Abstract
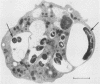
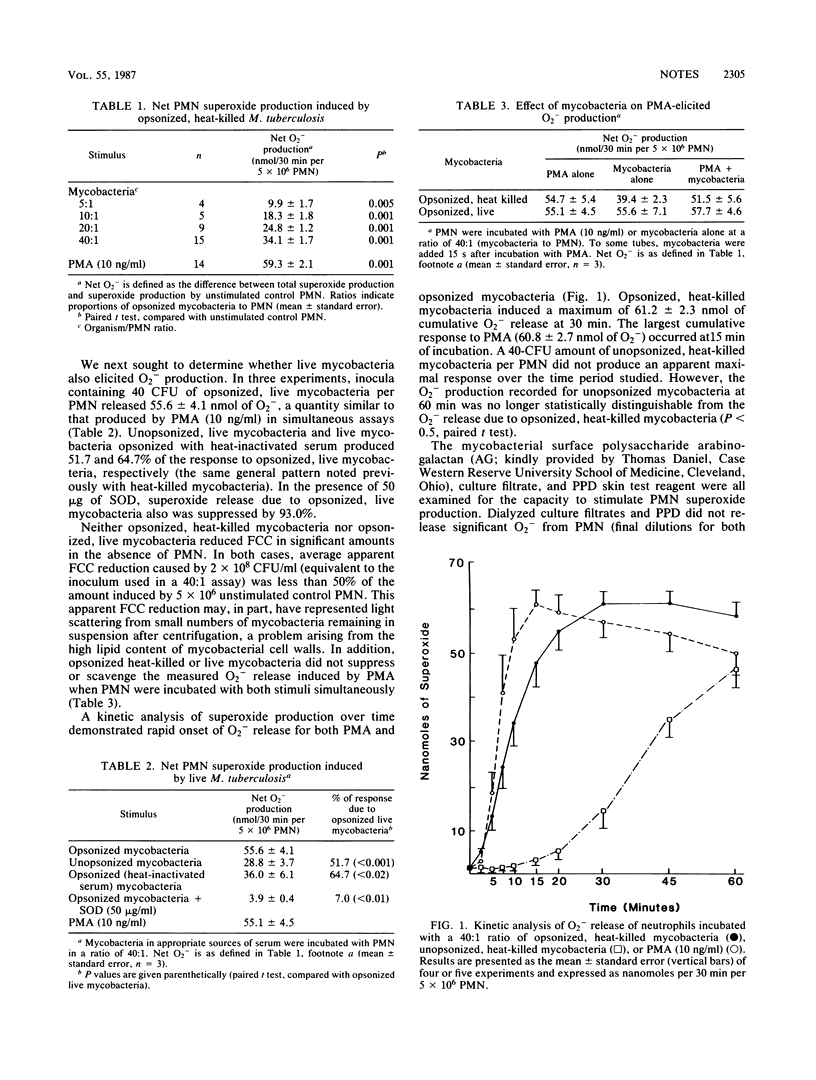
We examined the capacity of human neutrophils to develop a respiratory burst, as monitored by superoxide release, in response to interaction with Mycobacterium tuberculosis. Serum-opsonized, heat-killed mycobacteria induced significant release of superoxide from neutrophils after 30 min of exposure, with a maximum release of 34 +/- 1.7 nmol/30 min per 5 X 10(6) neutrophils occurring with a mycobacterium/neutrophil ratio of 40:1. Similar levels of superoxide release were induced by live mycobacteria. Neutrophil superoxide production was reduced significantly with exposure to unopsonized organisms or by substitution of heat-inactivated serum for opsonization. Mycobacterial components including culture filtrate, purified protein derivative, and the cell wall polysaccharide arabinogalactan failed to induce significant release of superoxide from neutrophils. Transmission electron microscopy demonstrated that more than 90% of the neutrophils had ingested heat-killed mycobacteria concomitant with the development of respiratory burst activity. These data suggest that the presumed failure of neutrophil killing of mycobacteria cannot be attributed to a lack of phagocytosis or respiratory burst activation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antony V. B., Sahn S. A., Antony A. C., Repine J. E. Bacillus Calmette-Guérin-stimulated neutrophils release chemotaxins for monocytes in rabbit pleural spaces and in vitro. J Clin Invest. 1985 Oct;76(4):1514–1521. doi: 10.1172/JCI112131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J. A., Hart P. D. Phagosome-lysosome interactions in cultured macrophages infected with virulent tubercle bacilli. Reversal of the usual nonfusion pattern and observations on bacterial survival. J Exp Med. 1975 Jul 1;142(1):1–16. doi: 10.1084/jem.142.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J. A., Hart P. D. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med. 1971 Sep 1;134(3 Pt 1):713–740. doi: 10.1084/jem.134.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chick T. W., Goldblum S. E., Smith N. D., Butler C., Skipper B. J., Winkelstein J. A., Cork L. C., Reed W. P. Pneumococcal-induced pulmonary leukostasis and hemodynamic changes: role of complement and granulocytes. J Lab Clin Med. 1984 Feb;103(2):180–192. [PubMed] [Google Scholar]
- Daniel T. M., Janicki B. W. Mycobacterial antigens: a review of their isolation, chemistry, and immunological properties. Microbiol Rev. 1978 Mar;42(1):84–113. doi: 10.1128/mr.42.1.84-113.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Densen P., Mandell G. L. Phagocyte strategy vs. microbial tactics. Rev Infect Dis. 1980 Sep-Oct;2(5):817–838. doi: 10.1093/clinids/2.5.817. [DOI] [PubMed] [Google Scholar]
- FLECK L. Recent investigations on leukergy. Tex Rep Biol Med. 1956;14(4):424–431. [PubMed] [Google Scholar]
- Fearon D. T., Ruddy S., Schur P. H., McCabe W. R. Activation of the properdin pathway of complement in patients with gram-negative of bacteremia. N Engl J Med. 1975 May 1;292(18):937–940. doi: 10.1056/NEJM197505012921802. [DOI] [PubMed] [Google Scholar]
- Goren M. B., D'Arcy Hart P., Young M. R., Armstrong J. A. Prevention of phagosome-lysosome fusion in cultured macrophages by sulfatides of Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2510–2514. doi: 10.1073/pnas.73.7.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackett P. S., Aber V. R., Lowrie D. B. Virulence and resistance to superoxide, low pH and hydrogen peroxide among strains of Mycobacterium tuberculosis. J Gen Microbiol. 1978 Jan;104(1):37–45. doi: 10.1099/00221287-104-1-37. [DOI] [PubMed] [Google Scholar]
- Kossack R. E., Guerrant R. L., Densen P., Schadelin J., Mandell G. L. Diminished neutrophil oxidative metabolism after phagocytosis of virulent Salmonella typhi. Infect Immun. 1981 Feb;31(2):674–678. doi: 10.1128/iai.31.2.674-678.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusunose E., Ichihara K., Noda Y., Kusunose M. Superoxide dismutase from Mycobacterium tuberculosis. J Biochem. 1976 Dec;80(6):1343–1352. doi: 10.1093/oxfordjournals.jbchem.a131407. [DOI] [PubMed] [Google Scholar]
- MASSEY V. The microestimation of succinate and the extinction coefficient of cytochrome c. Biochim Biophys Acta. 1959 Jul;34:255–256. doi: 10.1016/0006-3002(59)90259-8. [DOI] [PubMed] [Google Scholar]
- Misaki A., Seto N., Azuma I. Structure and immunological properties of D-arabino-D-galactans isolated from cell walls of Mycobacterium species. J Biochem. 1974 Jul;76(1):15–27. doi: 10.1093/oxfordjournals.jbchem.a130540. [DOI] [PubMed] [Google Scholar]
- Moulder J. W. Comparative biology of intracellular parasitism. Microbiol Rev. 1985 Sep;49(3):298–337. doi: 10.1128/mr.49.3.298-337.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrvik Q. N., Leake E. S., Wright M. J. Disruption of phagosomal membranes of normal alveolar macrophages by the H37Rv strain of Mycobacterium tuberculosis. A correlate of virulence. Am Rev Respir Dis. 1984 Feb;129(2):322–328. [PubMed] [Google Scholar]
- Quie P. G. Perturbation of the normal mechanisms of intraleukocytic killing of bacteria. J Infect Dis. 1983 Aug;148(2):189–193. doi: 10.1093/infdis/148.2.189. [DOI] [PubMed] [Google Scholar]
- Riley L. K., Robertson D. C. Brucellacidal activity of human and bovine polymorphonuclear leukocyte granule extracts against smooth and rough strains of Brucella abortus. Infect Immun. 1984 Oct;46(1):231–236. doi: 10.1128/iai.46.1.231-236.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley L. K., Robertson D. C. Ingestion and intracellular survival of Brucella abortus in human and bovine polymorphonuclear leukocytes. Infect Immun. 1984 Oct;46(1):224–230. doi: 10.1128/iai.46.1.224-230.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]