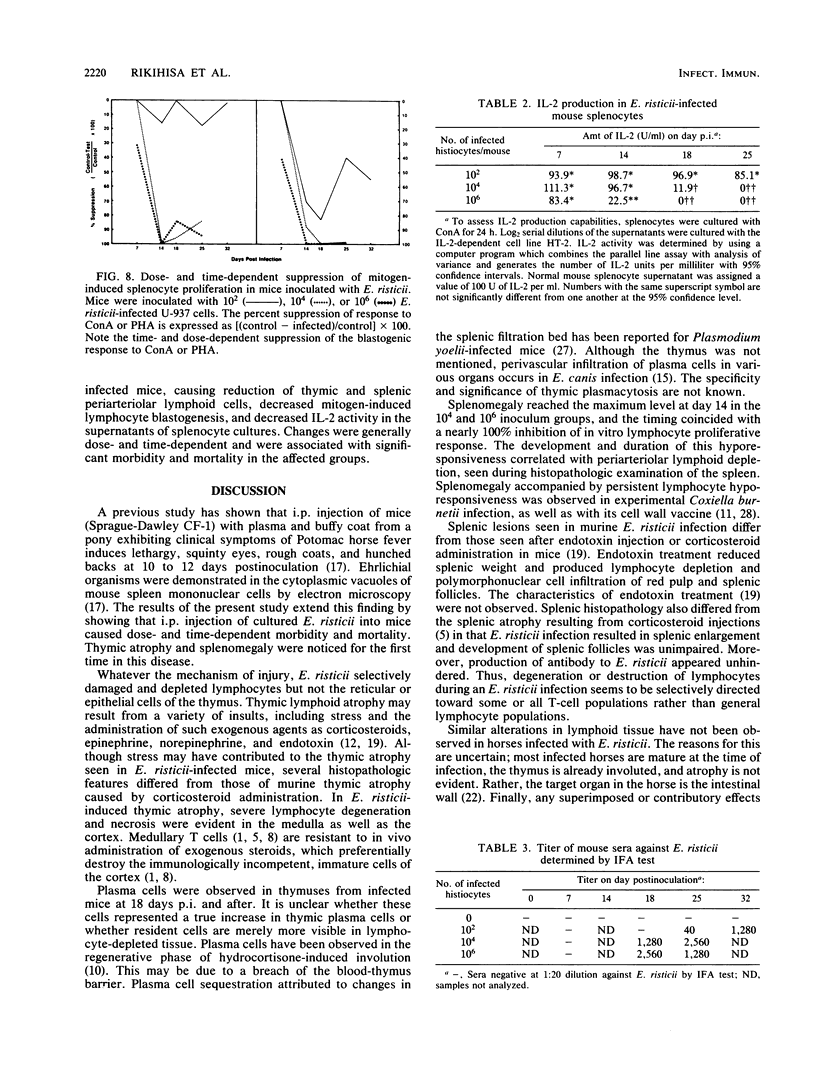
Abstract
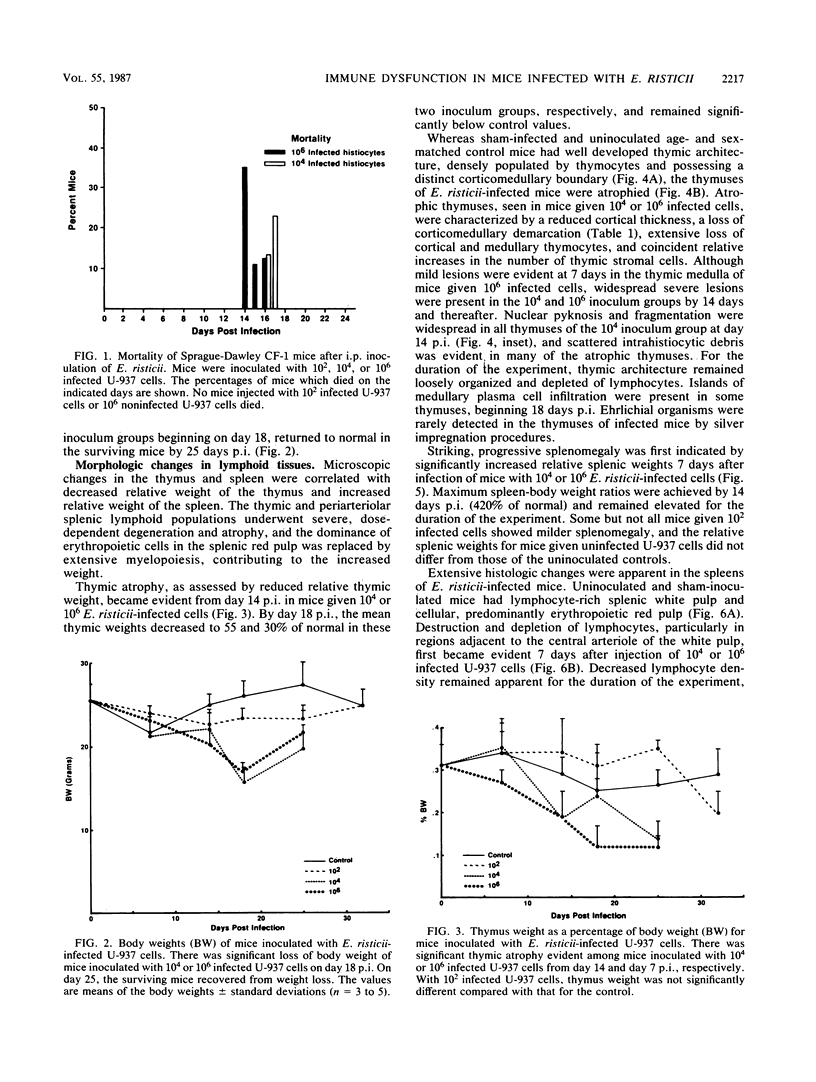
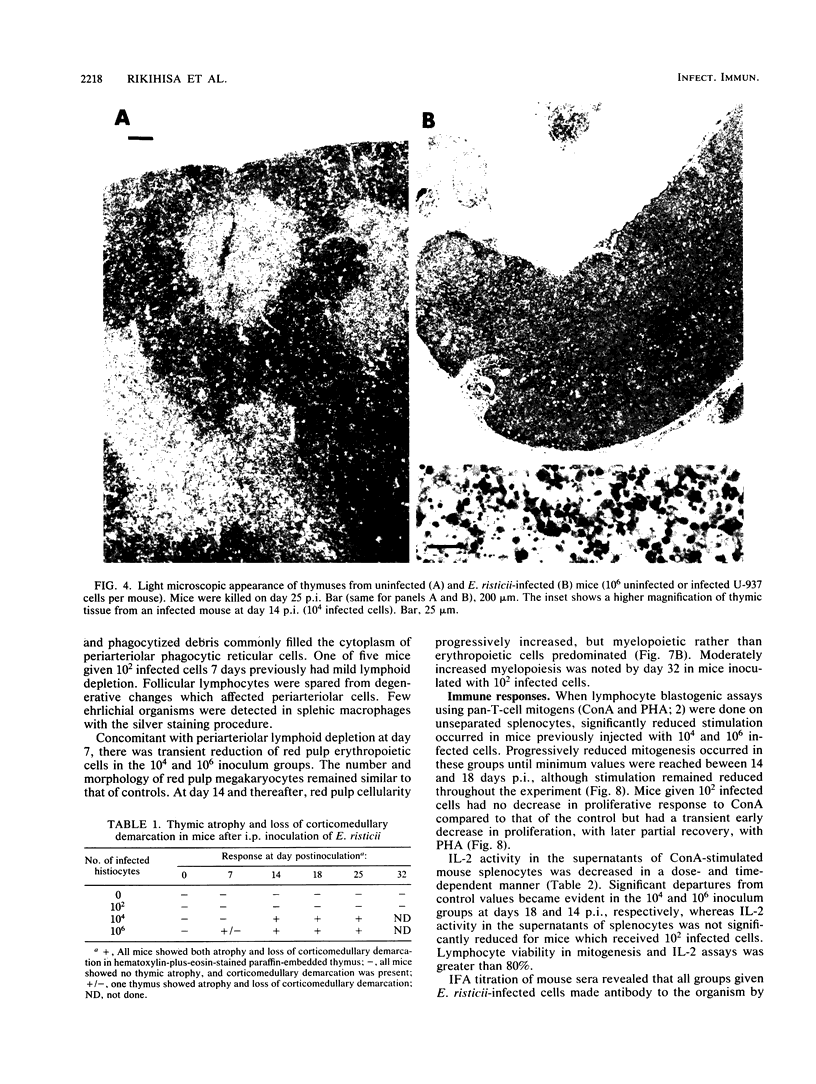
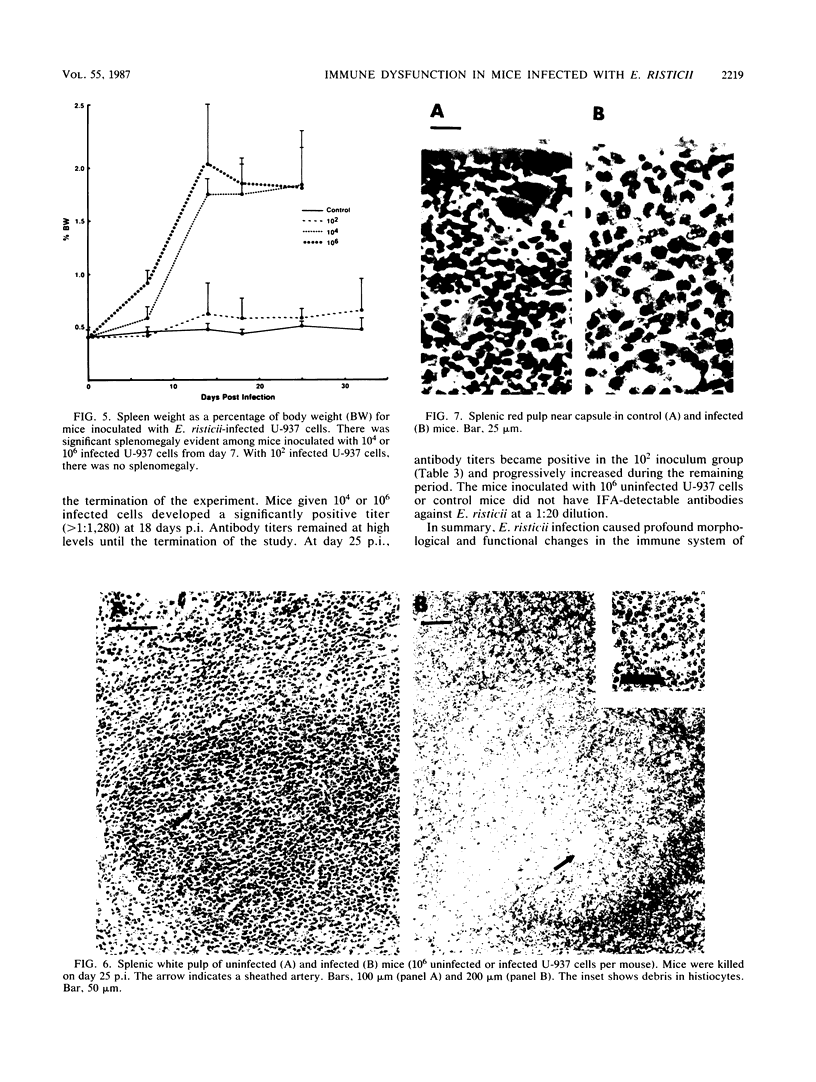
The histopathology of the thymus and spleen and the response of spleen cells to mitogenic stimuli were evaluated in Sprague-Dawley CF-1 mice infected with Ehrlichia risticii. Intraperitoneal injection of 10(4) or 10(6) E. risticii-infected U-937 cells into mice resulted in 100% morbidity and partial mortality. Thymic atrophy became significant between 1 and 2 weeks postinfection and remained for the duration of the study. The atrophy appeared associated with antecedent destruction and rarefaction of lymphocytes, resulting in the loss of corticomedullary demarcation. Splenomegaly was prominent; significantly increased weights were detected 7 days postinfection. Histopathologic examination revealed rarefaction of lymphocytes around central arteries, the presence of necrotic debris in histiocytes, and replacement of erythropoiesis by granulopoiesis in the red pulp. Marked and acute reduction of in vitro proliferative responses of spleen cells to concanavalin A (ConA) and phytohemagglutinin were observed in mice infected with 10(4) or 10(6) E. risticii-infected U-937 cells. Interleukin-2 activity in the supernatant of ConA-stimulated spleen cells was also severely reduced. Both changes were time- and dose-dependent and were not associated with decreased spleen cell viability. Neither morbidity nor mortality occurred in mice infected with 10(2) E. risticii-infected U-937 cells. Although there was temporal reduction in phytohemagglutinin-driven lymphocyte proliferation, reduction in neither ConA-driven lymphocyte proliferation nor interleukin-2 activity was observed with this dosage. All E. risticii-inoculated mice seroconverted between days 18 and 25, as detected by the indirect fluorescent-antibody procedure. The findings indicate for the first time the hypoimmune responsiveness and histopathologic changes in lymphoid organs associated with E. risticii infection.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blomgren H., Andersson B. Evidence for a small pool of immunocompetent cells in the mouse thymus. Exp Cell Res. 1969 Oct;57(2):185–192. doi: 10.1016/0014-4827(69)90140-2. [DOI] [PubMed] [Google Scholar]
- Burger C. J., Elgert K. D., Tyson J. J., Birch J. B. An improved data analysis method for interleukin 2 microassay. Comput Biol Med. 1986;16(5):377–390. doi: 10.1016/0010-4825(86)90005-3. [DOI] [PubMed] [Google Scholar]
- COWAN W. K., SORENSON G. D. ELECTRON MICROSCOPIC OBSERVATIONS OF ACUTE THYMIC INVOLUTION PRODUCED BY HYDROCORTISONE. Lab Invest. 1964 Apr;13:353–370. [PubMed] [Google Scholar]
- Claman H. N. Corticosteroids and lymphoid cells. N Engl J Med. 1972 Aug 24;287(8):388–397. doi: 10.1056/NEJM197208242870806. [DOI] [PubMed] [Google Scholar]
- Cohen J. J., Claman H. N. Thymus-marrow immunocompetence. V. Hydrocortisone-resistant cells and processes in the hemolytic antibody response of mice. J Exp Med. 1971 May 1;133(5):1026–1034. doi: 10.1084/jem.133.5.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. J. Hydrocortisone resistance of activated initiator cells in graft versus host reactions. Nature. 1971 Jan 22;229(5282):274–275. doi: 10.1038/229274a0. [DOI] [PubMed] [Google Scholar]
- Cordes D. O., Perry B. D., Rikihisa Y., Chickering W. R. Enterocolitis caused by Ehrlichia sp. in the horse (Potomac horse fever). Vet Pathol. 1986 Jul;23(4):471–477. doi: 10.1177/030098588602300418. [DOI] [PubMed] [Google Scholar]
- Damrow T. A., Williams J. C., Waag D. M. Suppression of in vitro lymphocyte proliferation in C57BL/10 ScN mice vaccinated with phase I Coxiella burnetii. Infect Immun. 1985 Jan;47(1):149–156. doi: 10.1128/iai.47.1.149-156.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durant S. In vivo effects of catecholamines and glucocorticoids on mouse thymic cAMP content and thymolysis. Cell Immunol. 1986 Oct 1;102(1):136–143. doi: 10.1016/0008-8749(86)90332-1. [DOI] [PubMed] [Google Scholar]
- Dutta S. K., Myrup A. C., Rice R. M., Robl M. G., Hammond R. C. Experimental reproduction of Potomac horse fever in horses with a newly isolated Ehrlichia organism. J Clin Microbiol. 1985 Aug;22(2):265–269. doi: 10.1128/jcm.22.2.265-269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt P. K., Huxsoll D. L., Walker J. S., Nims R. M., Taylor R., Andrews M. Pathology of canine ehrlichiosis (tropical canine pancytopenia). Am J Vet Res. 1973 Oct;34(10):1309–1320. [PubMed] [Google Scholar]
- Jenkins S. J., Jones N. K., Jenny A. L. Potomac horse fever agent in mice. Vet Rec. 1985 Nov 23;117(21):556–557. doi: 10.1136/vr.117.21.556. [DOI] [PubMed] [Google Scholar]
- Magnuson N. S., McGuire T. C., Banks K. L., Perryman L. E. In vitro and in vivo effects of corticosteroids on peripheral blood lymphocytes from ponies. Am J Vet Res. 1978 Mar;39(3):393–398. [PubMed] [Google Scholar]
- Pretzman C. I., Rikihisa Y., Ralph D., Gordon J. C., Bech-Nielsen S. Enzyme-linked immunosorbent assay for Potomac horse fever disease. J Clin Microbiol. 1987 Jan;25(1):31–36. doi: 10.1128/jcm.25.1.31-36.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y., Perry B. D. Causative ehrlichial organisms in Potomac horse fever. Infect Immun. 1985 Sep;49(3):513–517. doi: 10.1128/iai.49.3.513-517.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y., Perry B. D., Cordes D. O. Ultrastructural study of ehrlichial organisms in the large colons of ponies infected with Potomac horse fever. Infect Immun. 1985 Sep;49(3):505–512. doi: 10.1128/iai.49.3.505-512.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristic M., Holland C. J., Dawson J. E., Sessions J., Palmer J. Diagnosis of equine monocytic ehrlichiosis (Potomac horse fever) by indirect immunofluorescence. J Am Vet Med Assoc. 1986 Jul 1;189(1):39–46. [PubMed] [Google Scholar]
- Smith K. A. Interleukin 2. Annu Rev Immunol. 1984;2:319–333. doi: 10.1146/annurev.iy.02.040184.001535. [DOI] [PubMed] [Google Scholar]
- Steele K. E., Rikihisa Y., Walton A. M. Ehrlichia of Potomac horse fever identified with a silver stain. Vet Pathol. 1986 Jul;23(4):531–533. doi: 10.1177/030098588602300430. [DOI] [PubMed] [Google Scholar]
- Weiss L., Geduldig U., Weidanz W. Mechanisms of splenic control of murine malaria: reticular cell activation and the development of a blood-spleen barrier. Am J Anat. 1986 Jul;176(3):251–285. doi: 10.1002/aja.1001760303. [DOI] [PubMed] [Google Scholar]
- Williams J. C., Cantrell J. L. Biological and immunological properties of Coxiella burnetii vaccines in C57BL/10ScN endotoxin-nonresponder mice. Infect Immun. 1982 Mar;35(3):1091–1102. doi: 10.1128/iai.35.3.1091-1102.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemer E. L., Whitlock R. H., Palmer J. E., Spencer P. A. Clinical and hematologic variables in ponies with experimentally induced equine ehrlichial colitis (Potomac horse fever). Am J Vet Res. 1987 Jan;48(1):63–67. [PubMed] [Google Scholar]