Abstract
The ruslan (rus) mutant was previously identified in a behavioral screen for mutants defective in long-lasting memory, which consists of two consolidated memory types, anesthesia-resistant memory, and protein synthesis-dependent long-term memory (LTM). We demonstrate here that rus is a new allele of klingon (klg), which encodes a homophilic cell adhesion molecule. Klg is acutely required for LTM but not anesthesia-resistant memory formation, and Klg expression increases upon LTM induction. LTM formation also requires activity of the Notch cell-surface receptor. Although defects in Notch have been implicated in memory loss because of Alzheimer's disease, downstream signaling linking Notch to memory have not been determined. Strikingly, we found that Notch activity increases upon LTM induction and regulates Klg expression. Furthermore, Notch-induced enhancement of LTM is disrupted by a klg mutation. We propose that Klg is a downstream effector of Notch signaling that links Notch activity to memory.
Keywords: memory consolidation, ruslan, Alzheimer's disease
The Notch signaling pathway, which plays critical roles in cell fate specification and differentiation (1), is also important for protein synthesis-dependent long-term memory (LTM) formation (2–4). Upon ligand binding, the Notch receptor is proteolytically cleaved by presenilin-dependent γ-secretase (5, 6) to release a Notch intracellular domain (NICD), which activates gene expression in the nucleus. Over-expression of a dominant-negative Notch, which is defective for NICD function, inhibits LTM formation (3).
Presenilin-dependent γ-secretase also cleaves the amyloid precursor protein (APP), generating ß-amyloid peptide (Aß), and a transcriptionally active APP intracellular domain (AID) (7). Notably, mutations in presenilin, which cause early-onset familial Alzheimer's disease (AD) and selectively enhance production of the pathogenic 42-residue Aß peptide (Aß42), also impair NICD production (8). Furthermore, AID represses transcriptional activity of the NICD by binding to cytosolic inhibitors of Notch, Numb, and Numb-like (9). Although these observations suggest that a contributing factor to neuronal dysfunction and memory loss in some AD patients may be a decline in Notch signaling, downstream effectors linking Notch signaling to memory formation have not been identified.
Drosophila have 2 types of long-lasting consolidated memory, LTM and anesthesia-resistant memory (ARM). LTM of an aversive olfactory association is produced after spaced training, 10 training sessions with 15 min rest intervals between each training session, but not after massed training, 10 training sessions given successively with no rests between training sessions. ARM is produced after both spaced and massed training. LTM formation requires activity of the cyclic AMP-response element-binding transcription factor and depends on new protein synthesis. In contrast, ARM does not require cyclic AMP-response element-binding activity and can be produced under conditions where cellular protein synthesis is inhibited by 50% (10).
Previously, a large-scale behavioral screen identified 60 mutants with defective 1-day memory after spaced training (11). The ruslan (rus) mutation was identified in this screen and was proposed to be a mutation in the klingon (klg) gene, which encodes a member of the Ig superfamily of cell adhesion molecules (CAMs). CAMs play an important role in structural and functional synaptic plasticity, as well as in learning and memory. However, few CAMs have been implicated in consolidation to long-lasting memory (12, 13).
Although Klg can mediate homophilic adhesion and participates in the development of the R7 photoreceptor neuron (14), its function in the adult stage has not been defined. In the present study, we demonstrate that rus is an allele of klg and Klg is a CAM required for memory consolidation to LTM but not ARM. Furthermore, we provide evidence indicating that Klg is a downstream effector linking Notch activity to memory.
Results
ruslan Is a Klingon Mutant and Disrupts Consolidation to LTM.
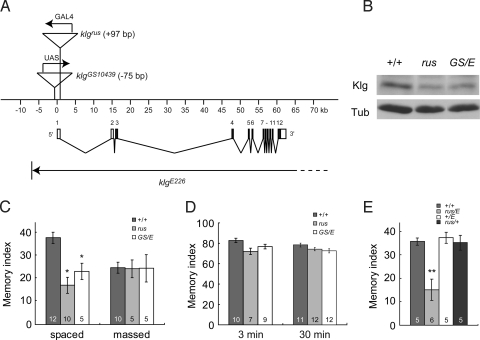
ruslan consists of an insertion of a P-GAL4 transposon into the first exon of the klingon (klg) gene (Fig. 1A), suggesting that ruslan is a new allele of klg [(11), J. Dubnau personal communication, T.T. unpublished data]. Therefore, we examined Klg expression in rus mutants and determined that Klg expression is reduced to ≈50% of WT in rus mutants (Fig. 1B). We next examined 1-day memory in a klg mutant. While homozygotes of 2 klg mutations, klgGS10439 [(15), http://gsdb.biol.metro-u.ac.jp/∼dclust/index.html] and klgE226 (14), are larval lethal, the klgGS10439/klgE226 heteroallelic mutant is viable and expresses similar amounts of Klg as rus mutants (see Fig. 1B). We observed a disruption of 1-day memory after spaced training in klgGS10439/klgE226 flies similar to rus flies (Fig. 1C). In contrast, we observed normal 1-day memory after massed training in both rus and klgGS10439/klgE226 flies (see Fig. 1C). Both mutant flies showed normal learning (3-min memory after single-cycle training), normal short-term memory (30-min memory after single cycle training) (Fig. 1D) and normal sensorimotor responses to odors and foot shocks used for training [supporting information (SI) Fig. S1]. These results indicate that rus and klg mutants are specifically defective for LTM and this memory defect is not because of impaired learning or sensorimotor responses.
Fig. 1.
ruslan (rus) is a new allele of klingon (klg). (A) A genomic map of the klg locus adapted from Butler et al. (14). The klgrus mutation results from an insertion of a P-GAL4 transposon in the first exon (+97 bp), oriented in the opposite direction to klg transcription [(11), J. Dubnau personal communication, T.T. unpublished data]. The klgGS10439 mutation results from an insertion of a P-UAS transposon 75 bp upstream of the proposed transcription start site [(15) http://gsdb.biol.metro-u.ac.jp/∼dclust/index.html]. The klg ORF is entirely deleted in the null klgE226 mutation, although the 3′ end of the deletion has not been determined (14). (B) Western blotting shows reduced expression of Klg protein (60 kDa) in adult heads of klgrus (rus) and klgGS10439/klgE226 flies (GS/E). α-tubulin (Tub) was used as an internal control. (C) Comparison of long-lasting memory produced by spaced or massed training. The klg mutations significantly reduce 1-day memory after spaced training. (*, P < 0.05 vs. WT by t test). (D) Olfactory learning and short-term memory (memory retention quantified 3 min and 30 min after a single training session, respectively) were not affected in klg mutants. (E) While 1-day memory after spaced training is normal in +/klgE226 (+/E) and rus/+ flies, memory defects in rus are not complemented in rus/klgE226 flies (**, P < 0.005 vs. WT by t test).
Both rus and klgE226 mutations are recessive for memory defects. However, rus/klgE226 heterozygous flies are defective for 1-day memory after spaced training (Fig. 1E), demonstrating that rus and klg are unable to complement each other. From these data, we conclude that rus is a new allele of klg (klgrus) and suggest that Klg is an essential CAM involved in LTM formation. Although the klgGS10439 mutation is also recessive for impaired memory, a klgrus/klgGS10439 line does not have LTM defects (Fig. S2). This is likely because the klgrus mutation results from insertion of a P-GAL4 transposon, while the klgGS10439 mutation results from insertion of a P-UAS transposon (see Fig. 1A). GAL4-dependent induction of klg expression rescues the LTM defects in klgrus/klgGS10439 flies.
Klg Protein Increases After LTM Induction and Adult klg Function Is Required for LTM Formation.
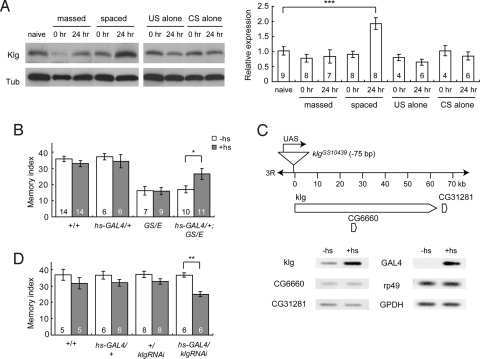
Because LTM formation requires new protein synthesis, we investigated whether Klg expression increases upon induction of LTM in WT flies. The amount of Klg protein in fly heads did not change immediately after spaced training but increased to a statistically significant level within 24 h (Fig. 2A and Fig. S3). Importantly, the increase in Klg was specific to LTM, because protein amounts did not change after massed training or after exposure to spaced training controls, including US (shock) or CS (odors) alone (see Fig. 2A). In contrast to protein amounts, we did not observed an increase in klg mRNA after spaced training (Fig. S4A). These results suggest that the increase in Klg upon LTM induction occurs at a posttranscriptional step.
Fig. 2.
Klg increases upon LTM induction and LTM depends on Klg expression. (A) Klg protein amounts, measured from fly head extracts, increase significantly 24 h after spaced training (***, P < 0.001), but not after massed training or after spaced application of the unconditioned stimulus (US) or conditioned stimuli (CS) alone. The ratio of Klg to α-tubulin (Tub) protein in naive flies was defined as 1.0. (B) A 37 °C heat-shock (+hs) for 15 min significantly improved 1-day memory after spaced training in hs-GAL4/+;klgGS10439/klgE226 (hs-GAL4/+;GS/E) flies (*, P < 0.04), indicating that the klg memory defect can be complemented by conditional expression of klg+. (C) Comparison of the expression of P-UAS downstream gene transcripts in hs-GAL4/+;klgGS10439/+ flies 3 h after the heat-shock (+hs). While heat-shock enhanced the expression of klg and GAL4 transcripts, it did not alter the expression of two other downstream genes, CG6660 and CG31281. RNA was isolated from Drosophila whole bodies and transcripts were quantified by semiquantitative RT-PCR. (D) RNAi-mediated silencing of klg disrupts LTM. A 37C°C heat-shock (+hs) for 30 min significantly disrupted 1-day memory after spaced training in hs-GAL4/UAS-klgRNAi (hs-GAL4/klgRNAi) flies (**, P < 0.005).
To address whether Klg is physiologically required for LTM formation, we next conditionally expressed a klg transgene (klg+) in the adult klg mutant (Fig. 2B). The klgGS10439 mutation results from a P-UAS transposon insertion (15) 75 bp upstream of the klg transcription start site, oriented in the same direction as klg transcription (see Fig. 1A and 2C). Consequently, klg can be inducibly expressed in adult mutants by crossing this line to one containing a GAL4 driver under heat-shock promoter (hs-GAL4) control (16).
When hs-GAL4/+;klgGS10439/klgE226 (hs-GAL4/+;GS/E) flies were transferred from 18 °C to 37 °C for 15 min, 3 h before training, 1-day memory after spaced training improved significantly compared to nonheat-shocked controls (see Fig. 2B). Although the P-UAS insertion in klgGS10439 flies is also upstream of the CG6660 and CG31281 genes, heat-shock increased expression of klg but not these other neighboring genes in hs-GAL4/+;GS/+ flies (see Fig. 2C), indicating that acutely induced Klg complements klg memory defects.
To further verify the physiological role of klg in LTM, we acutely inhibited klg expression using a heat-shock inducible klg RNAi construct in hs-GAL4/UAS-klgRNAi flies. We observed that a 37 °C heat-shock for 30 min, 5 h before training caused a significant decrease in 1-day memory after spaced training in these flies, compared to nonheat-shocked controls (Fig. 2D). In contrast, the same heat-shock regimen did not disrupt short-term memory in hs-GAL4/UAS-klgRNAi flies (Fig. S5). The above results taken together suggest that klg has an acute physiological role for LTM.
Klg Expression Is Regulated by Notch.
Similar to Klg, Notch is required for LTM formation (2–4) and development of the R7 photoreceptor neuron (17). These phenotypes led us to study possible interactions between Klg and Notch. To test whether Klg expression is regulated by Notch signaling, we measured Klg protein in transgenic flies expressing a dominant negative Notch transgene (hs-NΔcdc10rpts) or a WT Notch transgene (hs-N+) under heat-shock promoter control (3).
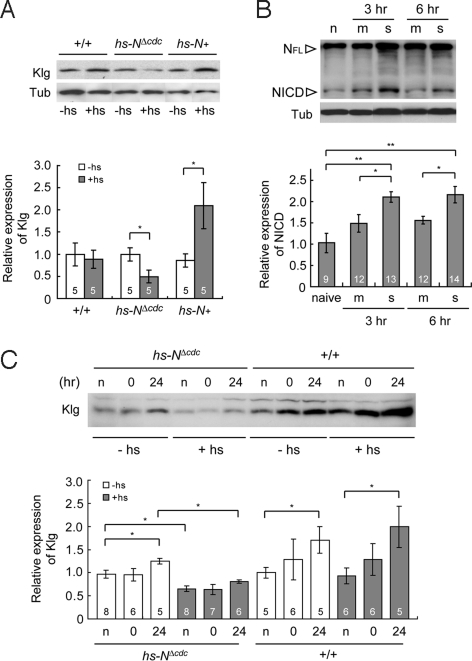
A previous study has demonstrated that a 37 °C heat-shock for 30 min disrupts LTM formation in transgenic hs-NΔcdc10rpts flies, while the same heat-shock enhances LTM formation in transgenic hs-N+ flies (3). Accordingly, we investigated Klg levels in transgenic hs-NΔcdc10rpts flies and hs-N+ flies 5 h after a 37 °C heat-shock for 30 min. As seen in Fig. 3A, Klg levels in hs-NΔcdc10rpts flies were significantly reduced after heat-shock compared to nonheat-shocked controls. Conversely, Klg expression was significantly increased in hs-N+ flies after the same heat-shock treatment compared to nonheat-shocked controls. In contrast to Klg protein, expression of klg transcripts was not dependent on Notch activity (Fig. S4B). Our results suggest that Notch regulates Klg protein expression at a posttranscriptional step, such as at protein synthesis and turnover.
Fig. 3.
Klg is regulated by Notch and LTM-dependent increases in Klg require Notch activity. (A) Heat-shock (+hs, 37 °C for 30 min) induction of a dominant-negative NΔcdc10rpts transgene significantly decreases Klg protein amounts in head extracts from hs-NΔcdc10rpts (hs-NΔcdc) flies. Conversely, Klg amounts are up-regulated by induction of a WT Notch transgene in hs-N+ flies. Flies were heat-shocked 5 h before fly head collection. The Klg/Tub ratio in nonheat-shocked WT flies was defined as 1.0. *P < 0.05. (B) Generation of the NICD upon LTM induction. The amounts of NICD in head extracts of naive flies (n) and flies harvested 3 and 6 h after massed (m) or spaced (s) training were measured. The amounts of NICD (120 kDa) and full length of Notch (NFL, 350 kDa) were determined using an anti-NICD monoclonal antibody. NICD levels increased significantly in spaced-trained flies compared to naive and massed-trained flies. The NICD/Tub ratio in naive WT flies was defined as 1.0. *, P < 0.03 and **, P < 0.005. (C) Induction of the dominant negative NΔcdc10rpts transgene in hs-NΔcdc flies suppresses the LTM-dependent increase in Klg protein in fly heads. The NΔcdc10rpts transgene was induced by heat-shocking flies for 30 min at 37 °C, 5 h before training (+hs). Heat-shocked naive flies were heat-shocked 5 h before head collection. Relative Klg amounts in naive flies and in spaced-trained flies at 0 and 24 h after training are indicated. *, P < 0.05.
The NΔcdc10rpts protein binds ligands normally but lacks NICD activity and is defective for signaling downstream from Notch (3). This led us to hypothesize that generation of the NICD may be important for LTM formation and we measured NICD protein levels after spaced training. We observed a significant increase in NICD amounts in the heads of spaced-trained flies 3 and 6 h after training, as compared to nontrained flies (Fig. 3B). We also observed a slight increase in NICD amounts in heads from mass trained flies, but this increase was not significantly different from untrained control flies and was significantly lower than in spaced-trained flies.
Because Klg protein levels are regulated by Notch, we next hypothesized that the increase in Klg protein after induction of LTM may also depend on Notch signaling activity. Thus, we investigated the amount of Klg in hs-NΔcdc10rpts flies after spaced training (Fig. 3C). In the absence of heat shock, hs-NΔcdc10rpts flies displayed a significant increase in Klg protein 24 h after spaced training, similar to WT flies. However, this increase was abolished when a 37 °C heat-shock was given for 30 min 5 h before training, suggesting that the LTM-associated increase in Klg requires NICD activity.
Klg Is Required for Notch-Dependent LTM Formation.
We next asked whether klg is required for Notch's effects on LTM formation. Acute over-expression of Notch in transgenic hs-N+ flies, induced by a 37 °C heat-shock for 30 min, has previously been shown to enhance 1-day memory after a single-cycle training session (3). Although aversive olfactory memory formed by single-cycle training is normally consolidated into ARM and not LTM (10), the enhanced 1-day memory in hs-N+ flies is blocked by the protein synthesis inhibitor, cycloheximide, indicating that Notch over-expression specifically enhances LTM (3). We therefore examined the effect of a klg mutation on the enhancement of LTM formation by Notch.
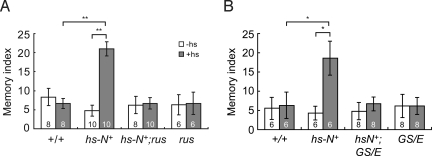
Significantly, memory enhancement upon Notch over-expression was prevented in klg mutants. While heat-shocked hs-N+ flies showed a greater than 4-fold increase in 1-day memory after single-cycle training, neither hs-N+;klgrus nor hs-N+;klgGS10439/klgE226 flies showed this increase (Fig. 4), demonstrating that regulation of Klg by Notch is an essential step for Notch-dependent LTM formation.
Fig. 4.
Enhancement of LTM by Notch over-expression is prevented in klg mutants. A 37 °C heat-shock for 30 min increases 1-day memory after single cycle training in hs-N+ flies. However, the same heat-shock does not increase 1-day memory in hs-N+;klgrus (hs-N+;rus) (A) and hs-N+;klgGS10439/KlgE226 (hs-N+;GS/E) (B) flies. Flies were heat-shocked 5 h before training. *, P < 0.03 and **, P < 0.005.
Discussion
In the present study, we demonstrate that the memory mutant, rus, is a previously uncharacterized allele of klg. klg was originally identified as a gene which encodes a homophilic CAM involved in photoreceptor development (14). We determined that, besides this developmental role, Klg is a CAM acutely required for LTM, using 2 independent genetic interventions: induced over-expression of klg+ in klg mutants (see Fig. 2B) and acute silencing of klg in WT flies (see Fig. 2D).
Functional roles of CAMs in learning and memory have been previously studied. For example, Drosophila Fas II, a homologue of vertebrate neural CAM, is a homophilic CAM that regulates synaptic stabilization and growth in an activity-dependent manner (18). Fas II function is also required for normal odor learning in the adult stage (19). Drosophila α-integrin, a molecule that mediates cell adhesion and signal transduction, is required for early-phase memory (20) and also regulates structural and functional synaptic plasticity (21). Likewise, neural CAM and integrin-mutant mice display impaired learning and memory as well as reduced long-term potentiation (22, 23). Besides roles in learning and early-phase memory, CAMs have also been implicated in memory consolidation. N-cadherin is synthesized upon induction of late-phase long-term potentiation, a putative cellular basis for LTM, and recruited to newly formed synapses (12). Functional modification of neural CAM by polysialic acid is proposed to be important for synaptic remodeling and memory consolidation (13).
Our results demonstrate that Klg is another CAM critical for memory consolidation. Interestingly, immunohistochemical experiments demonstrate extensive localization of Klg protein at the junctures between the neuropil and neuropil glia, including the junctures between the lobes and calyces of the mushroom bodies and the surrounding glial cells (data not shown). The α-lobes of the mushroom bodies have been implicated in LTM formation (24) and inhibition of Notch activity in the mushroom bodies impairs LTM formation (4). These results suggest that Klg may be involved in acute neuron-glia interactions required for LTM formation.
The importance of Notch signaling in memory formation has been demonstrated both in Drosophila and mice (2–4). Increasing Notch activity facilitates LTM formation, while reducing activity severely impairs LTM (3, 4). Because presenilin-dependent γ-secretase activity generates the transcriptionally active NICD, and familial AD-associated mutations in presenilin disrupt this step, a decline in Notch signaling activity has been proposed to be a causal factor in memory impairment in AD (25). In the present study, we provide evidence indicating that Klg is a downstream effector molecule linking Notch activity to memory formation. Furthermore, we demonstrate an increase in NICD amounts upon induction of LTM (spaced training). Although we also observed a slight increase in NICD amounts after massed training, this increase is significantly lower than after spaced training and does not cause an increase in Klg protein. These results are consistent with the observation that massed training is insufficient to generate LTM. Because the NICD is generated by a presenilin-dependent cleavage, our results further suggest that presenilin-dependent γ-secretase activity may be required for LTM formation. In support of this idea, a conditional double knockout of presenilins (PS1 and PS2) in the mouse forebrain results in severe impairment of spatial and contextual memory and impaired synaptic plasticity (26).
Although transcriptional activity of Notch regulates Klg protein levels, Notch is unlikely to directly regulate transcription of the klg gene, because the increase in Klg protein upon LTM induction is not accompanied by an increase in klg transcripts. These results suggest that in response to induction of LTM, Notch signaling is activated to regulate either synthesis or turnover of the Klg protein. Supporting this idea, we observed a delay in the increase of Klg protein compared to the increase of the NICD. While Klg protein gradually increases over 24 h after spaced training, the increase in the NICD reaches a plateau within 6 h after training. In addition to transcriptional regulation, recent studies have demonstrated the importance of strict regulation of protein synthesis and degradation in LTM formation (27–29). We suspect that the NICD activates transcription of a second factor, which in turn stabilizes or increases Klg protein amounts.
Materials and Methods
Fly Stocks.
Flies were raised under a 12 h:12 h, light:dark cycle at 25 °C and 60% humidity. w(CS10), derived from outcrossing w1118 to Canton-S for 10 generations, was used as the WT control as previously described (30). klgGS10439 and null klgE226 lines were gifts from T. Aigaki (Tokyo Metropolitan University, Japan) and Y. Hiromi (National Institute of Genetics, Japan), respectively; w;hs-GAL4/CyO (31) was a gift from Y. Hiromi and transgenic hs-N+ and hs-NΔcdc10 lines were gifts from Y. Zhong (Cold Spring Harbor Laboratory). The UAS-klgRNAi line (klg36162) was obtained from the Vienna Drosophila RNAi Center. Other lines used in this study were obtained from the Bloomington Stock Center of Indiana University. For all experiments, 3- to 7-day-old flies were used. All fly lines, except klgE226, were out-crossed to w(CS10) flies for at least 6 generations.
Fly Behavior.
All memory tests were performed in an environmental room maintained at 25 °C and 60% humidity.
Single-Cycle Training.
Standard single-cycle olfactory conditioning was performed as previously described (30, 32). Two aversive odors (OCT and MCH) were used for CS, and 1.5-s pulses of 60V DC electric shocks were used as the US. To test for memory retention, about 100 trained flies were tapped into the choice point of a T-maze, where they were exposed simultaneously to the CS+ (the odor that was paired to the US during training) and the CS− (unpaired with the US). A performance index was calculated so that a 50:50 distribution (no memory) yielded a performance index of zero and a 0:100 distribution away from the CS+ yielded a performance index of 100 (10).
Spaced and Massed Training.
Spaced and massed training sessions were performed as described previously (10). Spaced training consists of 10 single-cycle training sessions, with a 15-min rest interval between successive training cycles. Massed training consists of 10 cycles of training, where one session immediately follows the previous one. Memory was measured 1 day after spaced or massed training to evaluate LTM and ARM.
Sensorimotor Responses.
Peripheral control experiments, including odor acuity and shock reactivity assays, were performed as described previously (30, 32) to verify that sensitivity to the odors and shock were unaffected in our mutants. About 100 naive flies were tapped into the choice point of a T-maze in which they had to choose between an odor (OCT or MCH) and mineral oil (for olfactory acuity), or between a tube where they were electrically shocked and a tube where they were not shocked (shock reactivity). A performance index was calculated as previously described (10).
Western Blotting.
Rabbit anti-Klg polyclonal antibodies were generated against a 26-mer peptide sequence, CKGSGNPVPSIYWTKKSGANKSTARI, from the second IgG domain of the Klg protein in New Zealand White rabbits. Affinity-purified serum obtained from peptide cross-linked to a Hi-Trap affinity column (Amersham) recognized the same size Klg band as described previously (14). An affinity purified rabbit anti-Klg antibody (1:25) and a monoclonal antibody against NICD (1:100, C17.9C6 from Hybridoma bank) were used for quantification of Klg and NICD, respectively. A 1:1,000 dilution of mouse anti-α-tubulin antibody (#DM1a, Seikagaku Kogyo) was used for normalization. Head extracts were made in homogenization buffer [25 mM Hepes, 100 mM NaCl, 1 mM MgCl2, 1 mM CaCl2, 0.2% Trion X-100, 0.2% Nonidet P-40 and protease inhibitors (Roche)]. Immunoreactive bands were visualized by using ECL detection reagents (GE Healthcare), and protein levels were quantified using ImageJ (National Institutes of Health, http://rsbweb.nih.gov/ij/).
Quantification of Transcripts by RT-PCR.
Total RNA from Drosophila heads or whole bodies was extracted with TRIzol reagent (Invitrogen) and cDNA was synthesized using a High Capacity cDNA Archive Kit (Applied Biosystems) as described previously (33). For semiquantitative PCR, cDNAs were amplified for 25 cycles and for qPCR, an Applied Biosystems model 7500 machine was used. GPDH1 and rp49 transcripts were used for normalization. Primer sequences are shown in Table S1.
Heat-Shock Treatment.
Collected flies were maintained in an 18 °C incubator for at least 1 day before heat shock treatment to minimize leaky expression. For heat-shock, flies were transferred to preheated vials and submerged in a water bath maintained at 37 °C (for 15 min or 30 min). Heat-shocked flies were returned to food vials during the recovery period (3 h or 5 h).
Statistical Analyses.
All data are expressed as mean ± SEM. Sample sizes are indicated in each bar in all graphs. Graph Pad Prism version 4.01 was used for statistical analyses. Unless noted otherwise, data were analyzed by ANOVA followed by Bonferroni/Dunn posthoc comparisons.
Supplementary Material
Acknowledgments.
We thank Drs. Y. Hiromi, T. Aigaki, and Y. Zhong for fly stocks. We also thank H. Ueda for assistance with histology and Dr. T. Miyashita for help with statistical analyses and antibody generation. This work was funded by Grant-in-Aid for Scientific Research (B) 14380374 and a grant from The Uehara Memorial Foundation (to M.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0807665106/DCSupplemental.
References
- 1.Greenwald I. LIN-12/Notch signaling: lessons from worms and flies. Genes Dev. 1998;12:1751–1762. doi: 10.1101/gad.12.12.1751. [DOI] [PubMed] [Google Scholar]
- 2.Costa RM, Honjo T, Silva AJ. Learning and memory deficits in Notch mutant mice. Curr Biol. 2003;13:1348–1354. doi: 10.1016/s0960-9822(03)00492-5. [DOI] [PubMed] [Google Scholar]
- 3.Ge X, et al. Notch signaling in Drosophila long-term memory formation. Proc Natl Acad Sci USA. 2004;101:10172–10176. doi: 10.1073/pnas.0403497101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Presente A, Boyles RS, Serway CN, de Belle JS, Andres AJ. Notch is required for long-term memory in Drosophila. Proc Natl Acad Sci USA. 2004;101:1764–1768. doi: 10.1073/pnas.0308259100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ye Y, Lukinova N, Fortini ME. Neurogenic phenotypes and altered Notch processing in Drosophila presenilin mutants. Nature. 1999;398:525–529. doi: 10.1038/19096. [DOI] [PubMed] [Google Scholar]
- 6.Struhl G, Greenwald I. Presenilin is required for activity and nuclear access of Notch in Drosophila. Nature. 1999;398:522–525. doi: 10.1038/19091. [DOI] [PubMed] [Google Scholar]
- 7.Cao X, Sudhof TC. A transcriptionally [correction of transcriptively] active complex of APP with Fe65 and histone acetyltransferase Tip60. Science. 2001;293:115–120. doi: 10.1126/science.1058783. [DOI] [PubMed] [Google Scholar]
- 8.Moehlmann T, et al. Presenilin-1 mutations of leucine 166 equally affect the generation of the Notch and APP intracellular domains independent of their effect on Abeta 42 production. Proc Natl Acad Sci USA. 2002;99:8025–8030. doi: 10.1073/pnas.112686799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roncarati R, et al. The gamma-secretase-generated intracellular domain of beta-amyloid precursor protein binds Numb and inhibits Notch signaling. Proc Natl Acad Sci USA. 2002;99:7102–7107. doi: 10.1073/pnas.102192599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tully T, Preat T, Boynton SC, Del Vecchio M. Genetic dissection of consolidated memory in Drosophila. Cell. 1994;79:35–47. doi: 10.1016/0092-8674(94)90398-0. [DOI] [PubMed] [Google Scholar]
- 11.Dubnau J, et al. The staufen/pumilio pathway is involved in Drosophila long-term memory. Curr Biol. 2003;13:286–296. doi: 10.1016/s0960-9822(03)00064-2. [DOI] [PubMed] [Google Scholar]
- 12.Bozdagi O, Shan W, Tanaka H, Benson DL, Huntley GW. Increasing numbers of synaptic puncta during late-phase LTP: N-cadherin is synthesized, recruited to synaptic sites, and required for potentiation. Neuron. 2000;28:245–259. doi: 10.1016/s0896-6273(00)00100-8. [DOI] [PubMed] [Google Scholar]
- 13.Senkov O, et al. Polysialylated neural cell adhesion molecule is involved in induction of long-term potentiation and memory acquisition and consolidation in a fear-conditioning paradigm. J Neurosci. 2006;26:10888–10898. doi: 10.1523/JNEUROSCI.0878-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butler SJ, Ray S, Hiromi Y. Klingon, a novel member of the Drosophila immunoglobulin superfamily, is required for the development of the R7 photoreceptor neuron. Development. 1997;124:781–792. doi: 10.1242/dev.124.4.781. [DOI] [PubMed] [Google Scholar]
- 15.Aigaki T, Ohsako T, Toba G, Seong K, Matsuo T. The gene search system: its application to functional genomics in Drosophila melanogaster. J Neurogenet. 2001;15:169–178. doi: 10.3109/01677060109167374. [DOI] [PubMed] [Google Scholar]
- 16.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 17.Cooper MT, Bray SJ. R7 photoreceptor specification requires Notch activity. Curr Biol. 2000;10:1507–1510. doi: 10.1016/s0960-9822(00)00826-5. [DOI] [PubMed] [Google Scholar]
- 18.Schuster CM, Davis GW, Fetter RD, Goodman CS. Genetic dissection of structural and functional components of synaptic plasticity. II. Fasciclin II controls presynaptic structural plasticity. Neuron. 1996;17:655–667. doi: 10.1016/s0896-6273(00)80198-1. [DOI] [PubMed] [Google Scholar]
- 19.Cheng Y, et al. Drosophila fasciclinII is required for the formation of odor memories and for normal sensitivity to alcohol. Cell. 2001;105:757–768. doi: 10.1016/s0092-8674(01)00386-5. [DOI] [PubMed] [Google Scholar]
- 20.Grotewiel MS, Beck CD, Wu KH, Zhu XR, Davis RL. Integrin-mediated short-term memory in Drosophila. Nature. 1998;391:455–460. doi: 10.1038/35079. [DOI] [PubMed] [Google Scholar]
- 21.Rohrbough J, Grotewiel MS, Davis RL, Broadie K. Integrin-mediated regulation of synaptic morphology, transmission, and plasticity. J Neurosci. 2000;20:6868–6878. doi: 10.1523/JNEUROSCI.20-18-06868.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang HP, Lindberg FP, Wang HL, Huang AM, Lee EH. Impaired memory retention and decreased long-term potentiation in integrin-associated protein-deficient mice. Learn Mem. 1999;6:448–457. doi: 10.1101/lm.6.5.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cremer H, et al. Long-term but not short-term plasticity at mossy fiber synapses is impaired in neural cell adhesion molecule-deficient mice. Proc Natl Acad Sci USA. 1998;95:13242–13247. doi: 10.1073/pnas.95.22.13242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pascual A, Preat T. Localization of long-term memory within the Drosophila mushroom body. Science. 2001;294:1115–1117. doi: 10.1126/science.1064200. [DOI] [PubMed] [Google Scholar]
- 25.Costa RM, Drew C, Silva AJ. Notch to remember. Trends Neurosci. 2005;28:429–435. doi: 10.1016/j.tins.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Saura CA, et al. Loss of presenilin function causes impairments of memory and synaptic plasticity followed by age-dependent neurodegeneration. Neuron. 2004;42:23–36. doi: 10.1016/s0896-6273(04)00182-5. [DOI] [PubMed] [Google Scholar]
- 27.Ashraf SI, McLoon AL, Sclarsic SM, Kunes S. Synaptic protein synthesis associated with memory is regulated by the RISC pathway in Drosophila. Cell. 2006;124:191–205. doi: 10.1016/j.cell.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 28.Costa-Mattioli M, et al. eIF2alpha phosphorylation bidirectionally regulates the switch from short- to long-term synaptic plasticity and memory. Cell. 2007;129:195–206. doi: 10.1016/j.cell.2007.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimizu K, Phan T, Mansuy IM, Storm DR. Proteolytic degradation of SCOP in the hippocampus contributes to activation of MAP kinase and memory. Cell. 2007;128:1219–1229. doi: 10.1016/j.cell.2006.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamura T, et al. Aging specifically impairs amnesiac-dependent memory in Drosophila. Neuron. 2003;40:1003–1011. doi: 10.1016/s0896-6273(03)00732-3. [DOI] [PubMed] [Google Scholar]
- 31.Brand M, Jarman AP, Jan LY, Jan YN. Asense is a Drosophila neural precursor gene and is capable of initiating sense organ formation. Development. 1993;119(1):1–17. doi: 10.1242/dev.119.Supplement.1. [DOI] [PubMed] [Google Scholar]
- 32.Tully T, Quinn WG. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol [A] 1985;157:263–277. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- 33.Yamazaki D, et al. The Drosophila DCO mutation suppresses age-related memory impairment without affecting lifespan. Nat Neurosci. 2007;10:478–484. doi: 10.1038/nn1863. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.