Abstract
OBJECTIVE—NOD mice model human type 1 diabetes and are used to investigate tolerance induction protocols for islet transplantation in a setting of autoimmunity. However, costimulation blockade–based tolerance protocols have failed in prolonging islet allograft survival in NOD mice.
RESEARCH DESIGN AND METHODS—To investigate the underlying mechanisms, we studied the ability of costimulation blockade to prolong islet allograft survival in congenic NOD mice bearing insulin-dependent diabetes (Idd) loci that reduce the frequency of diabetes.
RESULTS—The frequency of diabetes is reduced in NOD.B6 Idd3 mice and is virtually absent in NOD.B6/B10 Idd3 Idd5 mice. Islet allograft survival in NOD.B6 Idd3 mice treated with costimulation blockade is prolonged compared with NOD mice, and in NOD.B6/B10 Idd3 Idd5, mice islet allograft survival is similar to that achieved in C57BL/6 mice. Conversely, some Idd loci were not beneficial for the induction of transplantation tolerance. Alloreactive CD8 T-cell depletion in (NOD × CBA)F1 mice treated with costimulation blockade was impaired compared with similarly treated (C57BL/6.H2g7 × CBA)F1 mice. Injection of exogenous interleukin (IL)-2 into NOD mice treated with costimulation prolonged islet allograft survival. NOD.B6 Idd3 mice treated with costimulation blockade deleted alloreactive CD8 T-cells and exhibited prolonged islet allograft survival.
CONCLUSIONS—Il2 is the Idd3 diabetes susceptibility gene and can influence the outcome of T-cell deletion and islet allograft survival in mice treated with costimulation blockade. These data suggest that Idd loci can facilitate induction of transplantation tolerance by costimulation blockade and that IL-2/Idd3 is a critical component in this process.
The NOD mouse is a model of type 1–like autoimmune diabetes and is used to study costimulation blockade–based transplantation tolerance within the context of autoimmunity (1–4). However, costimulation blockade protocols fail in NOD mice. To investigate further the cellular and genetic control of costimulation blockade–induced transplantation tolerance, we used NOD Idd congenic mice that have small introgressed regions of genetic intervals derived from diabetes-resistant C57 stocks. These mice exhibit varying degrees of protection from autoantibodies, insulitis, and diabetes (5). Using Idd congenic NOD mice, we have observed that islet allograft survival is improved by the addition of the diabetes-protective Idd3 locus (6,7).
Idd3 modulates infiltration of autoreactive lymphocytes into the islets (8), and there is compelling evidence that Idd3 is the interleukin (IL)-2 gene (9). In vivo stimulated NOD T-cells produce twofold less IL-2 mRNA than cells from NOD congenic mice having protective alleles at Idd3 (9,10). Neutralizing antibodies to IL-2 lead to accelerated disease in NOD mice (11), and targeted genetic disruption of IL-2 accelerates type 1–like autoimmune diabetes (9). Treatment with exogenous IL-2 inhibits diabetes development in NOD mice and improves T regulatory (Treg) function (12). IL-2 is also known to have a nonredundant role in CD8 T-cell activation–induced cell death via the CD95 (Fas) pathway (13), is required for the development of self-tolerance (14), and is essential for the induction of allograft tolerance by costimulation blockade (15). However, IL-2 is a double-edged sword, since administration of IL-2 in vivo can either enhance or depress a cytotoxic T lymphocyte (CTL) response (16).
In this study, we show that costimulation blockade fails to delete alloreactive CD8 T-cells in NOD mice. Genetic replacement of IL-2 in NOD.B6 Idd3 mice enhances alloreactive CD8 T-cell deletion and improves islet allograft survival. Finally, we show that Idd3 synergizes with genes within the Idd5 interval, leading to permanent islet allograft survival in a majority of NOD.B6/B10 Idd3 Idd5 mice treated with costimulation blockade.
RESEARCH DESIGN AND METHODS
C3H/He (H2k) mice were obtained from the National Cancer Institute (Frederick, MD), The Jackson Laboratory (Bar Harbor, ME), or Taconic Farms (Germantown, NY). NOD-Prkdcscid (NOD-scid) mice were obtained from The Jackson Laboratory. C57BL/6 (H2b), NOD/Mrk-TacfBR, NOD.B6 Idd3R450 (Taconic line 1098), NOD.CZECH Idd3 (Taconic line 1590), NOD.B6 Idd3R450 + B10 Idd5R444 (Taconic lines 1591 and 6109), NOD.B6 Idd3R450 + B10 Idd5R467 (Taconic line 1573), NOD.B10 Idd5R444 (Taconic line 1094), NOD.B6 Idd3 Idd10 Idd18R323 (Taconic line 1538), and NOD.B6 Idd10 Idd18(R2) (Taconic lines 1101 and 7754) were obtained from Taconic Farms. Because the experimental data using the NOD.B6 Idd3R450 (Taconic line 1098) and NOD.CZECH Idd3 (Taconic line 1590) congenic variants of Idd3 were comparable (9), these groups have been combined for presentation and are referred to in the text as NOD.B6 Idd3 mice. A schematic of the congenic intervals on mouse chromosomes is shown in Fig. 1. C57BL/6.NODc17 (H2g7, C57BL/6.H2g7) mice were developed by Edward Wakeland, University of Texas Southwestern Medical Center, Dallas, Texas (17). (KB5 CBA × C57BL/6.H2g7) F1 mice and (KB5 CBA × NOD) F1 mice were generated by a single intercross of the appropriate parental strains and were bred in our facility. The KB5 TCR transgene is expressed in CBA (H2k) mice by CD8+ T-cells and is specific for native H2-Kb (18).
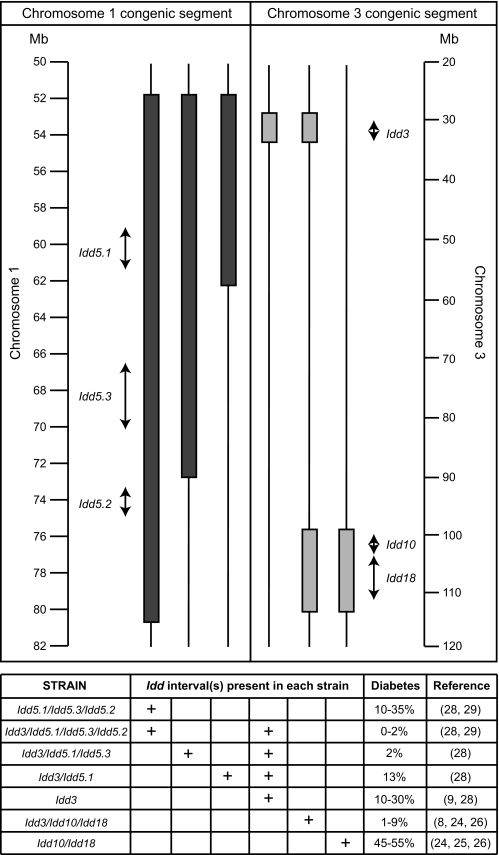
FIG. 1.
Schematic representation of candidate gene interval and chromosomal location. The filled bars represent B10-derived or B6-derived congenic segments on chromosomes 1 and 3, respectively. The arrows represent the size of each Idd interval as previously defined using additional congenic strains of mice: Idd3 (650 kb) (ref. 9), Idd10 (950 kb) (ref. 48), Idd18 (4.0 Mb) (ref. 29), Idd5.1 (2.1 Mb) (ref. 30), Idd5.2 (1.52 Mb) (ref. 30), and Idd5.3 (3.6 Mb) (ref. 31). The “diabetes” column indicates the percentage of females developing diabetes by 7 months of age. Where a range is indicated, this summarizes the results of a number of frequency studies performed over many years.
Animals were certified to be free of infectious pathogens, housed in microisolator cages within a specific pathogen-free facility, and given autoclaved food and acidified water ad libitum. All animal use was in accordance with the guidelines of the Animal Care and Use Committee of the University of Massachusetts Medical School and recommendations in the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Research Council, National Academy of Sciences, 1996).
Generation of KB5 synchimeras.
KB5 synchimeric mice were generated using a previously described procedure (18). Briefly, (CBA/J × NOD)F1 mice carrying a single copy of a B6-like allele of the IL-2 gene (19) and (CBA/J × C57BL/6.H2g7)F1 mice carrying two copies of a B6 or B6-like IL-2 gene were irradiated with 400 cGy from a 137Cs source (Gammacell 40; Atomic Energy of Canada, Ottawa, ON, Canada) and given a single intravenous injection of 0.5 × 106 (KB5 CBA × NOD)F1 or (KB5 CBA × C57BL/6.H2g7)F1 transgenic bone marrow cells, respectively. Mice were entered into experiments 8–12 weeks after bone marrow transplantation.
Antibodies and flow cytometry.
The KB5-specific clonotypic Desirè (DES) antibody was the gift of Dr. John Iacomini (Harvard Medical School, Boston, MA). Fluorescein isothiocyanate–conjugated anti-mouse IgG2a (clone R19-15) and PerCept-conjugated anti-mouse CD8α monoclonal antibodies (mAbs) (clone 53-6.7) were obtained from BD PharMingen (San Diego, CA). Isotype control mAbs including rat PerCP-conjugated IgG2a κ (clone R35-95) and mouse IgG2a κ anti-TNP (clone G155-178) were purchased from BD PharMingen.
Two-color flow cytometry analyses of lymph node and spleen cells were performed (20). Briefly, cells were incubated with anti-FcγRIII/II mAb (clone 2.4G2) to eliminate nonspecific Fc binding. Cells were washed, incubated with anti-DES antibody, washed, and incubated with fluorescein isothiocyanate–conjugated anti-mouse IgG2a mAb plus a mixture of conjugated mAbs. Whole blood was processed using fluorescence-activated cell sorter lysing solution (Becton Dickinson, Sunnyvale, CA). Labeled cells were washed, fixed with 1% paraformaldehyde-PBS, and analyzed using a FACScan instrument (Becton Dickinson). Lymphoid cells were gated according to their light-scattering properties, and 30–50 × 103 events were acquired for each analysis.
MR1 hamster anti-mouse CD154 mAb was produced as tissue culture supernatant and purified by affinity chromatography (National Cell Culture Center, Minneapolis, MN) (18). Contaminating endotoxin was uniformly <10 units/mg of mAb.
Histology.
Kidneys bearing islet grafts were fixed in Bouin's solution. Paraffin-embedded sections were prepared and stained with hematoxylin and eosin. Additional sections were stained for the presence of insulin and glucagon.
Tolerance induction and allograft transplantation.
Diabetes was induced in 6- to 8-week-old male mice by a single intraperitoneal injection of streptozotocin (150 mg/kg) (6). Animals were tested for glycosuria (test strips, Glucosin; Bayer, Elkhart, IN) twice weekly. Diabetes was confirmed by documenting plasma glucose concentration >250 mg/dl (Accu-Chek Active, Roche Diagnostics, Indianapolis, IN.). Mice hyperglycemic for at least 1 week were used in the experiments. Chemically diabetic mice were treated with our standard costimulation blockade protocol consisting of a single C3H/He donor-specific transfusion (DST) of 107 spleen cells injected intravenously on day −7 and with anti-CD154 mAb (0.5 mg/dose) on days −7, −4, 0, and +4 relative to transplantation on day 0 (6).
Islets isolated from C3H/He donors by collagenase digestion followed by density gradient separation were transplanted (20 islets/g body weight) into the renal subcapsular space of chemically diabetic recipients (21,22). Animals were tested for glycosuria twice weekly, and allograft rejection was defined as recurrent hyperglycemia (>250 mg/dl) on at least 2 consecutive days. Unilateral nephrectomy of the graft-bearing kidney was performed on all islet allograft recipients that were normoglycemic at the conclusion of an experiment to confirm allograft function.
(KB5 CBA × NOD)F1 or (KB5 CBA × C57BL/6.H2g7)F1 synchimeric mice were treated with C57BL/6 DST and anti-CD154 mAb (18).
Injection of IL-2 during costimulation blockade.
Recombinant murine IL-2 (0.8 μg, R&D systems, Minneapolis, MN) was injected intraperitoneally on days −7, −6, −5, −4, and −3 relative to analysis of KB5 DES+ CD8+ T-cells or transplantation on day 0. Concurrently, costimulation blockade consisting of DST on day −7 and injections of anti-CD154 mAb on days −7, −4, 0, and +4 were performed relative to transplantation on day 0.
In vivo cytotoxicity assay.
The in vivo cytotoxicity assay was performed as previously described (23). Briefly, 6- to 8-week-old male NOD, C57BL/6, or NOD.B6 Idd3 mice were treated on day −7 with DST and on days −7 and −4 with anti-CD154 mAb relative to depletion of natural killer (NK) cells on day −8 by injection of 1 mg anti-CD122 mAb (24). On day 0 (the normal day of islet transplantation), carboxyfluorescein diacetate succinimidyl ester–labeled splenocytes were adoptively transferred intravenously into naive recipient mice or into the indicated recipient mice. Spleens from recipient mice were harvested 20 h later, and the survival of each transferred population was assessed by flow cytometry. Specific lysis was calculated as described (23).
In vitro NK cell cytotoxicity assay.
The in vitro NK cell cytotoxicity assay was performed as previously described (25) using spleen cells from mice injected 24 h previously with 100 μg polyinosinic:polycytidylic (poly I:C) as effectors and 51Cr-labeled YAC-1 cells as targets. In some cohorts, NK cells were depleted by injection of 1 mg anti-CD122 mAb 1 day before poly I:C administration. Percent specific lysis was calculated as follows: % specific lysis = [(experimental lysis –spontaneous lysis)/(maximal lysis –spontaneous lysis)] × 100.
Statistical analysis.
Median duration of allograft survival is presented. Graft survival among groups was compared using the method of Kaplan and Meier. The equality of allograft survival distributions for animals in different treatment groups was tested using the log rank statistic. P values <0.05 were considered statistically significant. Data are presented as the mean ± 1 SD. Comparisons of two means used Student's t test with separate variance estimates. Comparisons of three or more means used one-way ANOVA and the least significant difference procedure for a posteriori contrasts.
RESULTS
Islet allograft rejection in chemically diabetic male NOD mice is not due to islet autoimmunity.
Islet allograft survival in chemically diabetic male NOD mice treated with our costimulation blockade protocol is relatively short (6). However, in those experiments, islet graft rejection could have resulted from islet autoimmunity or the failure to induce allograft tolerance. To investigate this, chemically diabetic NOD mice were transplanted with syngeneic NOD-scid islets. As reported previously (26), we observed through 150 days after islet transplantation that all mice (5/5) remained normoglycemic. Analysis of the islet-bearing kidney revealed an insulin-producing islet graft present at the time of necropsy with only a small amount of leukocytic infiltrate. These data suggest that islet allograft rejection in our model system results from failure to induce tolerance and not to the development of islet autoimmunity.
Islet allograft survival in NOD.B6 Idd10 Idd18 and NOD.B6 Idd3 Idd10 Idd18 congenic mice after treatment with DST and anti-CD154 mAb.
Islet allograft survival is prolonged in NOD mice bearing the diabetes-resistant Idd3 congenic interval (6). However, Idd3 is only partially protective but, when combined with certain other Idd loci, almost completely protects NOD mice from diabetes (Fig. 1). We hypothesized that combinations of Idd loci that are strongly protective against diabetes would enhance islet allograft survival. NOD mice congenic for the Idd10 Idd18 intervals have reduced incidence of diabetes (27,28) and, when combined with Idd3, have a very low frequency of diabetes (8,27,29).
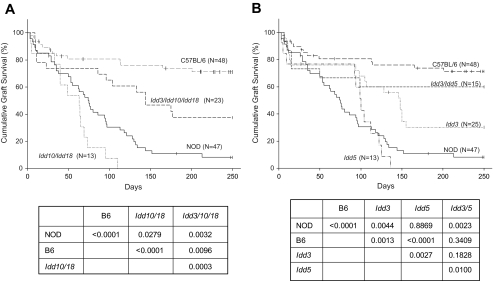
Confirming our previous report (6), islet allograft survival in NOD mice treated with costimulation blockade is short (median survival time [MST] was 74 days), whereas permanent islet allograft survival (MST >240 days) is observed in the majority of similarly treated C57BL/6 mice (Fig. 2A). We also confirmed that NOD mice bearing the Idd3 congenic interval exhibit islet allograft survival that is prolonged (MST = 140 days, [6]) compared with NOD mice but significantly shorter than that achieved in C57BL/6 mice (Fig. 2B). Surprisingly, we observed that NOD Idd10 Idd18 congenic mice exhibited shorter islet allograft survival (MST = 63 days) than that achieved in NOD mice (Fig. 2). Combination of the Idd10 Idd18 genetic intervals with the beneficial effects of Idd3 did not alter islet allograft survival compared with that achieved in NOD.B6 Idd3 congenic mice (NS, Fig. 2A and B).
FIG. 2.
Life table analysis of islet allograft survival in chemically diabetic congenic NOD mice. The 6- to 8-week-old male mice were treated with a DST plus anti-CD154 mAb. DST (107 C3H/He spleen cells) was given on day −7, and anti-CD154 mAb (0.5 mg/dose) was given on days −7, −4, 0, and +4 relative to transplantation with C3H/He islets on day 0. Vertical bars indicate mice removed from the study with intact grafts or alive with intact grafts at the conclusion of the period of observation. Comparative P values of islet allograft survival in the groups are shown. A: Idd10 Idd18 and Idd3 Idd10 Idd18 congenic NOD mice. B: Idd3, Idd5, and Idd3/Idd5 congenic NOD mice. Islet allograft survival in C57BL/6 and NOD mice shown in A is reproduced in B for ease of comparison with other strains.
Protective Idd5 and Idd3 alleles synergize to prolong islet allograft survival in chemically diabetic NOD mice treated with DST and anti-CD154 mAb.
We next studied the effects of Idd5 alone or in combination with Idd3. NOD mice bearing the Idd5 disease-resistant loci are partially protected from diabetes (30,31), and the addition of Idd3 protective alleles results in nearly complete disease suppression (31,32).
NOD.B10 Idd5 congenic mice treated with DST and anti-CD154 did not exhibit prolonged islet allograft survival (MST = 96 days) compared with that achieved in NOD mice (NS, Fig. 2B) and was significantly shorter than that achieved in NOD.B6 Idd3 congenic mice (P < 0.005, Fig. 2B). These data, combined with the NOD.B6 Idd10 Idd18 results, demonstrate that enhancement of islet allograft survival by Idd loci does not strictly correlate with the extent to which they suppress diabetes.
Strikingly, NOD mice bearing the diabetes-protective Idd3 and Idd5 congenic intervals exhibited prolonged islet allograft survival (MST >250 days), which was similar to that achieved in C57BL/6 mice (NS) and significantly greater than that achieved in NOD (P < 0.005) or NOD.B10 Idd5 mice (P < 0.01, Fig. 2B). In long-term surviving islet allografts, we routinely observed minimal to no mononuclear infiltration.
Islet allograft survival in Idd3 congenic NOD mice bearing different Idd5 subregions treated with costimulation blockade.
The Idd5 interval congenic strain used in this study contains at least three diabetes-resistant loci: Idd5.1, Idd5.2, and Idd5.3 (30,31). To begin to identify the congenic Idd5 interval that synergizes with Idd3 to prolong islet allograft survival in NOD mice treated with costimulation blockade, we tested two newly developed NOD congenic lines that carry the B6-derived Idd3 congenic interval as well as the B10-derived Idd5.1 or Idd5.1 plus Idd5.3 intervals (Fig. 1).
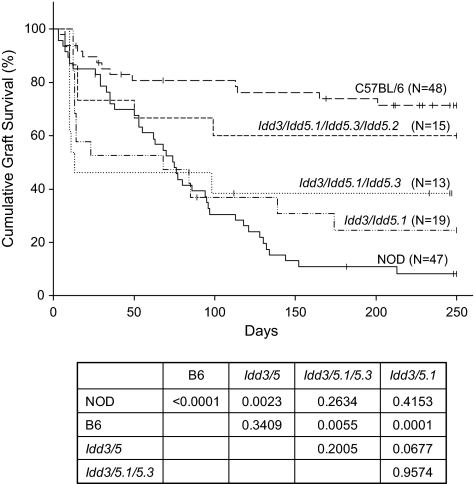
NOD.B6 Idd3 B10 Idd5.1 congenic mice treated with costimulation blockade exhibited islet allograft survival shorter (MST = 69 days) than that achieved in C57BL/6 mice (P < 0.0001) and not different from that achieved in NOD mice (NS, Fig. 3). Islet allograft survival was also not enhanced in NOD.B6 Idd3 B10 Idd5.1 Idd5.3 mice (MST = 13 days) over that achieved in NOD.B6 Idd3 B10 Idd5.1 congenic mice (NS, Fig. 3). These data suggest that expression of Idd5.2 encoding a nonfunctional protein is important for the beneficial effects of Idd5 in conjunction with Idd3 and that all three subregions are needed—or that Idd5.2 alone or in combo with Idd5.1 or Idd5.3 is beneficial. Interestingly, we observed that a proportion of animals maintained long-term graft function.
FIG. 3.
Life table analysis of islet allograft survival in chemically diabetic Idd3 congenic NOD mice bearing different Idd5 congenic intervals. Groups of 6- to 8-week-old chemically diabetic male mice were treated with a DST plus anti-CD154 mAb. A DST (107 C3H/He spleen cells) was given on day −7, and anti-CD154 mAb (0.5 mg/dose) was given on days −7, −4, 0, and +4 relative to transplantation with C3H/He islets on day 0. Vertical bars indicate mice removed from the study with intact grafts or alive with intact grafts at the conclusion of the period of observation. Islet allograft survival in C57BL/6, NOD, and NOD Idd3 Idd5 congenic mice shown in Fig. 2 is reproduced here for ease of comparison with other strains. Comparative P values of islet allograft survival in the groups are shown.
Failure of costimulation blockade treatment to delete alloreactive CD8+ T-cells in (NOD × KB5)F1 synchimeric mice is reversed by IL-2.
Idd3-mediated diabetes susceptibility in NOD mice is caused by an IL-2 allele that is transcribed at lower levels than variants contributing to disease resistance (9) and is important for tolerance induction (33). Therefore, we hypothesized that a deficiency in IL-2 production in NOD mice impairs host alloreactive CD8+ T-cell deletion.
To test this hypothesis, we modified our synchimera model system based on KB5 TCR transgenic alloreactive CD8+ T-cells (18). KB5 CBA mice were mated with NOD mice or with C57BL/6.H2g7 mice bearing the NOD major histocompatibility complex and were used to generate synchimeric mice (18). Synchimeric mice were treated with C57BL/6 DST and anti-CD154, and the circulating levels of KB5 transgenic alloreactive CD8+ T-cells were analyzed on day 0. Two groups of synchimeric mice were also given five daily injections of mouse rIL-2 beginning on the day of DST.
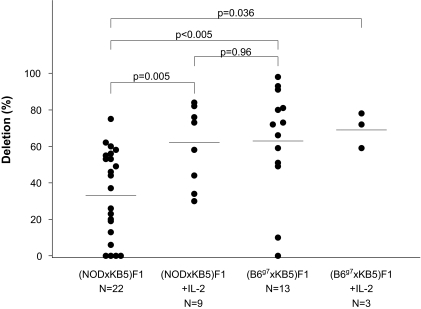
(KB5 CBA × C57BL/6.H2g7)F1 synchimeric mice treated with costimulation blockade exhibited marked deletion of their alloreactive CD8+ T-cells (63 ± 30%, Fig. 4). Similar results were seen in the (KB5 CBA × C57BL/6.H2g7)F1 synchimeric mice that received exogenous IL-2 in addition to the DST and anti-CD154 (70 ± 6%). In contrast, (KB5 CBA × NOD)F1 mice exhibited significantly less deletion of their alloreactive CD8+ T-cell population (34 ± 24%), which was restored by IL-2 treatment (63 ± 21%) to a level similar to that observed in (KB5 CBA × C57BL/6.H2g7)F1 mice (Fig. 4).
FIG. 4.
Scatter plot of alloreactive CD8+ T-cell deletion in synchimeric mice. (KB5 CBA × C57BL/6.H2g7)F1 mice and (KB5 CBA × NOD)F1 synchimeric mice were treated with a C57BL/6 DST on day −7 and anti-CD154 mAb on days −7 and −4 relative to analysis of their circulating levels of KB5 transgenic CD8+ T-cells on day 0 as described in research design and methods. P values are indicated by horizontal bars.
IL-2 improves islet allograft survival in NOD mice treated with costimulation blockade.
We next hypothesized that increased deletion of alloreactive CD8+ T-cells in (KB5 CBA × NOD)F1 mice treated with costimulation blockade plus IL-2 would lead to a difference in islet allograft survival. To test this, chemically diabetic NOD mice were treated with costimulation blockade and transplanted with C3H/He islets with or without peri-transplant injection of IL-2.
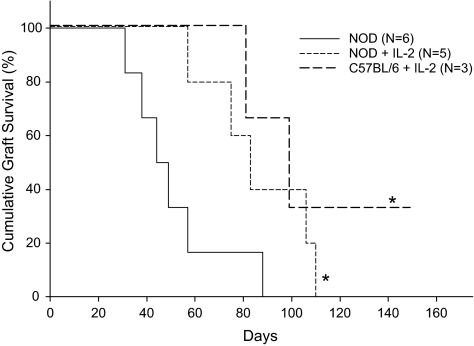
As expected (6), islet allograft survival in NOD mice treated with costimulation blockade was short (MST = 46 days, Fig. 5). In contrast, NOD mice treated with costimulation blockade and IL-2 exhibited slightly but significantly prolonged islet allograft survival (MST = 83 days), although all islet allografts were eventually rejected (Fig. 5).
FIG. 5.
Life table analysis of islet allograft survival in NOD mice treated with IL-2. Groups of 6- to 8-week-old chemically diabetic NOD mice were treated with a C3H/He DST on day −7 and anti-CD154 mAb (0.5 mg/dose) on days −7, −4, 0, and +4 relative to transplantation with C3H/He islets on day 0. One group of mice also received 0.8 μg recombinant murine IL-2 (R&D systems, Minneapolis, MN) intraperitoneally on days −7, −6, −5, −4, and −3 relative to islet transplantation on day 0. *P < 0.05 vs. NOD.
NOD.B6 Idd3 congenic mice exhibit a restored ability to delete alloreactive CD8 T-cells after costimulation blockade.
Having observed that the Idd3 locus improved islet allograft survival (Fig. 5) and that exogenous administration of IL-2 improved alloreactive CD8 T-cell deletion in our synchimeric model system (Fig. 4), we next tested directly our hypothesis that restoration of a normal IL-2 gene by the Idd3 locus in NOD mice would restore the ability of costimulation blockade to delete alloreactive CD8 T-cells. To directly identify alloreactive CD8 T-cell function in NOD.B6 Idd3 mice, we used an in vivo cytotoxicity assay (23). In NK cell–depleted mice, all in vivo cytotoxic activity is due to alloreactive CD8 T-cells (34).
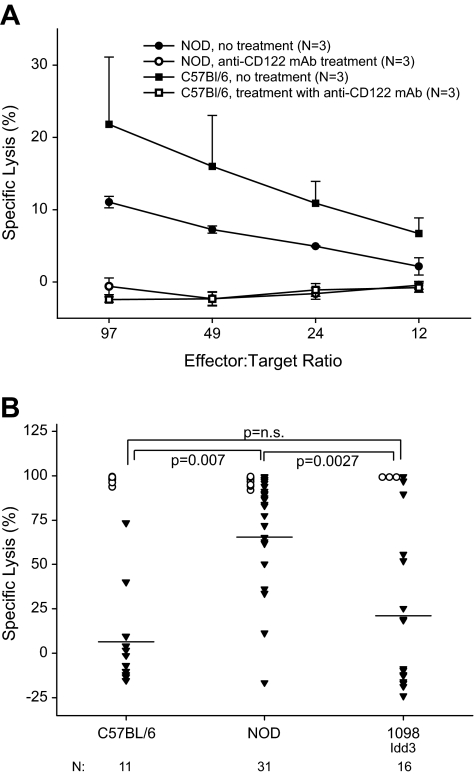
We first confirmed (24) that the anti-CD122 antibody would delete all NK cell cytotoxic activity in NOD mice (Fig. 6A). As expected, NK-depleted C57BL/6 mice treated with costimulation blockade exhibited low in vivo cytotoxicity, whereas alloreactive CD8 T-cell activity in NOD mice was high (Fig. 6B). In NK-depleted NOD.B6 Idd3 mice, in vivo cytotoxicity was significantly lower than that observed in NOD mice and was comparable to that observed in C57BL/6 mice (Fig. 6B). In all three strains, priming NK cell–depleted mice with a DST alone induced strong in vivo alloreactive CD8 T-cell cytotoxicity (Fig. 6B).
FIG. 6.
In vivo cytotoxicity activity of alloreactive CD8 T-cells in mice treated with costimulation blockade. A: NOD and C57BL/6 male mice 6–8 weeks of age were untreated or injected with 1.0 mg anti-CD122 mAb (clone TMβ1). Twenty-four hours later, all groups were injected with 100 μg poly I:C, and 20 h later, spleen cells were recovered for analyses of in vitro NK cell cytotoxicity activity on the NK-sensitive cell line YAC-1, as previously described (25). B: NOD, C57BL/6, and NOD.B6 Idd3 mice were treated with costimulation blockade, depleted of NK cells and injected with CFSE-labeled spleen cells for an in vivo cytotoxicity assay, as described in research design and methods. ○, DST; ▾, DST + anti-CD154. The number of mice tested in each group is indicated below each strain tested. NOD vs. NOD.B6 Idd3, P < 0.005; C57BL/6 vs. NOD, P < 0.01 C57BL/6 vs. NOD.B6 Idd3, NS.
DISCUSSION
We have confirmed in this article that islet allograft survival in NOD.B6 Idd3 mice treated with costimulation blockade is prolonged compared with NOD mice (1). We now document that some but not all Idd resistance loci synergize with Idd3 to enhance islet allograft survival. We further show that a major tolerance defect in NOD mice is the resistance of alloreactive CD8 T-cells to deletion by costimulation blockade, a defect that can be reversed by addition of the Idd3 gene or by administration of exogenous IL-2.
Disease-resistant alleles at the Idd10 Idd18 region provide moderate protection against diabetes (45–55%) (27,28,35), and together, the Idd3 Idd10 and Idd18 protective alleles confer almost complete resistance (8,27,29). We hypothesized that NOD.B6 Idd10 Idd18 mice and NOD.B6 Idd3 Idd10 Idd18 mice would demonstrate a stepwise improvement in costimulation blockade–induced islet allograft survival. Surprisingly, islet allograft survival in NOD.B6 Idd10 Idd18 mice was not increased over that achieved in NOD mice and did not increase over that achieved with Idd3 alone.
Genes located within the Idd10 and Idd18 intervals include Ptpn22, which is orthologous to PTPN22, a human gene that is associated with the development of diabetes and other autoimmune diseases (28,36). The disease-associated allele of human PTPN22 is a gain-of-function variant that in vitro suppresses TCR signaling in response to TCR/CD28 ligation to a greater extent than the more common allele (37). The functional outcome of TCR signaling in the PTPN22 gain-of-function variant reduces expression of IL-2. Studies that altered the allelic status of the Ptpn22 region in NOD congenic mice have demonstrated that the B6-derived interval confers susceptibility to type 1–like autoimmune diabetes (L.S.W., L.B.P., unpublished data), therefore providing a potential explanation for the inability of the B6-derived Idd10 Idd18 region to increase islet allograft survival.
Given the surprising results seen with the NOD.B6 Idd3 Idd10 Idd18 islet allograft studies, we next determined whether a synergistic effect could be found between Idd3 and Idd5. NOD.B10 Idd5 mice have a lower frequency of diabetes than NOD mice, and when combined with resistance alleles at Idd3, the frequency of spontaneous diabetes is <2% (30,32). Importantly, islet allograft survival in NOD.B6 Idd3 Idd5 mice was increased to levels achieved in C57BL/6 mice.
The Idd5 congenic strain used in our study contains at least three diabetes resistance genes, termed Idd5.1, Idd5.2, and Idd5.3 (30). The Idd5.1 gene is most likely a variant of Ctla4, with the diabetes-prone NOD allele producing less of the ligand-independent CTLA-4 (liCTLA-4) molecule than the resistant B10 allele (38). CTLA-4 is critical for the induction of tolerance using costimulation blockade (39). Slc11a1 (formerly known as Nramp1) is likely to be the causal Idd5.2 gene (40). Interestingly, the B10 diabetes-resistant Nramp1 allele encodes a nonfunctional protein (41). However, Idd5.2/Nramp1 is not required for the decreased diabetes frequency in NOD.B6 Idd3 B10 Idd5 mice, although the B10-derived Idd5.2 region is required to maintain the reduced insulitis present in NOD.B6 Idd3 B10 Idd5 mice (31). In addition, the non–NOD-derived Idd5.1/Ctla4 and Idd3 resistance alleles did not increase protection from diabetes compared with Idd3 alone (31). These results are consistent with our islet allograft survival data. The synergy observed between Idd5 and Idd3 that results in nearly complete protection from diabetes and insulitis and increases islet allograft survival to a C57BL/6-like frequency is dependent on the B10-derived Idd5.2 region, either alone or in combination with the other Idd5 loci. To extend this observation, additional congenic strain combinations would have to be developed and tested: Idd3/Idd5.3, Idd3/Idd5.2, and Idd3/Idd5.2/Idd5.3.
Idd3, which is partially protective of diabetes, significantly improves islet allograft survival in the NOD mouse, with the strongest effect seen in the NOD.B6 Idd3 B10 Idd5 congenic strain. The Idd3 effect likely results from differential expression of IL-2 that modulates CD4+CD25+ Treg cell function in NOD mice (9). IL-2 is also required for the development of self-tolerance and for costimulation blockade–induced allograft tolerance (10,15).
Based on the NOD.B6 Idd3 congenic data, we hypothesized that the inability to induce tolerance in NOD mice is due to a failure to efficiently delete host alloreactive CD8+ T-cells and that injection of exogenous IL-2 would correct this defect. As expected, (KB5 CBA × C57BL/6.H2g7)F1 synchimeric mice treated with costimulation blockade showed a marked deletion of alloreactive CD8+ T-cells compared with (KB5 CBA × NOD)F1 synchimeric mice. When (KB5 CBA × NOD)F1 synchimeric mice were treated with exogenous IL-2, alloreactive CD8+ T-cell deletion was significantly improved. These data suggest that NOD mice fail to efficiently delete alloreactive CD8+ T-cells because of insufficient IL-2 production.
To extend this finding, we observed that islet allograft survival in NOD mice treated with costimulation blockade plus IL-2 was slightly longer than in those receiving costimulation blockade alone, likely through enhancement of alloreactive CD8 T-cell apoptosis (16). This interpretation has previously been proposed based on the observation that coadministration of rapamycin plus IL-2 prevents spontaneous and recurrent autoimmunity in NOD mice through the ability of rapamycin to inhibit IL-2 T-cell proliferation but not IL-2–induced apoptosis (42). The fact that graft survival isn't as prolonged as that achieved in NOD.B6 Idd3 mice may be due to the transient administration of IL-2 and its short half-life, whereas the increased IL-2 achieved in NOD.B6 Idd3 mice is present throughout the animal's life. Alternatively, we had previously documented in CD8-deficient NOD and (NOD × C57BL/6)F1 mice that CD8+ T-cells are not solely responsible for the failure to induce prolonged allograft survival after costimulation blockade (7), implicating a role for Il2/Idd3 in other transplantation tolerance pathways, consistent with the known role of Idd3 in Treg function in NOD mice (9).
These data suggest that impaired production of IL-2 in NOD mice is a barrier to costimulation blockade–induced tolerance. IL-2 is indispensable for supporting the in vivo growth, survival, and function of naturally occurring Tregs (11,43–46), and because of their corrected Idd3 haplotype, NOD.B6 Idd3 mice have CD4+CD25+ Tregs with enhanced regulatory activity (9).
In summary, we have shown that the resistance to costimulation blockade–induced tolerance to islet allografts in NOD mice is in part due to the failure to efficiently delete alloreactive CD8 T-cells. Genetically, this can be overcome by the introgression of a normal Idd3 (i.e., Il2) gene that synergizes with one or more protective subregions within Idd5 to promote allograft tolerance. Speculatively, we propose that the B6 allele of Idd3 might also contribute to the apoptosis of islet-specific CD8 cells in spontaneous diabetes. Because SNP polymorphisms in the IL-2 receptor have been associated with a genetic predisposition for type 1 diabetes in humans (47), these data suggest that regulation of IL-2 may be important not only for diabetes but also for islet transplantation in type 1 diabetic individuals.
Acknowledgments
This study was supported in part by grants AR35506 and AI42669, an institutional Diabetes Endocrinology Research Center (DERC) grant (DK52530) from the National Institutes of Health, grant DK53006 from the National Institutes of Health, and a grant to L.D.S. from the Juvenile Diabetes Research Foundation (JDRF). T.P. was supported by a fellowship grant from JDRF. L.S.W. was supported by a joint grant from the JDRF and the Wellcome Trust. D.V.S. was supported by grants DK46266 and DK51090 from the National Institutes of Health, as well as by grants from the JDRF. L.D.S. was supported by JDRF Grant 1-2004-548.
The availability of NOD congenic mice through the Taconic Farms Emerging Models Program has been supported by grants from the Merck Genome Research Institute, the National Institute of Allergy and Infectious Diseases, and the JDRF. No other potential conflicts of interest relevant to this article were reported.
We thank Linda Paquin, Cindy Bell, Linda Leehy, Dan Rainbow, and Jean Leif for technical assistance.
Published ahead of print at http://diabetes.diabetesjournals.org on 4 November 2008.
The contents of this study are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Pearson T, Markees TG, Serreze DV, Pierce MA, Marron MP, Wicker LS, Peterson LB, Shultz LD, Mordes JP, Rossini AA, Greiner DL: Genetic disassociation of autoimmunity and resistance to costimulation blockade-induced transplantation tolerance in nonobese diabetic mice. J Immunol 171: 185–195, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Molano RD, Berney T, Li H, Cattan P, Pileggi A, Vizzardelli C, Kenyon NS, Ricordi C, Burkly LC, Inverardi L: Prolonged islet graft survival in NOD mice by blockade of the CD40-CD154 pathway of T-cell costimulation. Diabetes 50: 270–276, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Guo ZG, Wu T, Kirchhof N, Mital D, Williams JW, Azuma M, Sutherland DER, Hering BJ: Immunotherapy with nondepleting anti-CD4 monoclonal antibodies but not CD28 antagonists protects islet graft in spontaneously diabetic NOD mice from autoimmune destruction and allogeneic and xenogeneic graft rejection. Transplantation 71: 1656–1665, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Rossini AA, Greiner DL, Mordes JP: Induction of immunological tolerance for transplantation. Physiol Rev 79: 99–141, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Robles DT, Eisenbarth GS, Dailey NJM, Peterson LB, Wicker LS: Insulin autoantibodies are associated with islet inflammation but not always related to diabetes progression in NOD congenic mice. Diabetes 52: 882–886, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Pearson T, Weiser P, Markees TG, Serreze DV, Wicker LS, Peterson LB, Cumisky AM, Shultz LD, Mordes JP, Rossini AA, Greiner DL: Islet allograft survival induced by costimulation blockade in NOD mice is controlled by allelic variants of Idd3. Diabetes 53: 1972–1978, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Pearson T, Markees TG, Wicker LS, Serreze DV, Peterson LB, Mordes JP, Rossini AA, Greiner DL: NOD congenic mice genetically protected from autoimmune diabetes remain resistant to transplantation tolerance induction. Diabetes 52: 321–326, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Wicker LS, Todd JA, Prins JB, Podolin PL, Renjilian RJ, Peterson LB: Resistance alleles at two non-major histocompatibility complex-linked insulin-dependent diabetes loci on chromosome 3, Idd3 and Idd10, protect nonobese diabetic mice from diabetes. J Exp Med 180: 1705–1713, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamanouchi J, Rainbow D, Serra P, Howlett S, Hunter K, Garner VE, Gonzalez-Munoz A, Clark J, Veijola R, Cubbon R, Chen SL, Rosa R, Cumiskey AM, Serreze DV, Gregory S, Rogers J, Lyons PA, Healy B, Smink LJ, Todd JA, Peterson LB, Wicker LS, Santamaria P: Interleukin-2 gene variation impairs regulatory T cell function and causes autoimmunity. Nat Genet 39: 329–337, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Del Rio R, Noubade R, Subramanian M, Saligrama N, Diehl S, Rincon M, Teuscher C: SNPs upstream of the minimal promoter control IL-2 expression and are candidates for the autoimmune disease-susceptibility locus Aod2/Idd3/Eae3. Genes Immun 9: 115–121, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Setoguchi R, Hori S, Takahashi T, Sakaguchi S: Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med 201: 723–735, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang Q, Adams JY, Penaranda C, Melli K, Piaggio E, Sgouroudis E, Piccirillo CA, Salomon BL, Bluestone JA: Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity 28: 687–697, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Refaeli Y, Van Parijs L, London CA, Tschopp J, Abbas AK: Biochemical mechanisms of IL-2-regulated Fas-mediated T cell apoptosis. Immunity 8: 615–623, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Kramer S, Mamalaki C, Horak I, Schimpl A, Kioussis D, Hung T: Thymic selection and peptide-induced activation of T cell receptor-transgenic CD8 T cells in interleukin-2-deficient mice. Eur J Immunol 24: 2317–2322, 1994 [DOI] [PubMed] [Google Scholar]
- 15.Dai Z, Konieczny BT, Baddoura FK, Lakkis FG: Impaired alloantigen-mediated T cell apoptosis and failure to induce long-term allograft survival in IL-2-deficient mice. J Immunol 161: 1659–1663, 1998 [PubMed] [Google Scholar]
- 16.Shrikant P, Mescher MF: Opposing effects of IL-2 in tumor immunotherapy: promoting CD8 T cell growth and inducing apoptosis. J Immunol 169: 1753–1759, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Yui MA, Muralidharan K, Moreno-Altamirano B, Perrin G, Chestnut K, Wakeland EK: Production of congenic mouse strains carrying NOD-derived diabetogenic genetic intervals: an approach for the genetic dissection of complex traits. Mamm Genome 7: 331–334, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Iwakoshi NN, Markees TG, Turgeon NA, Thornley T, Cuthbert A, Leif JH, Phillips NE, Mordes JP, Greiner DL, Rossini AA: Skin allograft maintenance in a new synchimeric model system of tolerance. J Immunol 167: 6623–6630, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chesnut K, She JX, Cheng I, Muralidharan K, Wakeland EK: Characterizations of candidate genes for IDD susceptibility from the diabetes-prone NOD mouse strain. Mamm Genome 4: 549–554, 1993 [DOI] [PubMed] [Google Scholar]
- 20.Iwakoshi NN, Mordes JP, Markees TG, Phillips NE, Greiner DL, Rossini AA: Treatment of allograft recipients with donor specific transfusion and anti-CD154 antibody leads to deletion of alloreactive CD8+ T cells and prolonged graft survival in a CTLA4-dependent manner. J Immunol 164: 512–521, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Markees TG, Serreze DV, Phillips NE, Sorli CH, Noelle RJ, Woda BA, Greiner DL, Mordes JP, Rossini AA: NOD mice have a generalized defect in their response to transplantation tolerance induction. Diabetes 48: 967–974, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Seung E, Iwakoshi N, Woda BA, Markees TG, Mordes JP, Rossini AA, Greiner DL: Allogeneic hematopoietic chimerism in mice treated with sublethal myeloablation and anti-CD154 antibody: absence of graft-versus-host disease, induction of skin allograft tolerance, and prevention of recurrent autoimmunity in islet-allografted NOD/Lt mice. Blood 95: 2175–2182, 2000 [PubMed] [Google Scholar]
- 23.Brehm MA, Mangada J, Markees TG, Pearson T, Daniels KA, Thornley TB, Welsh RM, Rossini AA, Greiner DL: Rapid quantification of naive alloreactive T cells by TNF-alpha production and correlation with allograft rejection in mice. Blood 109: 819–826, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shultz LD, Banuelos SJ, Leif J, Appel MC, Cunningham M, Ballen K, Burzenski L, Greiner DL: Regulation of human short-term repopulating cell (STRC) engraftment in NOD/SCID mice by host CD122+ cells. Exp Hematol 31: 551–558, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Shultz LD, Schweitzer PA, Christianson SW, Gott B, Schweitzer IB, McKenna S, Mobraaten L, Rajan TV, Greiner DL, Leiter EH: Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J Immunol 154: 180–191, 1995 [PubMed] [Google Scholar]
- 26.Koulmanda M, Qipo A, Auchincloss H Jr, Smith RN: Effects of streptozotocin on autoimmune diabetes in NOD mice. Clin Exp Immunol 134: 210–216, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Podolin PL, Denny P, Lord CJ, Hill NJ, Todd JA, Peterson LB, Wicker LS, Lyons PA: Congenic mapping of the insulin-dependent diabetes (Idd) gene, Idd10, localizes two genes mediating the Idd10 effect and eliminates the candidate Fcgr1. J Immunol 159: 1835–1843, 1997 [PubMed] [Google Scholar]
- 28.Podolin PL, Denny P, Armitage N, Lord CJ, Hill NJ, Levy ER, Peterson LB, Todd JA, Wicker LS, Lyons PA: Localization of two insulin-dependent diabetes (Idd) genes to the Idd10 region on mouse chromosome 3. Mamm Genome 9: 283–286, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Lyons PA, Armitage N, Lord CJ, Phillips MS, Todd JA, Peterson LB, Wicker LS: Mapping by genetic interaction: high-resolution congenic mapping of the type 1 diabetes loci Idd10 and Idd18 in the NOD mouse. Diabetes 50: 2633–2637, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Wicker LS, Chamberlain G, Hunter K, Rainbow D, Howlett S, Tiffen P, Clark J, Gonzalez-Munoz A, Cumiskey AM, Rosa RL, Howson JM, Smink LJ, Kingsnorth A, Lyons PA, Gregory S, Rogers J, Todd JA, Peterson LB: Fine mapping, gene content, comparative sequencing, and expression analyses support Ctla4 and Nramp1 as candidates for Idd5.1 and Idd5.2 in the nonobese diabetic mouse. J Immunol 173: 164–173, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Hunter K, Rainbow D, Plagnol V, Todd JA, Peterson LB, Wicker LS: Interactions between Idd5.1/Ctla4 and other type 1 diabetes genes. J Immunol 179: 8341–8349, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Hill NJ, Lyons PA, Armitage N, Todd JA, Wicker LS, Peterson LB: NOD Idd5 locus controls insulitis and diabetes and overlaps the orthologous CTLA4/IDDM12 and NRAMP1 loci in humans. Diabetes 49: 1744–1747, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Langmuir PB, Rothstein DM, Strom TB, Turka LA, Sayegh MH: Th1 cytokines, programmed cell death, and alloreactive T cell clone size in transplant tolerance. J Clin Invest 109: 1471–1479, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oehen S, Brduscha-Riem K, Oxenius A, Odermatt B: A simple method for evaluating the rejection of grafted spleen cells by flow cytometry and tracing adoptively transferred cells by light microscopy. J Immunol Methods 207: 33–42, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Lyons PA, Wicker LS: Localising quantitative trait loci in the NOD mouse model of type 1 diabetes. Curr Dir Autoimmun 1: 208–225, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Bottini N, Musumeci L, Alonso A, Rahmouni S, Nika K, Rostamkhani M, MacMurray J, Meloni GF, Lucarelli P, Pellecchia M, Eisenbarth GS, Comings D, Mustelin T: A functional variant of lymphoid tyrosine phosphatase is associated with type I diabetes. Nat Genet 36: 337–338, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Vang T, Congia M, Macis MD, Musumeci L, Orru V, Zavattari P, Nika K, Tautz L, Tasken K, Cucca F, Mustelin T, Bottini N: Autoimmune-associated lymphoid tyrosine phosphatase is a gain-of-function variant. Nat Genet 37: 1317–1319, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Ueda H, Howson JM, Esposito L, Heward J, Snook H, Chamberlain G, Rainbow DB, Hunter KM, Smith AN, Di Genova G, Herr MH, Dahlman I, Payne F, Smyth D, Lowe C, Twells RC, Howlett S, Healy B, Nutland S, Rance HE, Everett V, Smink LJ, Lam AC, Cordell HJ, Walker NM, Bordin C, Hulme J, Motzo C, Cucca F, Hess JF, Metzker ML, Rogers J, Gregory S, Allahabadia A, Nithiyananthan R, Tuomilehto-Wolf E, Tuomilehto J, Bingley P, Gillespie KM, Undlien DE, Ronningen KS, Guja C, Ionescu-Tirgoviste C, Savage DA, Maxwell AP, Carson DJ, Patterson CC, Franklyn JA, Clayton DG, Peterson LB, Wicker LS, Todd JA, Gough SC: Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature 423: 506–511, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Markees TG, Phillips NE, Gordon EJ, Noelle RJ, Shultz LD, Mordes JP, Greiner DL, Rossini AA: Long-term survival of skin allografts induced by donor splenocytes and anti-CD154 antibody in thymectomized mice requires CD4+ T cells, interferon-gamma, and CTLA4. J Clin Invest 101: 2446–2455, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kissler S, Stern P, Takahashi K, Hunter K, Peterson LB, Wicker LS: In vivo RNA interference demonstrates a role for Nramp1 in modifying susceptibility to type 1 diabetes. Nat Genet 38: 479–483, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Fortier A, Min-Oo G, Forbes J, Lam-Yuk-Tseung S, Gros P: Single gene effects in mouse models of host: pathogen interactions. J Leukoc Biol 77: 868–877, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Rabinovitch A, Suarez-Pinzon WL, Shapiro AM, Rajotte RV, Power R: Combination therapy with sirolimus and interleukin-2 prevents spontaneous and recurrent autoimmune diabetes in NOD mice. Diabetes 51: 638–645, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Thornton AM, Donovan EE, Piccirillo CA, Shevach EM: Cutting edge: IL-2 is critically required for the in vitro activation of CD4+CD25+ T cell suppressor function. J Immunol 172: 6519–6523, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Bayer AL, Yu A, Adeegbe D, Malek TR: Essential role for interleukin-2 for CD4(+)CD25(+) T regulatory cell development during the neonatal period. J Exp Med 201: 769–777, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.D'Cruz LM, Klein L: Development and function of agonist-induced CD25+Foxp3+ regulatory T cells in the absence of interleukin 2 signaling. Nat Immunol 6: 1152–1159, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY: A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol 6: 1142–1151, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Todd JA, Walker NM, Cooper JD, Smyth DJ, Downes K, Plagnol V, Bailey R, Nejentsev S, Field SF, Payne F, Lowe CE, Szeszko JS, Hafler JP, Zeitels L, Yang JH, Vella A, Nutland S, Stevens HE, Schuilenburg H, Coleman G, Maisuria M, Meadows W, Smink LJ, Healy B, Burren OS, Lam AA, Ovington NR, Allen J, Adlem E, Leung HT, Wallace C, Howson JM, Guja C, Ionescu-Tirgoviste C, Simmonds MJ, Heward JM, Gough SC, Dunger DB, Wicker LS, Clayton DG: Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nat Genet 39: 857–864, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Penha-Goncalves C, Moule C, Smink LJ, Howson J, Gregory S, Rogers J, Lyons PA, Suttie JJ, Lord CJ, Peterson LB, Todd JA, Wicker LS: Identification of a structurally distinct CD101 molecule encoded in the 950-kb Idd10 region of NOD mice. Diabetes 52: 1551–1556, 2003 [DOI] [PubMed] [Google Scholar]