2003) is challenging, requiring access to substantial numbers of captive-raised young. Still, population level estimates of reaction norms (see van Noordwijk 1990; Stearns 1992; Helm & Gwinner 1999) can give an idea of the range of responses possible, and thereby a sense of the extent to which plasticity of responses to particular patterns of environmental cues is likely to be adaptive or not. In this context, the biggest concern may be the possibility that cue responses measured in any given individual have been determined substantially by the environment (e.g. latitude=photoperiod) at which it was born and developed.
It may be the reaction norm, rather than any particular response to a particular set of circumstances, that is under selection (see Visser & Lambrechts 1999). The question of how selection operates on reaction norms remains unresolved (see Coppack et al. 2001), and is of particular interest when contemplating how extremely flexible species, individuals of which may breed under dramatically different conditions in different years, will be affected by selection. Individual crossbills evidently breed on both the shortest and longest days of the year at specific locations. For instance, several individual male red crossbills (Groth's ‘Type 2’, one of the largest North American forms; Groth 1993) had breeding-size testes when captured in August 1988 in Central Washington State, and likewise had fully enlarged testes again when recaptured at the same site in January 1989 (population confirmed to be breeding at both times by capture of ovulating and incubating females, and by timing of appearance of dependent young; Hahn unpublished; Coombs-Hahn 1993). Further, as nomads, individual crossbills can breed at dramatically different latitudes in different years. Crossbills of the smallest North American form (Groth's ‘Type 3’; Groth 1993) commonly occur from northern California to coastal Alaska (a range of approx. 40°–60° N latitude), and individual birds probably breed at very different latitudes in different years. For instance, an adult female that we banded on the Olympic Peninsula of Washington State (47° N latitude) near the end of summer breeding in 2003 was subsequently recovered, and probably had bred in summer, near Juneau, Alaska (58° N latitude) 2 years later (Hahn, unpublished). Both the flexible reproductive timing and nomadism can thus lead to substantial, even extreme, variation in the conditions under which nestlings are raised; summer solstice photoperiod in northern California and southeast Alaska differs by approximately 3 hours (15 L : 9 D versus 18 L : 6 D), and photoperiods in January and July in the Washington Cascade Mountains differ by approximately 8 hours (8 L : 16 D versus 16 L : 8 D). In cases like this, local adaptation to particular photoperiodic and other environmental features clearly would be at best useless and at worst counter productive. In such cases, a system with suitably plastic photoperiodic responses (crossbills are definitely photoperiodic; Tordoff & Dawson 1965; Hahn 1995) would be essential, but given the potential for responses of one feature of the annual cycle to constrain modification of responses in others (e.g. timing of breeding onset versus moult completion versus migratory onset; see Coppack et al. 2001), the precise nature of the plasticity that would be favoured is not yet clear. It also seems sensible to consider carefully the effect that interannual variation in conditions at particular sites, leading to substantial differences in optimal breeding timing among years, might have on the reaction norms which are favoured by selection.
In closing this section, we wish to emphasize two additional things: First, it is probably not possible to overestimate the importance—thoroughly appreciated already by the researchers who have been studying integration of annual cycles of moult, migration and reproduction in stonechats and old world warblers (Gwinner, Berthold and colleagues), as well as by several Indian researchers (Chandola-Saklani, Kumar and colleagues), and several of the pioneers in photoperiodism studies (e.g. Alden Miller, Donald Farner, Albert Wolfson, William Hamner)—of characterizing cue responsiveness characteristics of taxa across a range of stimulus values (e.g. photoperiodic treatments and the like), rather than simply making comparisons among taxa under a single set of photoperiodic circumstances. Although simple common garden experiments under a single set of conditions are certainly informative, they fail to capture how cue response systems of different taxa vary in their potential outputs under different conditions (i.e. conditional plasticity). Clearly, obtaining some indication at least of mean population reaction norms (see also van Noordwijk 1990; Stearns 1992; see Helm & Gwinner 1999) gives a much clearer idea of how cue responsiveness differs among taxa, of the possibility that adaptive specialization has occurred, and of how conditional plasticity differences among taxa may affect behaviour in different environments. Performing experiments across a range of conditions also has somewhat more mundane advantage of simply reducing the likelihood of misclassification of taxa into particular trait categories. In our analysis, for instance, the original paper describing the response of the Baya weaver (Ploceus philippinus) to long days used a single long-day photoperiod treatment, 15 L : 9 D (Thapliyal & Saxena 1964), and reported no spontaneous regression of the gonads even after 15 months of exposure. Subsequent studies testing a range of photoperiods (Chandola & Chakravorty 1982; Chakravorty & Chandola-Saklani 1985) not only produced results on 15 L : 9 D identical to Thapliyal and Saxena's findings, but also demonstrated that complete spontaneous regression did occur on some other long-day photoperiods (e.g. 13 L : 11 D and 14 L : 10 D), and partial spontaneous regression occurs on 16 L : 8 D (Rani et al. 2005a).
Second, keeping clearly in mind the strengths and limitations of comparative approaches and attempting to establish perspectives at multiple comparative scales are extremely important. Detailed comparative studies of closely related taxa, such as the stonechats (see Helm et al. 2005 for review) and North American Zonotrichia (Lofts & Murton 1968; see also reviews by Farner et al. 1983; see Wingfield et al. 1996, 1997, 2003) are extremely powerful but can lead to misinterpretations if one does not step back to a slightly broader comparative perspective as well. For instance, failure to consider the prevalence of absolute refractoriness within songbirds generally could easily lead to the misconception that because Zonotrichia capensis hypoleuca is the most basally derived lineage among Zonotrichia (see above), its character state of no absolute refractoriness is therefore likely to represent the ancestral condition within the genus. In light of broader comparative data (figure 2), it is much more likely that Z. capensis (at least the tropical forms of Z. capensis) evolved a novelty in losing absolute refractoriness, whereas the remainder of the Zonotrichia lineage retained the ancestral refractoriness trait. Indeed, it will be useful to consider photoresponsiveness of songbirds in relation to other orders of birds that display phenomena such as relative refractoriness, but a comparative analysis at this level is beyond the scope of our current review.
7. Summary and synthesis
In this paper, we combine a comparative approach with explicit enunciation of three hypotheses—adaptive specialization, conditional plasticity and phylogenetic history—to examine adaptation of environmental cue response systems regulating reproductive timing in birds. The analysis within songbirds, in general, and cardueline finches, in particular, provides evidence for contributions of both phylogenetic history and adaptive specialization of these systems. We also present and discuss evidence of conditional plasticity that is probably present in all taxa to differing degrees. We suggest that the degree to which this plasticity is present in different taxa is of substantial significance to trade-offs and constraints affecting those taxa with respect to colonizing new habitats or adjusting (within individuals) or adapting (within populations) to ongoing changes in currently occupied habitats.
Conditional plasticity of photoperiod response systems has often been tacitly excluded from consideration in many previous studies. Characterizing inherent conditional plasticity would ideally use a reaction norm approach (see, e.g. Helm & Gwinner 1999), but, more practically, should at least compare taxon-level variation in responses across a range of cue values, such as maximum day length or rate of change of day length.
Perhaps one of the most illuminating undertakings for future research would be to expand and extend to additional taxa the types of detailed evaluations of numerous environmental cue response system features (a non-exhaustive list of promising features listed in table 1) that have so far been made in only a few taxa. Careful selection of these additional taxa based on their specific reproductive and migratory biology as well as their phylogenetic positions is important to maximize the meaningful insights these further studies will probably yield. For instance, it should be particularly illuminating to characterize in detail the cue response systems, including inherent conditional plasticity, of a few taxa whose phylogenetic relationships are well understood that differ in degree of nomadism versus site fidelity. Since site-faithful taxa would presumably be in situations more conducive to local adaptation (e.g. of photoperiodic cue response characteristics, or tendency to integrate non-photic cues such as food availability; see Lambrechts et al. 1996, 1997; see also Schoech & Hahn submitted) than would nomads, such comparisons may help to reveal more clearly the factors favouring, and trade-offs involved in, adaptive specialization optimizing reproductive performance in specific habitats (or locations) as opposed to conditional plasticity permitting adequate reproductive performance across a range of habitats (or locations). We concur with Helm et al. (2005) that continued experimentation in a comparative context is vital and certain to provide useful insight into the capacity of birds to adjust to a changing environment.
Acknowledgments
All of the original empirical work by the authors reported herein conformed to guidelines for ethical use of animals at their respective universities.
We thank J. Cornelius and G. Ball for their assistance when assembling references for this paper. We also thank J. McNamara, A. Houston and two anonymous reviewers for their critical comments that greatly improved the manuscript. NSF Grants IBN-0988470, 0196093 and 0310995 to T.P.H., and grants from NSERC Canada to S.A.M.-S. provided support for some of the work reported here.
Footnotes
One contribution of 14 to a Theme Issue ‘Adaptation to the annual cycle’.
References
- Adkisson C.S. Red crossbill (Loxia curvirostra) In: Poole A, Gill F, editors. The birds of North America. vol. 256. The Academy of Natural Sciences; The American Ornithologists'Union; Philadelphia, PA; Washington, DC: 1996. pp. 1–24. [Google Scholar]
- Arnaiz-Villena A, Guillen J, Ruiz-del-Valle V, Lowy E, Zamora J, Varela P, Stefani D, Allende L.M. Phylogeography of crossbills, bullfinches, grosbeaks, and rosefinches. Cell. Mol. Life Sci. 2001;58:1159–1166. doi: 10.1007/PL00000930. doi:10.1007/PL00000930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badyaev A.V. Avian life history variation along altitudinal gradients: an example with cardueline finches. Oecologia. 1997;111:365–374. doi: 10.1007/s004420050247. doi:10.1007/s004420050247 [DOI] [PubMed] [Google Scholar]
- Baker J.R. The evolution of breeding seasons. In: DeBeer G.B, editor. Evolution: essays on aspects of evolutionary biology. Clarendon Press; Oxford, UK: 1938. pp. 161–177. [Google Scholar]
- Ball G.F. The neural integration of environmental information by seasonally breeding birds. Am. Zool. 1993;33:185–199. [Google Scholar]
- Ball G.F, Hahn T.P. GnRH neuronal systems in birds and their relation to the control of seasonal reproduction. In: Parhar I.S, Sakuma Y, editors. GnRH Neurons: gene to behavior. Brain Shuppan Publishers; Tokyo, Japan: 1997. pp. 325–342. [Google Scholar]
- Beebe K, Bentley G.E, Hau M. A seasonally breeding tropical bird lacks absolute photorefractoriness in the wild, despite high photoperiodic sensitivity. Funct. Ecol. 2005;19:505–512. doi:10.1111/j.1365-2435.2005.00994.x [Google Scholar]
- Benkman C.W. Foraging rates and the timing of crossbill reproduction. Auk. 1990;107:376–386. [Google Scholar]
- Benkman C.W. White-winged crossbill (Loxia leucoptera) In: Poole A, Stettenheim P, Gill F, editors. The birds of North America. vol. 27. The Academy of Natural Sciences; The American Ornithologists'Union; Philadelphia, PA; Washington, DC: 1992. pp. 1–20. [Google Scholar]
- Bentley G.E, Wingfield J.C, Morton M.L, Ball G.F. Stimulatory effects on the reproductive axis in female songbirds by conspecific and heterospecific male song. Horm. Behav. 2000;37:179–189. doi: 10.1006/hbeh.2000.1573. doi:10.1006/hbeh.2000.1573 [DOI] [PubMed] [Google Scholar]
- Bentley G.E, Audage N.C, Hanspal E.K, Ball G.F, Hans T.P. Photoperiodic response of the hypothalamo–pituitary–gonad axis in male and female canaries, Serinus canaria. J. Exp. Zool. A. 2003;296:143–151. doi: 10.1002/jez.a.10245. doi:10.1002/jez.a.10245 [DOI] [PubMed] [Google Scholar]
- Burger J.W. On the relation of day length to the phases of testicular involution and inactivity of the spermatogenetic cycle of the starling. J. Exp. Zool. 1947;105:259–268. doi: 10.1002/jez.1401050207. doi:10.1002/jez.1401050207 [DOI] [PubMed] [Google Scholar]
- Chakravorty K, Chandola-Saklani A. Termination of seasonal breeding in a weaver finch, Ploceus philippinus: role of photoperiod. J. Exp. Zool. 1985;235:381–386. doi:10.1002/jez.1402350309 [Google Scholar]
- Chandola A, Chakravorty K. Termination of seasonal breeding in the photoperiodic weaver bird. J. Exp. Zool. 1982;222:169–172. doi:10.1002/jez.1402220208 [Google Scholar]
- Chandola-Saklani A, Thapliyal A, Negi K, Diyundi S.C, Choudhary B. Daily increments of light hours near vernal equinox synchronize circannual testicular cycle of tropical spotted munia. Chronobiol. Int. 2004;21:553–569. doi: 10.1081/cbi-200025991. doi:10.1081/CBI-200025991 [DOI] [PubMed] [Google Scholar]
- Chaturvedi C.M, Thapliyal J.P. Thyroid photoperiod and gonadal regression in the common myna Acridotheres tristis. Gen. Comp. Endocrinol. 1983;52:279–282. doi: 10.1016/0016-6480(83)90123-5. doi:10.1016/0016-6480(83)90123-5 [DOI] [PubMed] [Google Scholar]
- Cockrem J.F. Timing of seasonal breeding in birds, with particular reference to New Zealand birds. Reprod. Fertil. Dev. 1995;7:1–19. doi: 10.1071/rd9950001. doi:10.1071/RD9950001 [DOI] [PubMed] [Google Scholar]
- Coombs-Hahn, T. P. 1993 Integration of environmental cues to time reproduction in an opportunistic breeder, the red crossbill (Loxia curvirostra). PhD dissertation, University of Washington, Seattle.
- Coppack T, Both C. Predicting life-cycle adaptation of migratory birds to global climate change. Ardea. 2002;90:369–378. [Google Scholar]
- Coppack T, Pulido F. Photoperiodic response and the adaptability of avian life cycles to environmental change. Adv. Ecol. Res. 2004;35:131–150. [Google Scholar]
- Coppack T, Pulido F, Berthold P. Photoperiodic response to early hatching in a migratory bird species. Oecologia. 2001;128:181–186. doi: 10.1007/s004420100652. doi:10.1007/s004420100652 [DOI] [PubMed] [Google Scholar]
- Coppack T, Pulido F, Szisch M, Auer D.P, Berthold P. Photoperiodic response may facilitate adaptation to climatic change in long-distance migratory birds. Proc. R. Soc. B. 2003;270:S43–S46. doi: 10.1098/rsbl.2003.0005. doi:10.1098/rsbl.2003.0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsté P.H. Experimental modification of the sexual cycle of the greenfinch. J. Exp. Biol. 1947;24:20–35. doi: 10.1242/jeb.24.1-2.20. [DOI] [PubMed] [Google Scholar]
- Dawson A. Photoperiodic control of testicular regression and moult in male house sparrows Passer domesticus. Ibis. 1991;133:312–316. [Google Scholar]
- Dawson W.R. Pine siskin (Carduelis pinus) In: Poole A, Gill F, editors. The birds of North America. vol. 280. The Academy of Natural Sciences; The American Ornithologists' Union; Philadelphia, PA; Washington, DC: 1997. pp. 1–24. [Google Scholar]
- Dawson A, King V.M, Bentley G.E, Ball G.F. Photoperiodic control of seasonality in birds. J. Biol. Rhythm. 2001;16:366–381. doi: 10.1177/074873001129002079. doi:10.1177/074873001129002079 [DOI] [PubMed] [Google Scholar]
- Drent R.H, Daan S. The prudent parent: energetic adjustments in avian breeding. Ardea. 1980;68:225–252. [Google Scholar]
- Dolnik V.R. Fotoperiodicheskii kontrol sezonnykh tsiklov vesa tela, linki i polovoi aktivnosti u zyablikov (Fringilla coelebs) Zool. Zhur. 1975;54:1048–1056. [Google Scholar]
- Dolnik V.R. Izdatelstvo; Nauka, Moscow: 1976a. Migratsionnoe Sostioyanie Ptits. [Google Scholar]
- Dolnik V.R. Fotoperiodizm y ptits. In: Zaslavskii V.A, editor. Fotoperiodizm Zhivotnykh I Rastenii. Akademiya Nauk SSSR; Leningrad, Russia: 1976b. pp. 47–81. [Google Scholar]
- Donham R.S, Wilson F.E. Photorefractoriness in pinealectomized Harris' sparrows. Condor. 1970;72:101–102. doi:10.2307/1366482 [Google Scholar]
- Engels W.L. The influence of different daylengths on the tests of a transequatorial migrant, the bobolink (Dolichonyx oryzivorus) In: Withrow R, editor. Photoperiodism and related phenomena in plants and animals. American Association for the Advancement of Science Publication No. 55; Washington, DC: 1959. pp. 759–766. [Google Scholar]
- Engels W.L. Day-length and termination of photorefractoriness in the annual testicular cycle of the transequatorial migrant Dolichonyx (the bobolink) Biol. Bull. 1962;123:94–104. doi:10.2307/1539506 [Google Scholar]
- Erpino M.J. Seasonal cycle of reproductive physiology in the black-billed magpie. Condor. 1969;71:267–279. doi:10.2307/1366303 [Google Scholar]
- Farner D.S, Follett B.K. Reproductive periodicity in birds. In: Barrington E.J.W, editor. Hormones and evolution. Academic Press; New York, NY: 1979. pp. 829–872. [Google Scholar]
- Farner D.S, Gwinner E. Photoperiodicity, circannual and reproductive cycles. In: Epple A, Stetson M.H, editors. Avian endocrinology. Academic Press; New York, NY: 1980. pp. 331–366. [Google Scholar]
- Farner D.S, Mewaldt L.R. The natural termination of the refractory period in the white-crowned sparrow. Condor. 1955;57:112–116. doi:10.2307/1364552 [Google Scholar]
- Farner D.S, Serventy D.L. The timing of reproduction in birds in the arid regions of Australia. Anat. Rec. 1960;137:354. [Google Scholar]
- Farner D.S, Wilson A.C. A quantitative examination of testicular growth in the white-crowned sparrow. Biol. Bull. 1957;113:254–267. doi: 10.2307/1539953. doi:10.2307/1539083 [DOI] [PubMed] [Google Scholar]
- Farner D.S, Moore R.S, Donham M.C. Induction of testicular development in house sparrows, Passer domesticus, and white-crowned sparrows, Zonotrichia leucophrys gambelii, with very long days and continuous light. Physiol. Zool. 1981;54:372–378. [Google Scholar]
- Farner D.S, Donham R.S, Matt K.S, Mattocks P.W, Jr, Moore M.C, Wingfield J.C. The nature of photorefractoriness. In: Mikami S.I, Homma K, Wada M, editors. Avian endocrinology: environmental and ecological perspectives. Japan Scientific Society Press; Springer; Tokyo, Japan; Berlin, Germany: 1983. pp. 149–156. [Google Scholar]
- Felsenstein J. Phylogenies and the comparative method. Am. Nat. 1985;125:1–15. doi: 10.1086/703055. doi:10.1086/284325 [DOI] [PubMed] [Google Scholar]
- Follett B.K. Marshall's physiology of reproduction. In: Lamming G.E, editor. Birds. vol. 1. Longman Green; Edinburgh, UK: 1984. pp. 283–350. [Google Scholar]
- Follett B.K, Nicholls T.J. Photorefractoriness in Japanese quail: possible involvement of the thyroid gland. J. Exp. Zool. 1984;232:573–580. doi: 10.1002/jez.1402320325. doi:10.1002/jez.1402320325 [DOI] [PubMed] [Google Scholar]
- Fretwell S.D. Princeton University Press; Princeton, NJ: 1972. Populations in a seasonal environment. [PubMed] [Google Scholar]
- Groth J.G. Evolutionary differentiation in morphology, vocalizations, and allozymes among nomadic sibling species in the North American red crossbill (Loxia curvirostra) complex. Univ. Calif. Publ. Zool. 1993;127:1–143. [Google Scholar]
- Gwinner E, Dittami J. Photoperiodic responses in temperate zone and equatorial stonechats: a contribution to the problem of photoperiodism in tropical organisms. In: Gollett B.K, Ishii S, Chandola A, editors. The endocrine system and the environment. Japan Science Society Press; Springer; Tokyo, Japan; Berlin, Germany: 1985. pp. 279–294. [Google Scholar]
- Gwinner E, Dittami J, Gwinner H. Postjuvenile molt in East African and Central European stonechats (Saxicola torquata axillaris, S.t. rubicola) and its modification by photoperiod. Oecologia. 1983;60:66–70. doi: 10.1007/BF00379321. doi:10.1007/BF00379321 [DOI] [PubMed] [Google Scholar]
- Gwinner E, Dittam J, Beldhuis H.J.A. The seasonal development of photoperiodic responsiveness in an equatorial migrant, the garden warbler Sylvia borin. J. Comp. Physiol. A. 1988;162:389–396. doi:10.1007/BF00606125 [Google Scholar]
- Hahn T.P. Integration of photoperiodic and food cues to time changes in reproductive physiology by an opportunistic breeder, the red crossbill, Loxia curvirostra (Aves: Carduelinae) J. Exp. Zool. 1995;272:213–226. doi:10.1002/jez.1402720306 [Google Scholar]
- Hahn T.P. Cassin's Finch. (Carpodacus cassinii) In: Poole A, Gill F, editors. The Birds of North America. vol. 240. The Academy of Natural Sciences; The American Ornithologists' Union; Philadelphia, PA; Washington, DC: 1996. pp. 1–20. [Google Scholar]
- Hahn T.P. Reproductive seasonality in an opportunistic breeder, the red crossbill, Loxia curvirostra. Ecology. 1998;79:2365–2375. [Google Scholar]
- Hahn T.P, Ball G.F. Changes in brain GnRH associated with photorefractoriness in house sparrows (Passer domesticus) Gen. Comp. Endocrinol. 1995;99:349–363. doi: 10.1006/gcen.1995.1119. doi:10.1006/gcen.1995.1119 [DOI] [PubMed] [Google Scholar]
- Hahn T.P, Swingle J, Wingfield J.C, Ramenofsky M. Adjustments of the prebasic molt schedule in birds. Ornis Scand. 1992;23:314–321. doi:10.2307/3676655 [Google Scholar]
- Hahn T.P, Boswell T, Wingfield J.C, Ball G.F. Temporal flexibility in avian reproduction: patterns and mechanisms. Curr. Ornithol. 1997;14:39–80. [Google Scholar]
- Hahn T.P, Pereyra M.E, Sharbaugh S.M, Bentley G.E. Physiological responses to photoperiod in three cardueline finch species. Gen. Comp. Endocrinol. 2004;137:99–108. doi: 10.1016/j.ygcen.2004.02.014. doi:10.1016/j.ygcen.2004.02.014 [DOI] [PubMed] [Google Scholar]
- Hahn T.P, Katti M, Pereyra M.E, Ward G, MacDougall-Shackleton S.A. Effects of food availability on the reproductive system. In: Dawson A, Sharp P.J, editors. Functional avian endocrinology. Narosa Publishing House; New Delhi, India: 2005. pp. 167–180. [Google Scholar]
- Hamner W.M. Photoperiodic control of the annual testicular cycle in the house finch Carpodacus mexicanus. Gen. Comp. Endocrinol. 1966;7:224–233. doi:10.1016/0016-6480(66)90043-8 [Google Scholar]
- Hamner W.M. The photorefractory period of the house finch. Ecology. 1968;49:211–227. doi:10.2307/1934450 [Google Scholar]
- Harris M.O, Turek F.W. Photoperiodic control of the timing of testicular regression in white-throated sparrows. Gen. Comp. Endocrinol. 1982;46:124–129. doi: 10.1016/0016-6480(82)90172-1. doi:10.1016/0016-6480(82)90172-1 [DOI] [PubMed] [Google Scholar]
- Harvey P.H, Pagel M.D. Oxford University Press; Oxford, UK; New York, NY: 1991. The comparative method in evolutionary biology. [Google Scholar]
- Hau M. Timing of breeding in variable environments: tropical birds as model systems. Horm. Behav. 2001;40:281–290. doi: 10.1006/hbeh.2001.1673. doi:10.1006/hbeh.2001.1673 [DOI] [PubMed] [Google Scholar]
- Hau M, Wikelski M, Wingfield J.C. A neotropical forest bird can measure the slight changes in tropical photoperiod. Proc. R. Soc. B. 1998;265:89–95. doi:10.1098/rspb.1998.0268 [Google Scholar]
- Hau M, Wikelski M, Wingfield J.C. Visual and nutritional food cues fine-tune timing of reproduction in a neotropical rainforest bird. J. Exp. Zool. 2000;286:494–504. doi:10.1002/(SICI)1097-010X(20000401)286:5<494::AID-JEZ7>3.0.CO;2-3 [PubMed] [Google Scholar]
- Helm B, Gwinner E. Timing of postjuvenal molt in African (Saxicola torquata axillaris) and European (Saxicola torquata rubicola) stonechats: effects of genetic and environmental factors. Auk. 1999;116:589–603. [Google Scholar]
- Helm B, Gwinner E. Carry-over effects of day length during spring migration. J. Ornithol. 2005;146:348–354. doi:10.1007/s10336-005-0009-5 [Google Scholar]
- Helm B, Gwinner E, Trost L. Flexible seasonal timing and migratory behavior. Ann. NY Acad. Sci. 2005;1046:216–227. doi: 10.1196/annals.1343.019. doi:10.1196/annals.1343.019 [DOI] [PubMed] [Google Scholar]
- Hinde R.A, Steel E. The effect of male song on an estrogen-dependent behavior pattern in the female canary (Serinus canarius) Horm. Behav. 1976;7:293–304. doi: 10.1016/0018-506x(76)90035-0. doi:10.1016/0018-506X(76)90035-0 [DOI] [PubMed] [Google Scholar]
- Hinde R.A, Steel E. The influence of daylength and male vocalizations on the estrogen-dependent behavior of female canaries and budgerigars, with discussion of data from other species. In: Rosenblatt R.S, Hinde R, Beer C, Busnel M.-C, editors. Advances in the study of behavior. vol. 8. Academic Press; New York, NY: 1978. pp. 39–73. [Google Scholar]
- Huey R.B, Hertz P.E. Is a jack-of-all-temperatures a master of none? Evolution. 1984;38:441–444. doi: 10.1111/j.1558-5646.1984.tb00302.x. doi:10.2307/2408502 [DOI] [PubMed] [Google Scholar]
- Humphrey P, Parkes K. An approach to the study of molts and plumages. Auk. 1959;76:1–31. [Google Scholar]
- Jones P.J, Ward P. The level of reserve protein as the proximate factor controlling the timing of breeding and clutch size in the red-billed quelea, Quelea quelea. Ibis. 1976;118:547–574. [Google Scholar]
- Ketterson E.D, Nolan V., Jr Adaptation, exaptation and constraint: a hormonal perspective. Am. Nat. 1999;154(Suppl.):S4–S25. doi: 10.1086/303280. doi:10.1086/303280 [DOI] [PubMed] [Google Scholar]
- Komdeur J, Daan S. Breeding in the monsoon: semi-annual reproduction in the Seychelles warbler (Acrocephalus sechellensis) J. Ornithol. 2005;146:305–313. doi:10.1007/s10336-005-0008-6 [Google Scholar]
- Kumar B.S, Kumar V. Photoperiodic control of annual reproductive cycles in subtropical Brahminy myna, Sturnus pagodarum. Gen. Comp. Endocrinol. 1993;89:149–160. doi: 10.1006/gcen.1993.1018. doi:10.1006/gcen.1993.1018 [DOI] [PubMed] [Google Scholar]
- Kumar V, Tewary P.D. Photoperiodic testicular response and photorefractoriness in common Indian rosefinch. Environ. Control Biol. 1982;20:39–42. [Google Scholar]
- Lambrechts M.M, Perret P. A long photoperiod overrides non-photoperiodic factors in blue tits' timing of reproduction. Proc. R. Soc. B. 2000;267:585–588. doi: 10.1098/rspb.2000.1041. doi:10.1098/rspb.2000.1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrechts M.M, Perret P, Blondel J. Adaptive differences in the timing of egg laying between different populations of birds result from variation in photoresponsiveness. Proc. R. Soc. B. 1996;263:19–22. doi:10.1098/rspb.1996.0004 [Google Scholar]
- Lambrechts M.M, Blondel J, Maistre M, Perret P. A single response mechanism is responsible for evolutionary adaptive variation in a bird's laying date. Proc. Natl Acad. Sci. USA. 1997;94:5153–5155. doi: 10.1073/pnas.94.10.5153. doi:10.1073/pnas.94.10.5153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner S, Van't Hof T.J, Gahr M. Flexible reproduction in wild canaries is independent of photoperiod. Gen. Comp. Endocrinol. 2003;130:102–108. doi: 10.1016/s0016-6480(02)00574-9. doi:10.1016/S0016-6480(02)00574-9 [DOI] [PubMed] [Google Scholar]
- Lewis R.A, King J.R, Farner S. Photoperiodic responses of a subtropical population of the finch (Zonotrichia capensis hypoleuca) Condor. 1974;76:233–237. doi:10.2307/1366336 [Google Scholar]
- Ligon J.D. Green cones of the pinon pine stimulate late summer breeding in the pinon jay. Nature. 1974;250:80–82. doi: 10.1038/250080a0. doi:10.1038/250080a0 [DOI] [PubMed] [Google Scholar]
- Lofts B. Photoperiodism and the refractory period of reproduction in an equatorial bird Quelea quelea. Ibis. 1962;104:407–414. [Google Scholar]
- Lofts B. Evidence of an autonomous reproductive rhythm in an equatorial bird (Quelea quelea) Nature. 1964;201:523–534. doi: 10.1038/201523b0. doi:10.1038/201523b0 [DOI] [PubMed] [Google Scholar]
- Lofts B, Murton R.K. Photoperiodic and physiological adaptations regulating avian breeding cycles and their ecological significance. J. Zool. 1968;155:327–394. [Google Scholar]
- MacDougall-Shackleton S.A, Johnson R.E, Hahn T.P. Gray-crowned rosy-finch (Leucosticte tephrocotis) In: Poole A, Gill F, editors. The birds of North America. vol. 559. The Birds of North America, Inc; Philadelphia, PA: 2000. [Google Scholar]
- MacDougall-Shackleton S.A, Deviche P.J, Crain R.D, Ball G.F, Hahn T.P. Seasonal changes in brain GnRH immunoreactivity and song-control nuclei volumes in an opportunistically breeding songbird. Brain Behav. Evol. 2001;58:38–48. doi: 10.1159/000047260. doi:10.1159/000047260 [DOI] [PubMed] [Google Scholar]
- MacDougall-Shackleton S.A, Pereyra M.E, Katti M, Hahn T.P. GnRH, photorefractoriness, and breeding schedules of cardueline finches. In: Dawson A, Sharp P.J, editors. Functional avian endocrinology. Narosa Publishing House; New Delhi, India: 2005. pp. 97–110. [Google Scholar]
- Marsh R.H, MacDougall-Shackleton S.A, Hahn T.P. Photorefractoriness and neural response to day length in American goldfinches, Carduelis tristis. Can. J. Zool. 2002;80:2100–2107. doi:10.1139/z02-208 [Google Scholar]
- Marshall A.J. Internal and environmental control of breeding. Ibis. 1959;101:456–478. [Google Scholar]
- Marshall A.J. Environmental factors other than light involved in the control of sexual cycles in birds and mammals. In: Benoit J, Assenmacher I, editors. La photregulation de la reproduction chez les oiseaux et les mammiferes. Editions du Centre National de la Recherche Scientifique; Paris, France: 1970. pp. 53–64. [Google Scholar]
- Marshall A.J, Coombs C.J.F. The interaction of environmental, internal and behavioural factors in the rook Corvus frugilegus Linnaeus. Proc. Zool. Soc. London. 1957;128:545–589. [Google Scholar]
- Marshall A.J, Disney H.J.de.S. Photostimulation of an equatorial bird (Quelea quelea Linneaus) Nature. 1956;177:143–144. doi: 10.1038/177143a0. doi:10.1038/177143a0 [DOI] [PubMed] [Google Scholar]
- Marshall A.J, Disney H.J. de. S. Experimental induction of the breeding season in a xerophilous bird. Nature. 1957;180:647–649. doi: 10.1038/180647a0. doi:10.1038/180647a0 [DOI] [PubMed] [Google Scholar]
- Marten J.A, Johnson N.K. Genetic relationships of North American cardueline finches. Condor. 1986;88:409–420. doi:10.2307/1368266 [Google Scholar]
- McNamara J.M, Welham R.K, Houston A.I, Daan S, Tinbergen J.M. The effects of background mortality on optimal reproduction in a seasonal environment. Theor. Popul. Biol. 2004;65:361–372. doi: 10.1016/j.tpb.2003.10.006. doi:10.1016/j.tpb.2003.10.006 [DOI] [PubMed] [Google Scholar]
- Misra M, Rani S, Singh S, Kumar V. Regulation of seasonality in the migratory male blackheaded bunting (Emberiza melanocephala) Reprod. Nutr. Dev. 2004;44:341–352. doi: 10.1051/rnd:2004039. doi:10.1051/rnd:2004039 [DOI] [PubMed] [Google Scholar]
- Monroe B.L, Jr, Sibley C.G. Yale University Press; New Haven, CT: 1993. A world checklist of birds. [Google Scholar]
- Moore M.C, Donham R.S, Farner D.S. Physiological preparation for autumnal migration in white-crowned sparrows. Condor. 1982;84:410–419. doi:10.2307/1367445 [Google Scholar]
- Moore I.T, Wada H, Perfito N, Busch D.S, Hahn T.P, Wingfield J.C. Territoriality and testosterone in an equatorial population of rufous-collared sparrows, Zonotrichia capensis. Anim. Behav. 2004;67:411–420. doi:10.1016/j.anbehav.2003.03.021 [Google Scholar]
- Morton M.L, Pereyra M.E, Baptista L.F. Photoperiodically induced ovarian growth in the white-crowned sparrow (Zonotrichia leucophrys gambelii) and its augmentation by song. Comp. Biochem. Physiol. A. 1985;80:93–97. doi:10.1016/0300-9629(85)90684-X [Google Scholar]
- Newton I. Taplinger; New York, NY: 1973. Finches. [Google Scholar]
- Nicholls T.J, Goldsmith A.R, Dawson A. Photorefractoriness in birds and comparison with mammals. Physiol. Rev. 1988;68:133–176. doi: 10.1152/physrev.1988.68.1.133. [DOI] [PubMed] [Google Scholar]
- Partecke J, Van't Hof T, Gwinner E. Differences in the timing of reproduction between urban and forest European blackbirds (Turdus merula): result of phenotypic flexibility or genetic differences? Proc. R. Soc. B. 2004;271:1995–2001. doi: 10.1098/rspb.2004.2821. doi:10.1098/rspb.2004.2821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten M.A, Fugate M. Systematic relationships among the emberizid sparrows. Auk. 1998;115:412–424. [Google Scholar]
- Pereyra M.E, Sharbaugh S.M, Hahn T.P. Interspecific variation in photo-induced GnRH plasticity among nomadic cardueline finches. Brain Behav. Evol. 2005;66:35–49. doi: 10.1159/000085046. doi:10.1159/000085046 [DOI] [PubMed] [Google Scholar]
- Perfito N, Bentley G, Hau M. Tonic activation of brain GnRH immunoreactivity despite reduction of peripheral reproductive parameters in opportunistically breeding zebra finches. Brain Behav. Evol. 2006;67:123–134. doi: 10.1159/000090977. doi:10.1159/000090977 [DOI] [PubMed] [Google Scholar]
- Perrins C.M. The timing of birds' breeding seasons. Ibis. 1970;112:242–255. [Google Scholar]
- Phillmore L.S, Hoshooley J.S, Hahn T.P, MacDougall-Shackleton S.A. A test of absolute photorefractoriness and photo-induced neural plasticity of song control regions in black-capped chickadees (Poecile atricapillus) Can. J. Zool. 2005;83:747–753. doi:10.1139/z05-070 [Google Scholar]
- Rani S, Singh S, Kumar V. The pineal clock affects behavioral circadian rhythms but not photoperiodic induction in the Indian weaver bird (Ploceus philippinus) J. Ornithol. 2005a;146:355–364. doi:10.1007/s10336-005-0005-9 [Google Scholar]
- Rani S, Singh S, Misra M, Malik S, Singh B.P, Kumar V. Daily light regulates seasonal responses in the migratory male redheaded bunting (Emberiza bruniceps) J. Exp. Zool. A. 2005b;303:541–550. doi: 10.1002/jez.a.187. doi:10.1002/jez.a.187 [DOI] [PubMed] [Google Scholar]
- Robinson J.E, Follett B.K. Photoperiodism in Japanese quail: the termination of seasonal breeding by photorefractoriness. Proc. R. Soc. B. 1982;215:95–116. doi: 10.1098/rspb.1982.0030. doi:10.1098/rspb.1982.0030 [DOI] [PubMed] [Google Scholar]
- Rowan W. Relation of light to bird migration and developmental changes. Nature. 1925;115:494–495. [Google Scholar]
- Rowan W. On photoperiodism, reproductive periodicity and the annual migrations of birds and certain fishes. Proc. Boston Soc. Nat. Hist. 1926;38:147–189. [Google Scholar]
- Runfeldt S, Wingfield J.C. Experimentally prolonged sexual activity in female song sparrows delays termination of reproductive activity in their untreated mates. Anim. Behav. 1985;33:403–410. doi:10.1016/S0003-3472(85)80064-6 [Google Scholar]
- Schoech, S. J. & Hahn, T. P. Submitted. Latitude affects degree of advancement in laying by birds in response to food supplementation: a meta-analysis. [DOI] [PubMed]
- Schwab R.G, Lott D.F. Testis growth and regression in starlings (Sturnus vulgaris) as a function of the presence of females. J. Exp. Zool. 1969;171:39–42. doi:10.1002/jez.1401710106 [Google Scholar]
- Sharp P.J. Strategies in avian breeding cycles. Anim. Reprod. Sci. 1996;42:505–513. doi:10.1016/0378-4320(96)01556-4 [Google Scholar]
- Sibley C.G, Ahlquist J.E. Yale University Press; New Haven, CT: 1990. Phylogeny and classification of birds: a study in molecular evolution. [Google Scholar]
- Sibley C.G, Monroe B.L., Jr . Yale University Press; New Haven, CT; London, UK: 1990. Distribution and taxonomy of birds of the world. [Google Scholar]
- Silverin B. Reproductive adaptations to breeding in the North. Am. Zool. 1995;35:191–202. [Google Scholar]
- Silverin G, Viebke P.A. Low temperatures affect the photoperiodically induced LH and testicular cycles differently in closely related species of tits (Parus spp.) Horm. Behav. 1994;28:199–206. doi: 10.1006/hbeh.1994.1017. doi:10.1006/hbeh.1994.1017 [DOI] [PubMed] [Google Scholar]
- Silverin B, Viebke P.A, Westin J. An artificial simulation of the vernal increase in day length and its effects on the reproductive system in three species of tits (Parus spp.), and modifying effects of environmental factors. Condor. 1989;91:598–608. doi:10.2307/1368110 [Google Scholar]
- Silverin B, Massa R, Stokkan K.A. Photoperiodic adaptation to breeding at different latitudes in great tits. Gen. Comp. Endocrinol. 1993;90:14–22. doi: 10.1006/gcen.1993.1055. doi:10.1006/gcen.1993.1055 [DOI] [PubMed] [Google Scholar]
- Sossinka R. Der Einfluss von Dursteperioden auf die Schilddrusse- und Gonaden-aktvitat und ihre Bedeuten fur die Brutperiodik des Zebrafinken (Taeniopygia castanotis Gould) J. Ornithol. 1974;115:128–141. doi:10.1007/BF01643286 [Google Scholar]
- Stearns S.C. Oxford University Press; Oxford, UK: 1992. The evolution of life histories. [Google Scholar]
- Storey C.R, Nicholls T.J. Some effects of manipulation of daily photoperiod on rate of onset of a photorefractory state in canaries (Serinus canarius) Gen. Comp. Endocrinol. 1976;30:204–208. doi: 10.1016/0016-6480(76)90101-5. doi:10.1016/0016-6480(76)90101-5 [DOI] [PubMed] [Google Scholar]
- Storey C.R, Nicholls T.J. A photoperiodically induced cycle of gonadotropin secretion in intact, hemi castrated and fully castrated male bullfinches Pyrrhula pyrhhula. Ibis. 1982;124:55–60. [Google Scholar]
- Styrsky J.D, Berthold P, Robinson W.D. Endogenous control of migration and calendar effects in an intratropical migrant, the yellow–green vireo. Anim. Behav. 2004;67:1141–1149. doi:10.1016/j.anbehav.2003.07.012 [Google Scholar]
- Svensson E. Natural selection on avian breeding time: causality, fecundity-dependent, and fecundity-independent selection. Evolution. 1997;51:1276–1283. doi: 10.1111/j.1558-5646.1997.tb03974.x. doi:10.2307/2411056 [DOI] [PubMed] [Google Scholar]
- Tewary P.D, Dixit A.S. Photoperiodic control of the ovarian cycle in the rosefinch, Carpodacus erythrinus. J. Exp. Zool. 1983;228:537–542. doi:10.1002/jez.1402280313 [Google Scholar]
- Tewary P.D, Kumar V. VPhotoperiodic responses of a subtropical migratory finch, the black-headed bunting (Emberiza melanocephala) Condor. 1982;84:168–171. doi:10.2307/1367661 [Google Scholar]
- Tewary P.D, Tripathi P.M. Circadian photoperiodicity in termination of photorefractoriness in the yellow-throated sparrow. Experientia. 1985;41:135–1351. doi:10.1007/BF01952090 [Google Scholar]
- Tewary P.D, Kumar V, Prasad B.N. Influence of photoperiod in a subtropical migratory finch, the common Indian rosefinch Carpodacus erythrinus. Ibis. 1983;125:115–120. [Google Scholar]
- Thapliyal J.P, Saxena R.N. Absence of a refractory period in the common weaver bird. Condor. 1964;66:199–208. doi:10.2307/1365644 [Google Scholar]
- Tordoff H.B, Dawson W.R. The influence of daylength on reproductive timing in the red crossbill. Condor. 1965;67:416–422. doi:10.2307/1365634 [Google Scholar]
- van Noordwijk A.J. The methods of genetical ecology applied to the study of evolutionary change. In: Wohrmann K, Jain S.K, editors. Population biology: ecological and evolutionary viewpoints. Springer; Berlin, Germany: 1990. pp. 291–319. [Google Scholar]
- Vaugien L. Sur le comportement sexual singulaier de la Peruch ondulee, maintenue a l'obscurite. Comp. Acad. Sci. 1952;234:1489. [Google Scholar]
- Vaugien L. Sur l'apparition de la maturite sexuelle des jeunes perruches ondulees males soumises a diverses conditions d'eclairement: Le developpement testiculaire est plus rapide dans l'obscurite complete. Bull. Biol. 1953;87:274–286. [Google Scholar]
- Verhulst S, Nilsson J.-Å. The timing of birds' breeding seasons: a review of experiments that manipulated timing of breeding. Phil. Trans. R. Soc. B. 2008;363:399–410. doi: 10.1098/rstb.2007.2146. doi:10.1098/rstb.2007.2146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M.E, Lambrechts M.M. Information constraints in the timing of reproduction in temperate zone birds: great and blue tits. Proc. Int. Ornithol. Cong. 1999;22:249–264. [Google Scholar]
- Visser M.E, van Noordwijk A.J, Tinbergen J.M, Lessells C.M. Warmer springs lead to mis-timed reproduction in great tits (Parus major) Proc. R. Soc. B. 1998;265:1867–1870. doi:10.1098/rspb.1998.0514 [Google Scholar]
- Visser M.E, Both C, Lambrechts M.M. Global climate change leads to mistimed avian reproduction. Adv. Ecol. Res. 2004;35:89–110. [Google Scholar]
- Wilson F.E, Donham R.S. Daylength and control of seasonal reproduction in male birds. In: Stetson M.H, editor. Processing of environmental information in vertebrates. Springer; Berlin, Germany: 1988. pp. 101–120. [Google Scholar]
- Wilson F.E, Follett B.K. Plasma and pituitary luteinizing hormone in intact and castrated tree sparrows (Spizella arborea) during a photoinduced gonadal cycle. Gen. Comp. Endocrinol. 1974;23:82–93. doi: 10.1016/0016-6480(74)90056-2. doi:10.1016/0016-6480(74)90056-2 [DOI] [PubMed] [Google Scholar]
- Wingfield J.C. Fine temporal adjustments of reproductive function. In: Epple A, Stetson M.H, editors. Avian endocrinology. Academic Press; New York, NY: 1980. pp. 367–389. [Google Scholar]
- Wingfield J.C. Environmental and endocrine control of reproduction: an ecological approach. In: Mikami S.I, Homma K, Wada M, editors. Avian endocrinology: environmental and ecological perspectives. Japan Scientific Society Press; Springer; Tokyo, Japan; Berlin, Germany: 1983. pp. 265–288. [Google Scholar]
- Wingfield J.C. Control of testicular cycles in the song sparrow, Melospiza melodia melodia: interaction of photoperiod and an endogenous program? Gen. Comp. Endocrinol. 1993;92:388–401. doi: 10.1006/gcen.1993.1176. doi:10.1006/gcen.1993.1176 [DOI] [PubMed] [Google Scholar]
- Wingfield J.C, Farner D.S. Endocrinology of reproduction in wild species. Avian Biol. 1993;9:163–327. [Google Scholar]
- Wingfield J.C, Hahn T.P, Levin R.N, Honey P. Environmental predictability and control of gonadal cycles in birds. J. Exp. Zool. 1992;261:214–231. doi:10.1002/jez.1402610212 [Google Scholar]
- Wingfield J.C, Hahn T.P, Doak D. Integration of environmental factors regulating transitions of physiological state, morphology and behaviour. In: Sharp P, editor. Avian endocrinology. Journal of Endocrinology Ltd; Bristol, UK: 1993. pp. 111–122. [Google Scholar]
- Wingfield J.C, Hahn T.P, Wada M, Astheimer L.B, Schoech S. Interrelationship of daylength and temperature in the control of gonadal development, body mass and fat depots in white-crowned sparrows, Zonotrichia leucophrys gambelii. Gen. Comp. Endocrinol. 1996;101:242–255. doi: 10.1006/gcen.1996.0027. doi:10.1006/gcen.1996.0027 [DOI] [PubMed] [Google Scholar]
- Wingfield J.C, Hahn T.P, Wada M, Schoech S. Effects of day length and temperature on gonadal development, body mass and fat depots in white-crowned sparrows, Zonotrichia leucophrys pugetensis. Gen. Comp. Endocrinol. 1997;107:44–62. doi: 10.1006/gcen.1997.6894. doi:10.1006/gcen.1997.6894 [DOI] [PubMed] [Google Scholar]
- Wingfield J.C, Hahn T.P, Maney D.L, Schoech S, Wada M, Morton M.L. Effects of temperature on photoperiodically-induced reproductive development, circulating plasma luteinizing hormone and thyroid hormones, body mass, fat deposition and molt in mountain white-crowned sparrows, Zonotrichia leucophrys oriantha. Gen. Comp. Endocrinol. 2003;131:143–158. doi: 10.1016/s0016-6480(02)00648-2. doi:10.1016/S0016-6480(02)00648-2 [DOI] [PubMed] [Google Scholar]
- Wolfson A. The occurrence and regulation of the refractory period in the gonadal and fat cycles of the junco. J. Exp. Zool. 1952;121:311–326. doi:10.1002/jez.1401210204 [Google Scholar]
- Wolfson A. The role of light and darkness in the regulation of spring migration and reproductive cycles in birds. In: Withrow R.B, editor. Photoperiodism. American Association for the Advancement of Science Publication 55; Washington, DC: 1959. pp. 679–716. [Google Scholar]
- Zink R.M. Patterns of genic and morphologic variation among sparrows in the genera Zonotrichia, Melospiza, Junco and Passerella. Auk. 1982;99:632–649. [Google Scholar]
- Zink R.M, Blackwell R.C. Patterns of allozyme, mitochondrial DNA, and morphometric variation in four sparrow genera. Auk. 1996;113:59–67. [Google Scholar]
- Zink R.M, Dittman D.L, Rootes W.L. Mitochondrial DNA variation and the phylogeny of Zonotrichia. Auk. 1991;108:578–584. [Google Scholar]