Abstract
Brain-derived neurotrophic factor (BDNF) has been implicated in mechanisms of synaptic plasticity such as long-term potentiation (LTP), but its role in associative learning remains largely unknown. In the present study, we investigated the function of BDNF and its receptor tropomyosin-related kinase B (TrkB) in an in vitro model of classical conditioning. Conditioning resulted in a significant increase in BDNF and phospho (p)-Trk expression. Bath application of antibodies directed against TrkB, but not TrkA or TrkC, abolished acquisition of conditioning, as did a receptor tyrosine kinase inhibitor K252a and an inhibitor of nitric oxide synthase 7-nitroindazole. Significantly, injections of BDNF Ab into the nerve roots of presynaptic axonal projections or postsynaptic motor neurons prevented acquisition of conditioning, suggesting that BDNF is required on both sides of the synapse for modification to occur. The presynaptic proteins synaptophysin and synapsin I were increased upon conditioning or BDNF application. Furthermore, BDNF application alone mimicked conditioning-induced synaptic insertion of GluR1 and GluR4 AMPAR subunits into synapses, which was inhibited by co-application of BDNF and K252a. Data also show that extracellular signal-regulated kinase (ERK) was activated in BDNF-treated preparations. We conclude that coordinate pre- and postsynaptic actions of BDNF are required for acquisition of in vitro classical conditioning.
Keywords: BDNF, classical conditioning, turtles, GluR4, AMPAR trafficking, eyeblink
Introduction
Brain-derived neurotrophic factor (BDNF), initially identified as an essential differentiation and survival factor during CNS development, plays an important role in activity-dependent synaptic plasticity (for reviews see, Bramham and Messaoudi, 2005; Carvalho et al., 2007). Long-term potentiation (LTP), a well-studied model of synaptic plasticity, is impaired in the hippocampus when BDNF and TrkB function is suppressed by gene knockout (Korte et al., 1995; Xu et al., 2000; Zakharenko et al., 2003), antisense techniques (Ma et al., 1998), or antibody (Ab) manipulations (Figurov et al., 1996; Kang et al., 1997; Chen et al., 1999). The deficits in LTP resulting from BDNF knockdown could be rescued by addition of recombinant BDNF to medium (Patterson et al., 1996) or adenovirus-mediated overexpression of BDNF (Korte et al., 1996). Studies have also suggested that BDNF itself could induce (Messaoudi et al., 1998) or facilitate (Kovalchuk et al., 2002) LTP expression in the hippocampus. Additional findings that high-frequency stimulation or more natural theta patterns of afferent activity upregulates BDNF expression further corroborate its role in synaptic plasticity (Gooney and Lynch, 2001; Balkowiec and Katz, 2002). Mechanisms underlying the function of BDNF in plasticity include presynaptic (Gottschalk et al., 1998; Pozzo-Miller et al., 1999; Jovanovic et al., 2000; Xu et al., 2000; Zakharenko et al., 2003; Tyler et al., 2006) or postsynaptic actions (Levine et al., 1998; Lin et al., 1998; Di Luca et al., 2001; Kovalchuk et al., 2002) as well as coordinate pre- and postsynaptic processes (Alder et al., 2005; Gartner et al., 2006). Despite a great deal of progress on the function of BDNF in LTP, investigation of its role in associative learning has only more recently begun to be examined. Results from a contextual learning study show that BDNF mRNA is increased in the hippocampus (Hall et al., 2000) and the amygdala after fear conditioning (Rattiner et al., 2004). Much less information is available about the role BDNF plays in other forms of classical conditioning, although it is likely to be involved (Gruart et al., 2007).
An in vitro model of classical conditioning displays responses characteristic of eyeblinks recorded from the abducens nerve in turtles after paired stimulation of the auditory (the “tone” conditioned stimulus, CS) and trigeminal (the “airpuff” unconditioned stimulus, US) nerves (for a review, see Keifer, 2003). Our initial studies of conditioning used an isolated brain stem-cerebellum preparation (Keifer et al., 1995). However, subsequent studies found that an isolated brain stem preparation alone without the cerebellum could acquire robust CRs, although these had a significantly shorter onset latency compared to intact preparations (Anderson and Keifer, 1997, 1999; Keifer and Clark, 2003). These findings are similar to those obtained from rabbits with cerebellar cortex lesions (Perrett et al., 1993). Our current studies use a brain stem preparation in which to examine cellular mechanisms of CR acquisition while analysis of mechanisms controlling CR timing are undertaken using preparations with an intact cerebellum (Keifer, 2003). More recently we have shown that conditioning is associated with two waves of synaptic AMPAR insertion in abducens motor neurons. Initially, GluR1-containing AMPARs are trafficked to synapses to unsilence them (Mokin et al., 2007). This is followed by NMDA-dependent synaptic delivery of GluR4-containing AMPARs that is associated with the acquisition of conditioned responses (CRs; Mokin and Keifer, 2004; Mokin et al., 2006). Synaptic incorporation of GluR4 subunits is accomplished through interactions with the immediate-early gene-encoded protein Arc and the actin cytoskeleton (Mokin et al., 2006). Furthermore, studies demonstrate that protein kinase C (PKC) activation of ERK MAPK signal transduction pathways regulate GluR4 synaptic insertion during conditioning, whereas ERK signaling, but not PKC, regulates insertion of GluR1 (Keifer et al., 2007; Zheng and Keifer, submitted). Apart from these postsynaptic modifications during conditioning, expression of the presynaptic vesicle-associated protein synaptophysin was also consistently enhanced during the acquisition of conditioning (Mokin and Keifer, 2004; Mokin et al., 2006; Mokin et al., 2007). Given the evidence implicates a role for BDNF in pre- and postsynaptic modifications in LTP, the present study was carried out to examine BDNF-induced mechanisms of synaptic plasticity during classical conditioning in this in vitro preparation. The results show that bath application of pharmacological agents that suppress function of BDNF-TrkB abolish acquisition of conditioning. Moreover, injections of BDNF Ab into the nerve roots containing axons of presynaptic projections or postsynaptic motor neurons prevent acquisition of conditioning, suggesting that BDNF is required on both sides of the synapse for modification to occur. Additionally, the presynaptic proteins synaptophysin and synapsin I were increased upon conditioning or BDNF application, as was synaptic incorporation of GluR1 and GluR4 AMPARs. Finally, data show that ERK MAPK was activated in BDNF-treated preparations. These findings indicate that coordinate pre- and postsynaptic actions of BDNF are required for acquisition of in vitro classical conditioning and may act through ERK-mediated mechanisms.
EXPERIMENTAL PROCEDURES
Conditioning procedures
Freshwater pond turtles Pseudemys scripta elegans obtained from commercial suppliers were anesthetized by hypothermia until torpid and decapitated. Protocols involving the use of animals complied with the guidelines of the National Institutes of Health and the Institutional Animal Care and Use Committee. The brain stem was transected at the levels of trochlear and glossopharyngeal nerves, and the cerebellum was removed as described previously (Anderson and Keifer, 1999). Therefore, this preparation consisted only of the pons, containing the pontine blink circuitry, with the cerebellar circuitry and the red nucleus removed. The brainstem was continuously bathed in physiological saline (2–4 ml/min) containing (in mM): 100 NaCl, 6 KCl, 40 NaHCO3, 2.6 CaCl2, 1.6 MgCl2, and 20 glucose, which was oxygenated with 95% O2/5% CO2 and maintained at room temperature (22–24 °C) at pH 7.6 (Anderson and Keifer, 1999). Suction electrodes were used for stimulation and recording of cranial nerves. The US was an approximately two times threshold single shock stimulus applied to the trigeminal nerve; the CS was a 100 Hz, 1 s train stimulus applied to the ipsilateral posterior root of the eighth nerve that was below threshold amplitude required to produce activity in the abducens nerve (Keifer et al., 1995; Anderson and Keifer, 1999). The latter nerve will be referred to as the auditory nerve as it carries predominantly auditory fibers. Neural activity was recorded from the ipsilateral abducens nerve which projects to the extraocular muscles controlling movements of the eye, nictitating membrane, and eyelid. The CS-US interval was 20 ms which is defined as the time between the offset of the CS and the onset of the US. This brief trace delay interval was found to be optimal for conditioning, however, conditioning is not supported using longer trace intervals (Keifer, 2001). Intertrial interval between the paired stimuli was 30 s. A pairing session consisted of 50 CS-US presentations followed by a 30 min rest period in which there was no stimulation. Conditioned responses were defined as abducens nerve activity that occurred during the CS and exceeded the amplitude of double the baseline recording level. Conditioned preparations were those that received paired CS-US stimulation whereas pseudoconditioned preparations received the same number of CS and US exposures that were explicitly unpaired using a CS-US interval randomly selected between 300 ms and 25 s. At the end of the conditioning experiments, preparations for immunocytochemistry were immersion fixed in cold 0.5% paraformaldehyde whereas those for protein analysis were frozen in liquid nitrogen and stored at −70 °C.
Pharmacology
To examine the function of BDNF-TrkB in conditioning, preparations were incubated overnight (~15 h) in oxygenated physiological saline containing 5 µg/ml TrkB antibody (20542, Santa Cruz Biotechnology, Santa Cruz, CA), TrkA Ab (20539, Santa Cruz), or TrkC Ab (14025, Santa Cruz), followed by perfusion with normal physiological saline during the conditioning procedures (Kang et al., 1997). BDNF (4554, Santa Cruz) stock solution was added immediately before each experiment to physiological saline to a final concentration of 100 ng/ml and applied to preparations 30 min prior to conditioning. K252a (Calbiochem, La Jolla, CA), a cell-permeable protein kinase inhibitor with actions on receptor tyrosine kinases, and its related but weaker form K252b, which does not enter cells as efficiently as K252a, was dissolved in DMSO and added to physiological saline to a final concentration of 200 nM and administered 2 h prior to conditioning and continued throughout the training procedures. 7-nitroindazole (7-NI; Calbiochem), a selective nitric oxide synthase (NOS) inhibitor was prepared as a stock solution of 0.2 M in DMSO, frozen at −20 °C, and later diluted to 200 µM in physiological saline and applied to preparations 30 min prior to and throughout the conditioning procedures. In all cases, the final concentration of DMSO in the bathing medium was 0.1 %, which had no effect on the physiological responsiveness of the preparations.
Immunocytochemistry for BDNF
For BDNF immunostaining of the pontine blink-related circuitry, naïve brain stems were immersion fixed in 3% paraformaldehyde. Tissue sections were cut at 30 µm on a microtome and preincubated in 10% goat serum for 1 h. Sections were incubated in primary antibody to BDNF (1:2,000; 20981, Santa Cruz) overnight at 4 °C with gentle shaking, rinsed, and incubated for 2 h in Cy3-conjugated secondary antibody (1:100). After incubation, sections were rinsed, mounted on slides, and coverslipped.
Western blot analysis
Brain stems were frozen in liquid nitrogen at the end of the physiological experiments and stored at −70 °C. Tissue was homogenized in NP-40 buffer containing protease and phosphatase inhibitors, and the homogenates placed on an orbital shaker for 2 h, and centrifuged at 10,000 g for 20 min. The supernatants were aspirated and protein concentrations determined by the Bradford protein assay. Equal amounts of protein sample were denatured in loading buffer containing 125 mM Tris-HCl (pH 6.8), 20% glycerol, 6% SDS, and 5% β-mercaptoethanol, boiled for 3 min, and subjected to 8–15% gradient SDS-PAGE. The proteins were transferred to PVDF membranes and blocked with 5% nonfat dry milk in TBST (20 mM Tris at pH 7.6, 150 mM NaCl, and 0.1% Tween-20) for 1 h. Membranes were probed using the following primary antibodies: BDNF (1:500, 20981, Santa Cruz), phospho-TrkA that detects levels of all Trks (A–C) phosphorylated at Tyr490 (p-Trk; 1:500, 9141S, Cell Signaling Technology, Danvers, MA), synaptophysin (1:500, S5768, Sigma, St. Louis, MO), synapsin (1:1,000; 106002, Synaptic Systems, Göttingen, Ger), phospho-ERK that recognizes dually phosphorylated Thr183/Tyr185 (p-ERK; 1:1,000; V803A, Promega, Madison, WI), ERK (1:5,000; 3053, Chemicon, Temecula, CA), phospho-p38 that recognizes dually phosphorylated Thr180/Tyr182 (p-p38; 1:1,000; V121A, Promega), and p38 (1:1,000; 535, Santa Cruz). The membranes were rinsed with TBST and incubated with HRP-conjugated or fluorescently-labeled secondary antibodies for 2 h at room temperature. Proteins were detected using the ECL-Plus chemiluminescence system (Amerisham Pharmacia, Piscataway, NJ) and immunoreactive signals were captured on Kodak X-omatic AR film and quantified by computer assisted densitometry. In some cases, the Odyssey infrared imaging system (Li-Cor Bioscience) was used for detection and quantification of proteins. Each membrane was reprobed for loading controls with β-actin (1:1,000; Chemicon). Quantification of total protein was determined relative to β-actin, whereas phospho-protein was determined relative to total protein for the same experiments. Ratios of total protein/β-actin or phospho-protein/total protein were obtained for each experiment and averaged. Data are displayed as a percentage of normalized values from pseudoconditioned or naive controls.
Antero- and retrograde microinjections of BDNF antibodies
Anterograde microinjections were achieved by inserting a micropipette into the cut end of the auditory nerve. The abducens nerve was targeted for retrograde microinjections. The micropipette was connected to a syringe pump by tubing which was loaded with 200 µg/ml BDNF Ab (20981, Santa Cruz) or NGF Ab (549, Santa Cruz). The antibodies were delivered at a constant rate for 6 h (total volume, 10µl). Following the injections, the brain stem underwent the conditioning procedures. At the end of these experiments, brain stems were immersion fixed and processed for immunocytochemistry using secondary antibodies alone to visualize the injected primary antibodies.
Glutamate receptor localization, confocal imaging, and data analysis
Tissue sections were cut at 30 µm and preincubated in 10% normal goat serum. Sections were incubated in primary antibody overnight at 4 °C with gentle shaking. The primary antibodies used were a polyclonal antibody raised in rabbit that recognizes the GluR1 subunit of AMPA receptors (1:100; 1504, Chemicon), a polyclonal antibody raised in goat that recognizes the GluR4 subunit of AMPA receptors (1:100; 7614, Santa Cruz), and a monoclonal antibody raised in mouse that recognizes synaptophysin (1:1,000; S5768, Sigma). Specificities of all antibodies were confirmed by Western blot. After the primary antibodies, sections were rinsed and incubated with secondary antibodies for 2 h at room temperature. The secondary antibodies were a Cy3-conjugated goat anti-rabbit IgG (1:100) for GluR1, a Cy3-conjugated rabbit anti-goat IgG (1:100) for GluR4, and a Cy2-conjugated goat anti-mouse IgG (1:50) for synaptophysin (Jackson ImmunoReserach, West Grove, PA). After incubation in the secondaries, sections were rinsed, mounted on slides and coverslipped. Images of labeled neurons in the principal or accessory abducens motor nuclei were obtained using an Olympus Fluoview 500 laser-scanning confocal microscope. Tissue samples were scanned using a 60 × 1.4 NA oil immersion objective with dual excitation using a 488 nm argon laser and a 543 nm HeNe laser. Quantification of punctate staining of at least two-fold greater intensity above background was performed using stereological procedures (Mokin and Keifer, 2006) with MetaMorph software (Universal Imaging, Downingtown, PA). Images of two consecutive optical sections were taken using confocal microscopy. Protein puncta were counted in one optical section (sample section) if they were not present in the optical section immediately below the sample section (look-up section) and if they were within the inclusion boundaries of the unbiased counting frame. Colocalized staining indicating the presence of glutamate receptor subunits at synaptic sites was determined when red and green puncta were immediately adjacent to one another or if they were overlapping. All data were analyzed using Statview software (SAS, Cary, NC) by ANOVA and are presented as means ± SEM except where indicated.
RESULTS
Localization of basal BDNF in abducens blink-related pathways
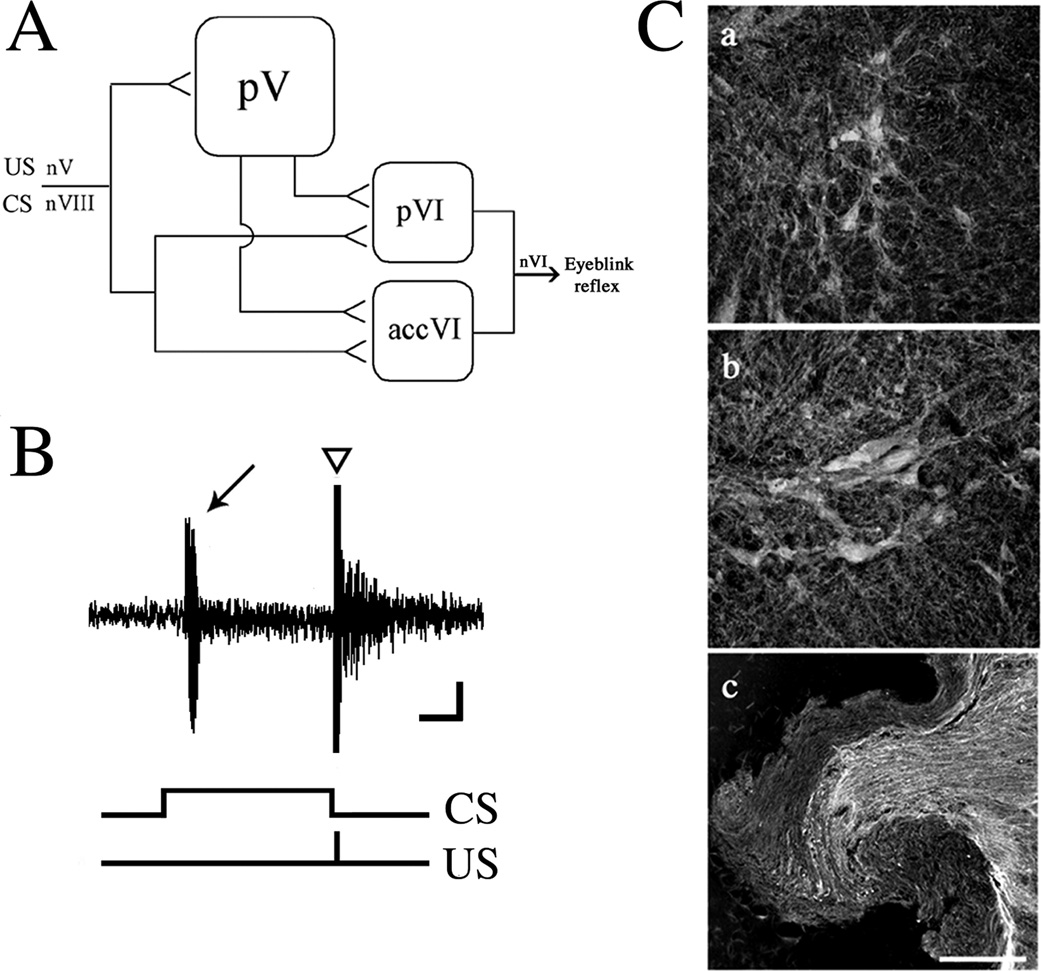
Our previous tract tracing studies showed that the pontine blink-related pathways consisted of the principal sensory trigeminal nucleus, cochlear/vestibular nuclear complexes, and the abducens motor nuclei (Fig. 1A; Zhu and Keifer, 2004). Briefly, both the principal (pVI) and accessory (accVI) abducens motor nuclei receive direct convergent inputs from the trigeminal (US) and auditory nerves (CS; Keifer and Mokin, 2004). They also receive inputs from the principal sensory trigeminal nucleus (pV). Finally, the principal nucleus receives projections from the vestibular nuclei while the accessory nucleus receives inputs from the cochlear nuclei (not indicated in Fig. 1A). The abducens motor nuclei in turn project to the extraocular muscles controlling movements of the eye, nictitating membrane, and eyelid. As turtles do not have muscles of facial expression, such as the orbicularis oculi that controls eyelid movements in mammals, the facial nucleus does not contribute to blinking in this species. Instead, they have the pyramidalis muscle, in addition to retractor bulbi, that directly controls movement of the nictitating membrane and eyelid that is innervated by the abducens nerve. Electrical stimulation of the trigeminal nerve evokes a burst discharge characteristic of a neural correlate of an eyeblink response (Fig. 1B, triangle; Keifer, 1993), whereas paired stimulation of the trigeminal and auditory nerves results in acquisition of CRs (Fig. 1B, arrow). In addition to activity-dependent secretion, BDNF is also expressed independent of activity levels. To investigate sites of basal BDNF expression in the pontine blink circuitry, immunostaining was performed. In naïve (untrained) preparations, intensely-labeled BDNF immunopositive neurons were detected in the principal (Fig. 1Ca) and accessory abducens motor nuclei (Fig. 1Cb). BDNF immunolabeling was observed both in the soma and dendrites. Abducens motor neurons immunopositive for BDNF have also been described in the cat (Benitez-Temino et al., 2004). Axons of the auditory nerve were also intensely labeled for BDNF (Fig. 1Cc). The trigeminal nerve was minimally labeled. Immunopositive neurons for BDNF were also observed in the principal sensory trigeminal nucleus and in the cochlear and vestibular nuclei (data not shown). Therefore, expression of BDNF in both auditory nerve axon terminals, abducens motor neurons, and elsewhere in the pontine blink circuitry, indicates that BDNF is localized both pre- and postsynaptically and may exert its effects at both sites.
Fig. 1. Postsynaptic abducens motor neurons and presynaptic auditory axons in the turtle are immunopositive for BDNF.
(A) schematic diagram of eyeblink-related pathways in pond turtles (see text for details). Abbreviations: nV, trigeminal nerve; nVIII, auditory nerve; pV, principal sensory trigeminal nucleus; pVI, principal abducens nucleus; accVI, accessory abducens nucleus; nVI, abducens nerve. Calibration bar = 0.25 s, 50 µV. (B) A representative recording from a conditioned preparation in which a conditioned response (CR, arrow) was recorded as a burst discharge followed by an unconditioned response (UR, triangle). The occurrence of the CS and US are indicated. (C) Immunocytochemical labeling of principal (a) and accessory (b) abducens motor neurons, and axons in the auditory nerve (c) for BDNF from naïve tissue. Scale bar = 100 µm.
Role for BDNF-TrkB in acquisition of in vitro classical conditioning
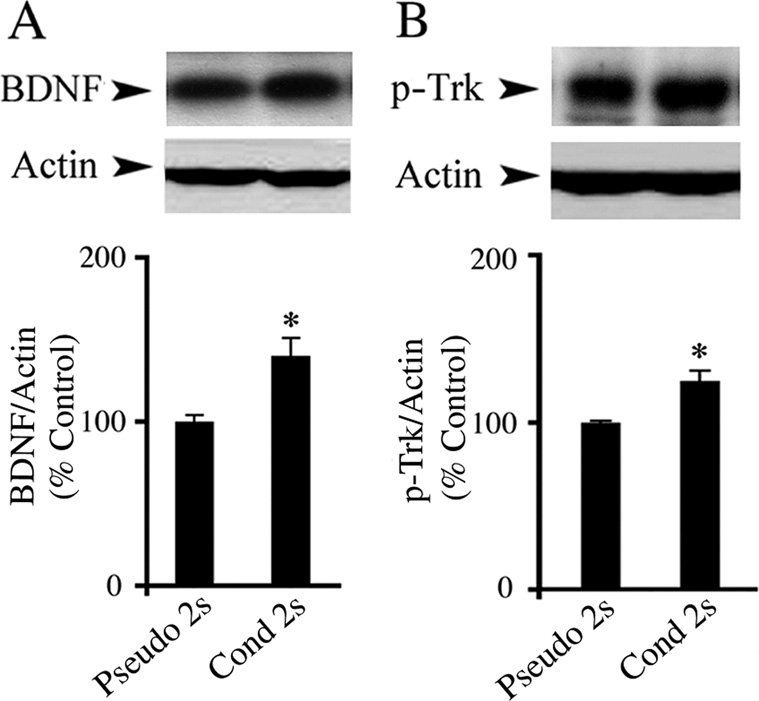
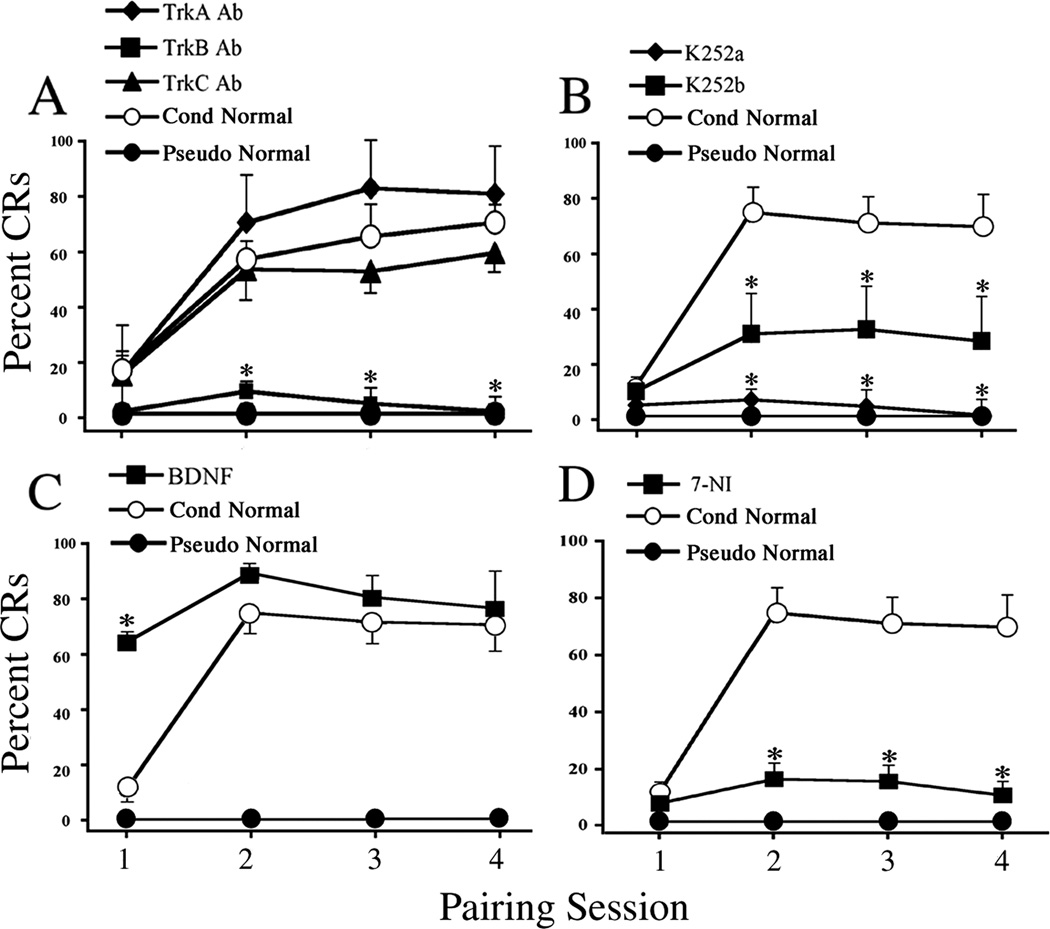
To investigate the expression of BDNF-TrkB in acquisition of conditioning, preparations were conditioned for two pairing sessions and subjected to Western blot analysis. Conditioning resulted in a significant increase in BDNF protein expression relative to the pseudoconditioned group (Fig. 2A; n = 4/group, F(1,6) = 11.5, P = 0.01). We next tested whether the Trk receptors were activated upon two sessions of conditioning stimuli. As shown in Fig. 2B, the levels of p-Trk, as indicated by antibodies to p-TrkA which recognize phosphorylation of Tyr490 of all Trk receptors, were significantly augmented above pseudoconditoned levels (n = 8/group, F(1,13) = 25.5, P = 0.0002). These results suggest that BDNF signaling occurs during the acquisition phase of in vitro classical conditioning. To further establish a role for BDNF-TrkB in conditioning, preparations were challenged with selective blocking agents. The physiological results show that preparations incubated in TrkA or TrkC Ab overnight, which are selective for NGF and NT-3 receptors, respectively, underwent conditioning and expressed levels of CRs that did not differ statistically from preparations incubated in normal physiological saline (Fig. 3A; n = 5/group, F(2,14) = 1.7, P = 0.213). However, in preparations exposed to TrkB Ab, acquisition of conditioning was abolished, having only an average of 9 ± 4% CRs in session two (n = 6, F(2,13) = 17.3, P < 0.0001, TrkA and TrkC vs TrkB Ab groups). To further confirm the results using antibodies, K252a, a receptor tyrosine kinase inhibitor, was employed. Pretreatment of preparations with K252a for 2 h resulted in no CR acquisition (Fig. 3B; n = 5, F(1,11) = 34, P = 0.0001, K252a vs Cond). Application of K252b also significantly attenuated the percentage of CRs but to a lesser extent than K252a (Fig. 3B; n = 6, F(1,12) = 7.1, P = 0.02, K252b vs Cond). These results suggest that BDNF-TrkB is required for the acquisition of conditioning. We then reasoned that if BDNF-TrkB signaling is important for conditioning, then pre-exposure of preparations to BDNF might be expected to facilitate acquisition. BDNF was bath applied 30 min before training. As shown in Fig. 3C, BDNF treatment significantly increased the percentage of CRs in the first pairing session to 65 ± 4%, compared to 12 ± 3% CRs in the conditioned group, thereby promoting earlier expression of CRs during the training procedure (n = 3, F(1,9) = 54.5, P < 0.0001). Finally, since BDNF was found to be localized both pre- and postsynaptically in the abducens blink circuit, we determined whether conditioning required the generation of the gaseous signaling molecule nitric oxide (NO). Pretreatment of preparations for 30 min with 7-NI, a selective NOS inhibitor, blocked the acquisition of conditioning compared to the conditioned group (Fig. 3D; n = 5, F(1, 11) = 25.4, P = 0.0004). Taken together, the data provide strong evidence that BDNF-TrkB signaling accompanied by the synthesis of NO is critical for the acquisition of in vitro classical conditioning.
Fig. 2. Western blot analysis shows increased BDNF and p-Trk expression after two sessions of in vitro conditioning.
(A) BDNF expression was significantly increased after two pairing sessions of conditioning as compared to the pseudoconditioned group. (B) Preparations also exhibited significantly enhanced expression of p-Trk. * indicates significant differences compared with the Pseudo 2s group. For all figures, P values and the number of preparations tested in each group are given in the text.
Fig. 3. BDNF-TrkB and nitric oxide are required for acquisition of in vitro conditioning.
(A) Preparations treated with TrkA or TrkC Ab (5 µg/ml) overnight were able to undergo conditioning and express CRs similar to conditioned preparations, while conditioning was not established in preparations treated with TrkB Ab (5 µg/ml) and was comparable to the pseudoconditioned group. (B) CRs were abolished by K252a treatment (200 nM), and were also significantly attenuated by K252b (200 nM) compared to conditioning. (C) BDNF treatment (100 ng/ml) prior to training significantly facilitated the acquisition of CRs in the first pairing session compared to conditioning in normal saline. (D) Application of 7-NI, a selective NOS inhibitor, attenuated the acquisition of conditioning. * indicates significant differences compared with the conditioned group.
Attenuation of conditioning by antero- or retrogradely injected BDNF antibodies
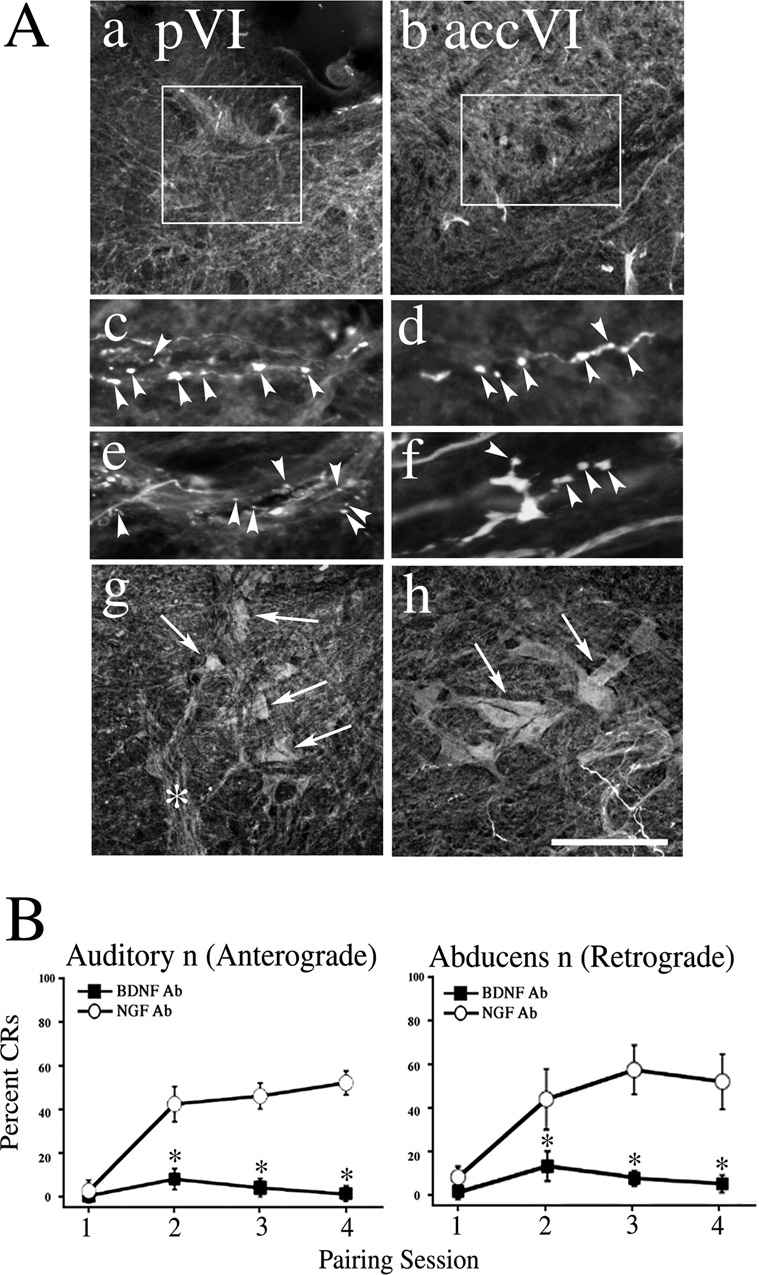
Since the immunocytochemistry showed that BDNF is localized both presynaptically in auditory nerve terminals and postsynaptically in abducens motor neurons, we next determined whether either pre- or postsynaptic BDNF exerts a functional effect on conditioning. Anti-BDNF antibodies were delivered into the auditory nerve for anterograde transport or into the abducens nerve for retrograde transport to suppress the function of BDNF during conditioning while control preparations were injected with NGF antibodies. In all cases, a strong trigeminal nerve-evoked UR and auditory nerve-evoked "startle" response was recorded from the abducens nerve indicating that synaptic transmission was unaffected by the manipulation. The results from the auditory nerve microinjections to examine presynaptic BDNF action are shown in Fig. 4. As shown in the photomicrographs in Fig. 4Aa–f, anterograde injections resulted in labeling of auditory axons and nerve terminals (arrowheads) in both the principal (Fig. 4Aa,c,e) and accessory (Fig. 4Ab,d.f) abducens motor nuclei. The percentage of CRs was significantly attenuated in the BDNF Ab injected preparations compared to the NGF Ab-treated group (Fig. 4B, left panel; n = 7, F(1,12) = 13.3, P = 0.003). Similarly, injections of BDNF Ab into the abducens nerve for retrograde transport also resulted in a marked reduction of the percentage of CRs compared with the NGF Ab-treated group (Fig. 4B, right panel; n = 6, F(1,11) = 6.8, P = 0.02). In these cases, the soma and dendritic processes of principal (Fig. 4Ag) and accessory (Fig. 4Ah) abducens motor neurons were intensely labeled (arrows). These data suggest that BDNF is required both pre- and postsynaptically for the acquisition of in vitro conditioning.
Fig. 4. BDNF antibodies microinjected into the auditory (anterograde transport) or abducens nerve (retrograde transport) attenuate the acquisition of in vitro conditioning.
(A) Following the physiological experiments, preparations were processed and stained with corresponding secondary antibodies to visualize the primary antibodies to BDNF microinjected into the auditory nerve. Photomicrographs show auditory axons projecting to the principal (pVI; Aa) and accessory (accVI; Ab) abducens motor nuclei, and labeled terminal boutons (Ac,e, principal nucleus; Ad,f, accessory nucleus; arrowheads). The boxes in Aa and Ab indicate the location of boutons. In contrast, photomicrographs from microinjections of the abducens nerve show intense labeling of principal (Ag) and accessory (Ah) abducens motor neurons and dendrites (arrows). The asterisk in Ag indicates the labeled abducens nerve. (B) Preparations injected with BDNF Ab failed to acquire CRs while those treated with NGF Ab displayed conditioning. In the graphs, * indicates significant differences between BDNF Ab and NGF Ab treated groups. Scale bar = 200 µm in Aa,b, 20 µm in Ac–f, 100 µm in Ag,h.
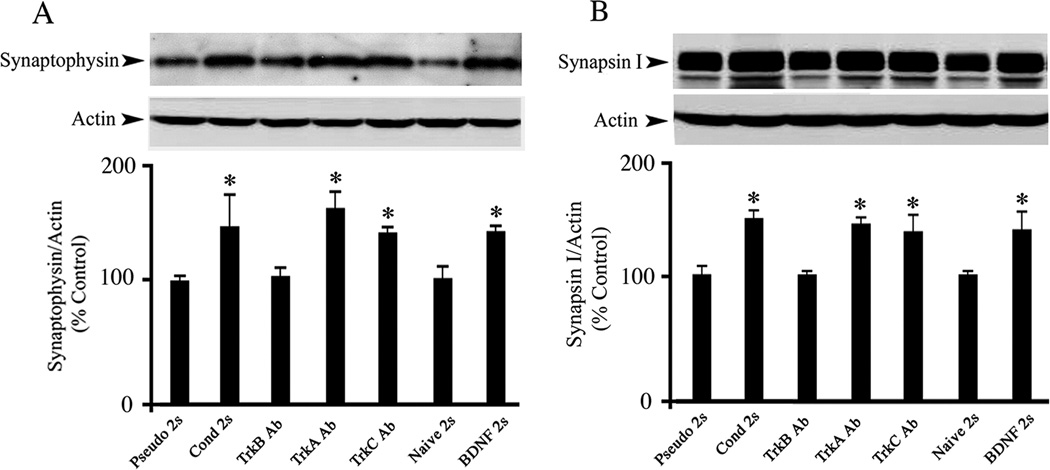
BDNF-induced expression of presynaptic proteins synaptophysin and synapsin I
In previous studies we consistently observed that levels of the presynaptic protein synaptophysin were significantly elevated during conditioning (Mokin and Keifer, 2004; Mokin et al., 2006; Mokin et al., 2007). Western blotting was performed to assess whether BDNF induces a similar enhancement of presynaptic proteins. Compared with the pseudoconditioned group, synaptophysin levels in preparations conditioned for two sessions were increased by approximately 60% (Fig. 5A; n = 4/group, F(1,6) = 6.2, P = 0.04). Increased expression of synaptophysin was also observed in preparations incubated in TrkA or TrkC Ab overnight and subsequently conditioned for two sessions (n= 3/group, F(2,7) = 45.0, P = 0.0001). In contrast, TrkB Ab-treated preparations did not display a detectable increase in synaptophysin levels compared to pseudoconditioning (n = 3, F(1,5) = 0.007, P = 0.93). To test whether such enhancement could be induced by BDNF application, exogenous BDNF was bath applied for 80 min, equivalent to two pairing sessions. BDNF treatment resulted in a significant increase in synaptophysin expression to levels comparable to that of the conditioned group (Fig. 5A; n = 5, F(1,7) = 16.8, P = 0.004, BDNF vs Naïve). In addition to synaptophysin, another synaptic vesicle-associated protein, synapsin I, was also examined. The results obtained for synapsin I were remarkably similar to those for synaptophysin as shown in Fig 5B. Preparations conditioned for two sessions displayed increased expression of synapsin I compared to pseudoconditioning (Fig. 5B; n = 4/group, F(1,6) = 16.4, P = 0.006), which was mimicked by addition of BDNF for two sessions (n = 5, F(1,7) = 6.2, P = 0.04, BDNF vs Naïve). Preparations treated with TrkA or TrkC Ab overnight also showed increased expression of synapsin I (n = 3/group, F(2,7) = 12.7, P = 0.004) while those incubated in TrkB Ab did not (n = 3, F(1,5) = 0.8, P = 0.41). These results indicate that BDNF expression contributes to the enhancement of presynaptic proteins during the acquisition of conditioning.
Fig. 5. Similar to conditioning, bath application of BDNF results in enhanced expression of two presynatic proteins, synaptophysin and synapsin I, which is blocked by TrkB Ab but not TrkA or TrkC Abs.
Western blot analysis for synaptophysin (A) and synapsin I (B) is shown relative to β-actin. In comparison to the pseudoconditioned groups, two sessions of conditioning resulted in elevated expression of synaptophysin and synapsin I, which was mimicked by BDNF application (100 ng/ml) for a time period equivalent to two sessions. Treatment with TrkB Ab (5 µg/ml) during two sessions of conditioning blocked expression of both proteins while TrkA or TrkC Ab (5 µg/ml) treatment did not. Naive preparations were incubated in normal saline alone. * indicates significant differences between Pseudo 2s and the other treatment groups.
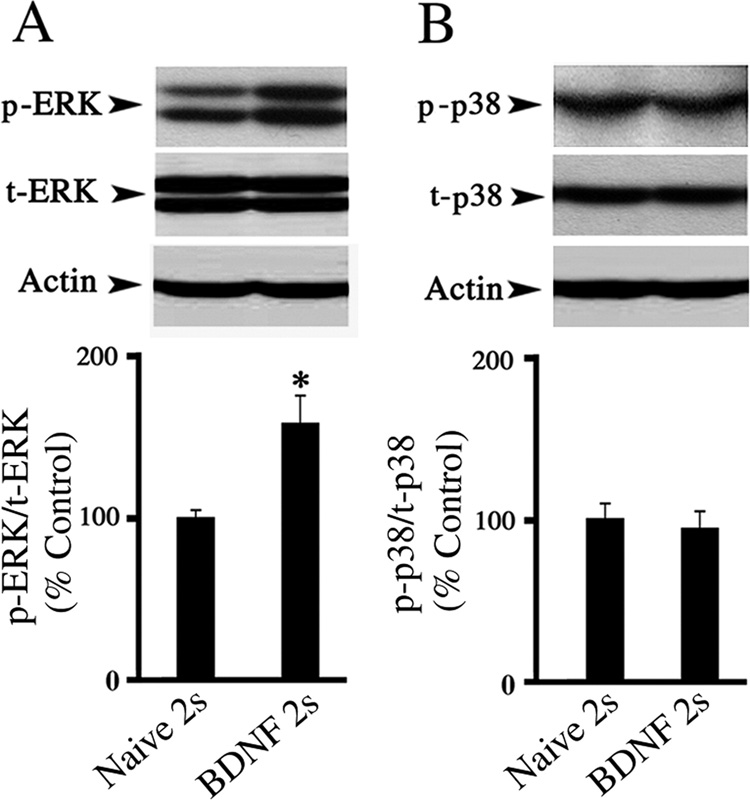
Enhanced expression of ERK in response to BDNF treatment
We previously found that ERK MAPK was activated early in conditioning after two pairing sessions and that p38 MAPK was activated later after five pairing sessions (Keifer et al., 2007). Therefore, we next examined whether BDNF application for the equivalent of two sessions activates ERK, but not p38 MAPK. As expected, BDNF promoted expression of the phosphorylated form of ERK with respect to total ERK (Fig. 6A; n = 10, F(1,13) = 6.8, P = 0.02). In contrast, p-p38 remained unaltered in preparations exposed to BDNF for the same time period (Fig. 6B; n = 10, F(1,14) = 0.14, P = 0.71). These data demonstrate that BDNF application for the equivalent of two sessions selectively induces ERK, but not p38, MAPK expression.
Fig. 6. Treatment with BDNF promotes p-ERK, but not p-p38, MAPK expression.
(A) Phospho-ERK MAPK was significantly elevated compared to total ERK in preparations treated with BDNF for the equivalent of two sessions compared with the naïve group. (B) No significant change in the level of phospho-p38 relative to total p38 was detected between the naïve and BDNF groups. * indicates significant difference between Naïve 2s and BDNF 2s groups.
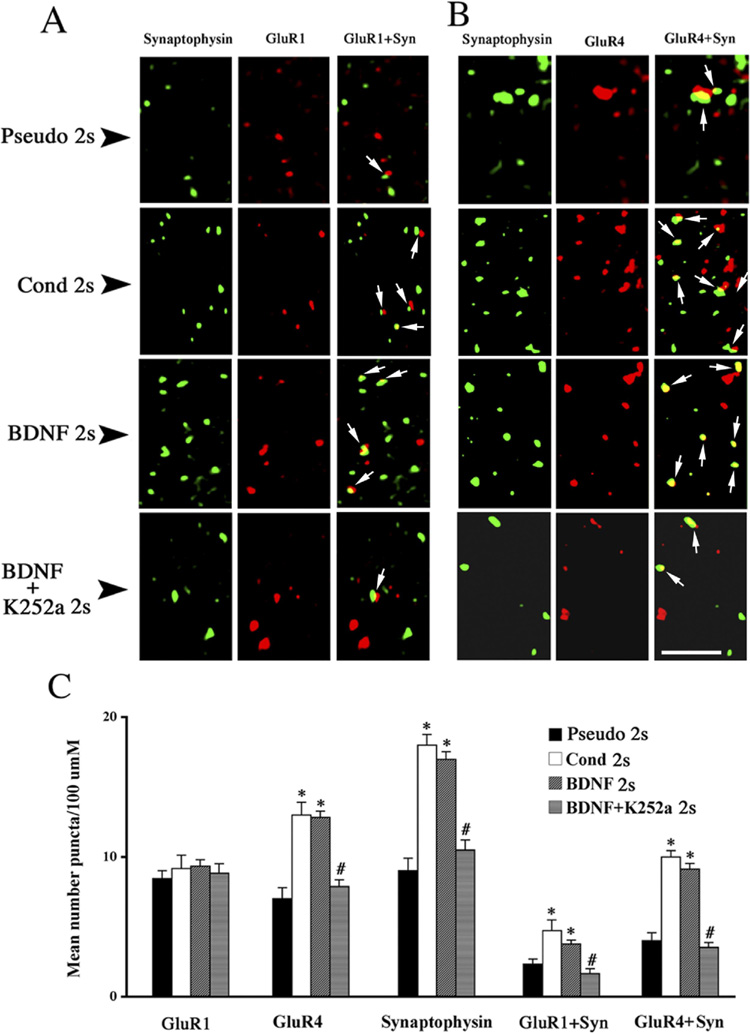
BDNF-induced synaptic delivery of GluR1- and GluR4-containing AMPARs
Conditioning in this model has been shown to involve transient delivery of synaptic GluR1-containing AMPARs followed by synaptic insertion of GluR4 subunits (Mokin et al., 2007). Here, using immunostaining and confocal imaging of abducens motor neurons, we examined whether BDNF application results in the synaptic delivery of either GluR1 or GluR4 subunits, or both. Images of abducens motor neurons double-labeled for GluR1 or GluR4 (red) and synaptophysin (green) and quantitative data are illustrated in Fig. 7. Levels of GluR1 subunits remained unchanged after two pairing sessions of conditioning, after treatment with BDNF for the same time period, or after co-application of BDNF with K252a (Fig. 7A,C, GluR1; n = 3/group, F(3,182) = 1.8, P = 0.14). Colocalization of GluR1 with synaptophysin in preparations conditioned for two sessions was significantly increased above pseudoconditioned levels (Fig. 7A,C, GluR1 + Syn; n = 3, F(1,65) = 17.3, P < 0.0001), a finding that closely matches the results obtained for BDNF treatment (Fig. 7A,C; n = 3, F(1,118) = 11.4, P = 0.001). Following K252a, the BDNF-induced enhancement in colocalization was abolished (Fig. 7A,C; n = 3, F(1,128) = 11.4, P = 0.001). BDNF treatment for two sessions also resulted in a significant increase in punctate staining for synaptophysin comparable to levels induced by conditioning for two sessions (Fig. 7A,C, Syn; n = 3/group, F(2,260) = 47.7, P < 0.0001). The effect of BDNF treatment on synaptophysin was counteracted by co-application of BDNF and K252a (Fig. 7A,C; n = 3, F(1,232) = 103.8, P < 0.0001). In contrast to unaltered expression of GluR1 AMPAR subunits, GluR4 subunit levels were substantially increased after conditioning, and similar results were obtained following BDNF treatment (Fig. 7B,C, GluR4; n = 3/group, F(2,136) = 19.9, P < 0.0001). This effect of BDNF on GluR4 was attenuated by co-application with K252a (Fig. 7B,C; n = 3, F(1,121) = 50.5, P < 0.0001). Double labeling for GluR4 and synaptophysin also showed a significant increase in colocalization after two sessions of conditioning (Fig. 7B,C, GluR4 + Syn; n = 3, F(1.64) = 19.0, P < 0.0001), which was mimicked by exogenous application of BDNF (Fig. 7B,C; n = 3, F(1, 121) = 53.6, P < 0.0001), and blocked by co-application of BDNF and K252a (Fig. 7B,C; n = 3, F(1,121) = 98.5, P < 0.0001, BDNF vs BDNF+K252a). Collectively, these findings strongly support the conclusion that BDNF initiates postsynaptic signaling mechanisms during early stages of in vitro conditioning that result in synaptic incorporation of GluR1 and GluR4 AMPARs.
Fig. 7. Localization studies using confocal microscopy reveal that BDNF mimics enhanced synaptic delivery of GluR1- and GluR4-containing AMPAs observed during early stages of conditioning.
(A) Confocal images of abducens motor neurons showing punctate staining for GluR1 subunits (red) and synaptophysin (green) from each of the four groups examined: pseudoconditioned (Pseudo 2s), conditioned (Cond 2s), BDNF-treated (BDNF 2s), and BDNF with K252a-treated (BDNF + K252a 2s) preparations. (B) Confocal images abducens motor neurons showing punctate staining for GluR4 subunits (red) and synaptophysin (green) from similarly treated preparations as shown in A. In A and B, arrows indicate colocalization of AMPAR subunits with synaptophysin. (C) Quantitative analysis of GluR1, GluR4, synaptophysin, GluR1 + synaptophysin, and GluR4 + synaptophysin staining from the different treatment groups. * indicates significant differences between Pseudo 2s and Cond 2s or BDNF 2s groups; # indicates significant difference between BDNF 2s and BDNF + K252a 2s groups. Scale bar = 2 µm.
DISCUSSION
Role of BDNF in in vitro classical conditioning
The present study shows that BDNF is involved in in vitro conditioning by using TrkB Ab to functionally block the initiation of BDNF-TrkB signaling cascades. Incubation of preparations with TrkB Ab overnight abolished the acquisition of CRs, while TrkA and TrkC Ab had no significant effect on conditioning. Additionally, both BDNF expression and TrkB activation were upregulated after two sessions of conditioning. Further evidence for the role of BDNF in in vitro conditioning comes from results of K252a and K252b in which preparations treated with K252a prevented CR acquisition while K252b had a weaker effect. Our findings that BDNF pretreatment significantly promotes earlier expression of CRs, also suggest a role for BDNF in conditioning. Consistent with our findings on conditioning, LTP induction increases BDNF expression in the dentate gyrus of the hippocampus (Gooney and Lynch, 2001), fear conditioning results in enhanced expression of BDNF mRNA and activation of TrkB receptors (Rattiner et al., 2004), and activation of TrkB underlies long-term synaptic facilitation and memory in Aplysia (Sharma et al., 2006). BDNF infusion appears to enhance synaptic transmission (BDNF-LTP; Messaoudi et al., 2002, see also Lu et al., 2008). Analysis of LTP in hippocampal slices further demonstrated that application of repetitive depolarizing pulses failed to induce LTP in TrkB-IgG-incubated slices, whereas TrkA and TrkC-IgG had no effect (Gubellini et al., 2005, also Korte et al., 1998). TrkB Ab application was also shown to impair LTP induced by theta-burst or paired stimulation, leaving basal synaptic transmission and shortterm plasticity intact (Kang et al., 1997). Mice with a TrkB-deletion also demonstrated marked impairment in LTP at Schaffer collateral-CA1 synapses (Xu et al., 2000). In contrast to the effects of BDNF-TrkB manipulation on synaptic transmission, application of NT-3 resulted in no effect on LTP in the neonatal hippocampus (Figurov et al., 1996), and LTP could not be compromised in adult hippocampal slices deficient in the NT-3 gene or incubated in NT-3 antibodies (Chen et al., 1999; Ma et al., 1999). However, one study showed that application of NT-3 induced LTP (Kang and Schuman, 1995). As suggested by Ma et al. (1999), this discrepancy may be derived from the use of high concentrations of NT-3 because NT-3 could activate TrkB receptors under these conditions. From studies such as these, it is clear that BDNF-TrkB has a critical role in the induction of synaptic plasticity mechanisms using a number of different models and species. The action of other neurotrophins can not be excluded, however, the findings are inconclusive. For example, transgenic mice in which TrkC was overexpressed showed enhanced hippocampal LTP but reduced trace conditioning (Sahun et al., 2007).
Pre- and postsynaptic effects of BDNF
Whether BDNF has a role in pre- or postsynaptic mechanisms of synaptic plasticity has been a subject of considerable controversy. It was reported that BDNF participated exclusively in pre- or postsynaptic modulation of synaptic efficacy (Gottschalk et al., 1998; Kovalchuk et al., 2000; Zakharenko et al., 2003). For example, using restricted genetic deletion in mice, BDNF was selectively eliminated either from neurons in the entire hippocampus or only from postsynaptic CA1 neurons. Such mice expressed a presynaptic form of LTP that required BDNF originating from presynaptic CA3 neurons (Zakharenko et al., 2003). Several recent studies, however, also suggest coordinate pre- and postsynaptic actions of BDNF in synaptic enhancement (Alder et al., 2005; Gartner et al., 2006). Gartner et al. (2006) injected Sindbis virus into the CA1 or CA3 region to interfere with BDNF-initiated PLCγ signaling specifically at pre- or postsynaptic sites, suggesting that concurrent pre- and postsynaptic stimulation of PLCγ is required for LTP. Evidence from the present study suggests that BDNF effects on in vitro classical conditioning induce both pre- and postsynaptic mechanisms. This conclusion is based on several lines of evidence including localized microinjection of BDNF Ab, Western blot and immunocytochemical data. The expression of two presynaptic proteins, synaptophysin and synapsin I, was found to be increased in preparations conditioned for two sessions, which was blocked by TrkB Ab, but not TrkA and TrkC Ab. Enhanced levels of these two presynaptic proteins were also observed in BDNF-treated preparations. The increase in synaptophysin expression was further confirmed by immunocytochemical findings showing increased synaptophysin puncta on abducens motor neurons after BDNF treatment, which was counteracted by co-application of BDNF and K252a. In addition to presynaptic proteins, postsynaptic GluR4 puncta, as well as synaptic incorporation of GluR1- and GluR4-containing AMPARs, was also increased after BDNF treatment, demonstrating a role for BDNF in postsynaptic modifications during in vitro conditioning. Another strong argument for the pre-and postsynaptic actions of BDNF comes from experiments in which either antero- or retrograde injections of BDNF Ab resulted in prevention of CR acquisition. Antero- and retrograde injections of BDNF Ab were used to selectively suppress the function of pre- and postsynaptic BDNF. Antibodies were taken up by nerve roots and transported to either pre- or postsynaptic targets. Immunocytochemical data verified these injections by showing that BDNF Ab was confined to either presynaptic terminals or postsynaptic abducens motor neurons. A wealth of evidence has suggested that BDNF is transported transsynaptically. Moreover, BDNF could act on TrkB receptors localized pre- and/or postsynaptically regardless of the source of BDNF release. In our experiments, BDNF could have been released presynaptically and diffused to the postsynaptic side (Kohara et al., 2001), or postsynaptically to affect the presynaptic side (for reviews, see Tao and Poo, 2001; Magby et al., 2006). However, even if transsynaptic effects of BDNF normally occur in this preparation, functional BDNF from the opposite side of the synapse was insufficient to rescue conditioning following BDNF suppression on one side.
BDNF-induced trafficking of GluR1 and GluR4 AMPARs
We reported previously that preexisting GluR1 AMPAR subunits are delivered to silent synapses during early stages of conditioning, followed by synthesis and insertion of GluR4 subunits that support CR acquisition (Mokin et al., 2007). In the present study, bath application of BDNF for the equivalent of two pairing sessions induced the same postsynaptic glutamate receptor modifications that was observed after two sessions of conditioning. BDNF treatment led to synaptic insertion of both GluR1 and GluR4 subunits while the synthesis of GluR4, but not GluR1 subunits (as determined by absolute number of puncta), was found to be increased in BDNF-treated preparations. These effects could be blocked by K252a, further supporting the role for BDNF in trafficking of GluR1 and GluR4 AMPAR subunits. BDNF-induced AMPAR trafficking has been observed elsewhere (Caldeira et al., 2007), but the molecular mechanisms involved remain largely unknown. It was reported recently that delivery of AMPARs induced by BDNF is dependent on Ca2+ influx from IP3-sensitive internal stores (Nakata and Nakamura, 2007). Our previous results have indicated that CR acquisition after two pairing sessions induced the expression of ERK, but not P38 MAPK, and conditioning-related synaptic insertion of GluR4 AMPAR subunits was inhibited by the MEK-ERK MAPK antagonist PD98059 (Keifer et al., 2007). Consistent with these results, the present observations indicate that BDNF treatment resulted in enhanced expression of ERK, but not P38 MAPK. More recent findings implicate ERK in synaptic incorporation of GluR1-containing AMPARs early in conditioning, followed by PKC and ERK-mediated GluR4 subunit insertion (Zheng and Keifer, submitted), consistent with the present findings that BDNF induces ERK expression and synaptic insertion of both GluR1 and GluR4 subunits. BDNF-induced activation of ERK is also required for hippocampal LTP (Ying et al., 2002) and long-term facilitation in Aplysia (Sharma et al., 2006).
Possible cooperative effects of BDNF and NO in in vitro conditioning
It has been shown that NO, like BDNF, contributes widely to synaptic plasticity through pre- and postsynaptic modifications (for a review, see Prast and Philippu, 2001). NO promotes neurotransmitter release through a cGMP-dependent mechanism (Arancio et al., 1996), and may modulate postsynaptic signaling cascades necessary for the induction of synaptic potentiation (Ko and Kelly, 1999). Interestingly, NO behaves in a manner similar to the bidirectional signaling properties of BDNF (Arancio et al., 1996; Shibuki and Kimura, 1997). Like BDNF that could be elicited by glutamate (Griesbeck et al., 1999), NO release is generally linked to the activation of NMDA receptors, which induces influx of Ca2+ and consequently activation of NOS (for a review, see Garthwaite and Boulton, 1995). Besides the involvement of BDNF, the present study using the NOS inhibitor 7-NI also suggests that NO is required for in vitro conditioning.
Acknowledgements
We thank Dr. Frances Day for assistance with the confocal microscopy. Supported by National Institutes of Health grants NS-051187 and P20 RR-015567 which is designated as a Center of Biomedical Research Excellence (COBRE) to J. K.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alder J, Thakker-Varia S, Crozier RA, Shaheen A, Plummer MR, Black IB. Early presynaptic and late postsynaptic components contribute independently to brain-derived neurotrophic factor-induced synaptic plasticity. J Neurosci. 2005;25:3080–3085. doi: 10.1523/JNEUROSCI.2970-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CW, Keifer J. The cerebellum and red nucleus are not required for in vitro classical conditioning of the turtle abducens nerve response. J Neurosci. 1997;17:9736–9745. doi: 10.1523/JNEUROSCI.17-24-09736.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CW, Keifer J. Properties of conditioned abducens nerve responses in a highly reduced in vitro brain stem preparation from the turtle. J Neurophysiol. 1999;81:1242–1250. doi: 10.1152/jn.1999.81.3.1242. [DOI] [PubMed] [Google Scholar]
- Arancio O, Kiebler M, Lee CJ, Lev-Ram V, Tsien RY, Kandel E, Hawkins RD. Nitric oxide acts directly in the presynaptic terminal to produce long-term potentiation in cultured hippocampal neurons. Cell. 1996;87:1025–1035. doi: 10.1016/s0092-8674(00)81797-3. [DOI] [PubMed] [Google Scholar]
- Balkowiec A, Katz DM. Cellular mechanisms regulating activity-dependent release of native brain-derived neurotrophic factor from hippocampal neurons. J Neurosci. 2002;22:10399–10407. doi: 10.1523/JNEUROSCI.22-23-10399.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benítez-Temiño B, Morcuende S, Mentis GZ, de la Cruz RR, Pastor AM. Expression of Trk receptors in the oculomotor system of the adult cat. J Comp Neurol. 2004;473:538–552. doi: 10.1002/cne.20095. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol. 2005;76:99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Caldeira MV, Melo CV, Pereira DB, Carvalho R, Correia SS, Backos DS, Carvalho AL, Esteban JA, Duarte CB. Brain-derived neurotrophic factor regulates the expression and synaptic delivery of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor subunits in hippocampal neurons. J Biol Chem. 2007;282:12619–12628. doi: 10.1074/jbc.M700607200. [DOI] [PubMed] [Google Scholar]
- Carvalho AL, Caldeira MV, Santos SD, Duarte CB. Role of the brain-derived neurotrophic factor at glutamatergic synapses. Br J Pharmacol. 2007 doi: 10.1038/sj.bjp.0707509. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Kolbeck R, Barde YA, Bonhoeffer T, Kossel A. Relative contribution of endogenous neurotrophins in hippocampal long-term potentiation. J Neurosci. 1999;19:7983–7990. doi: 10.1523/JNEUROSCI.19-18-07983.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Luca M, Gardoni F, Finardi A, Pagliardini S, Cattabeni F, Battaglia G, Missale C. NMDA receptor subunits are phosphorylated by activation of neurotrophin receptors in PSD of rat spinal cord. Neuroreport. 2001;12:1301–1305. doi: 10.1097/00001756-200105080-00049. [DOI] [PubMed] [Google Scholar]
- Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- Garthwaite J, Boulton CL. Nitric oxide signaling in the central nervous system. Annu Rev Physiol. 1995;57:683–706. doi: 10.1146/annurev.ph.57.030195.003343. [DOI] [PubMed] [Google Scholar]
- Gärtner A, Polnau DG, Staiger V, Sciarretta C, Minichiello L, Thoenen H, Bonhoeffer T, Korte M. Hippocampal long-term potentiation is supported by presynaptic and postsynaptic tyrosine receptor kinase B-mediated phospholipase Cγ signaling. J Neurosci. 2006;26:3496–3504. doi: 10.1523/JNEUROSCI.3792-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooney M, Lynch MA. Long-term potentiation in the dentate gyrus of the rat hippocampus is accompanied by brain-derived neurotrophic factor-induced activation of TrkB. J Neurochem. 2001;77:1198–1207. doi: 10.1046/j.1471-4159.2001.00334.x. [DOI] [PubMed] [Google Scholar]
- Gottschalk W, Pozzo-Miller LD, Figurov A, Lu B. Presynaptic modulation of synaptic transmission and plasticity by brain-derived neurotrophic factor in the developing hippocampus. J Neurosci. 1998;18:6830–6839. doi: 10.1523/JNEUROSCI.18-17-06830.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesbeck O, Canossa M, Campana G, Gartner A, Hoener MC, Nawa H, Kolbeck R, Thoenen H. Are there differences between the secretion characteristics of NGF and BDNF? Implications for the modulatory role of neurotrophins in activity-dependent neuronal plasticity. Microc Res Technol. 1999;45:262–275. doi: 10.1002/(SICI)1097-0029(19990515/01)45:4/5<262::AID-JEMT10>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Gruart A, Sciarretta C, Valenzuela-Harrington M, Delgado-Garcia JM, Minichiello L. Mutation at the TrkB PLCγ-docking site affects hippocampal LTP and associative learning in conscious mice. Learn Mem. 2007;14:54–62. doi: 10.1101/lm.428307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubellini P, Ben-Ari Y, Gaiarsa JL. Endogenous neurotrophins are required for the induction of GABAergic long-term potentiation in the neonatal rat hippocampus. J Neurosci. 2005;25:5796–5802. doi: 10.1523/JNEUROSCI.0824-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nat Neurosci. 2000;3:533–535. doi: 10.1038/75698. [DOI] [PubMed] [Google Scholar]
- Jovanovic JN, Czernik AJ, Fienberg AA, Greengard P, Sihra TS. Synapsins as mediators of BDNF-enhanced neurotransmitter release. Nat Neurosci. 2000;3:323–329. doi: 10.1038/73888. [DOI] [PubMed] [Google Scholar]
- Kang H, Schuman EM. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- Kang H, Welcher AA, Shelton D, Schuman EM. Neurotrophins and time: Different roles for TrkB signaling in hippocampal long-term potentiation. Neuron. 1997;19:653–664. doi: 10.1016/s0896-6273(00)80378-5. [DOI] [PubMed] [Google Scholar]
- Keifer J. In vitro eye-blink reflex model: role of excitatory amino acids and labeling of network activity with sulforhodamine. Exp Brain Res. 1993;97:239–253. doi: 10.1007/BF00228693. [DOI] [PubMed] [Google Scholar]
- Keifer J. In vitro eye-blink classical conditioning is NMDA receptor dependent and involves redistribution of AMPA receptor subunit GluR4. J Neurosci. 2001;21:2434–2441. doi: 10.1523/JNEUROSCI.21-07-02434.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keifer J. In vitro classical conditioning of the turtle eyeblink reflex: approaching cellular mechanisms of acquisition. Cerebellum. 2003;2:55–61. doi: 10.1080/14734220310015610. [DOI] [PubMed] [Google Scholar]
- Keifer J, Armstrong KE, Houk JC. In vitro classical conditioning of abducens nerve discharge in turtles. J Neurosci. 1995;15:5036–5048. doi: 10.1523/JNEUROSCI.15-07-05036.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keifer J, Clark TG. Abducens conditioning in in vitro turtle brain stem without cerebellum requires NMDA receptors and involves upregulation of GluR4-containing AMPA receptors. Exp Brain Res. 2003;151:405–410. doi: 10.1007/s00221-003-1494-5. [DOI] [PubMed] [Google Scholar]
- Keifer J, Mokin M. Distribution of anterogradely labeled trigeminal and auditory nerve boutons on abducens motor neurons in turtles: Implications for in vitro classical conditioning. J Comp Neurol. 2004;471:144–152. doi: 10.1002/cne.20032. [DOI] [PubMed] [Google Scholar]
- Keifer J, Zheng Z, Zhu D. MAPK signaling pathways mediate AMPA receptor trafficking in an in vitro model of classical conditioning. J Neurophysiol. 2007;97:2067–2074. doi: 10.1152/jn.01154.2006. [DOI] [PubMed] [Google Scholar]
- Ko GY, Kelly PT. Nitric oxide acts as a postsynaptic signaling molecule in calcium/calmodulin-induced synaptic potentiation in hippocampal CA1 pyramidal neurons. J Neurosci. 1999;19:6784–6794. doi: 10.1523/JNEUROSCI.19-16-06784.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohara K, Kitamura A, Morishima M, Tsumoto T. Activity-dependent transfer of brain-derived neurotrophic factor to postsynaptic neurons. Science. 2001;291:2419–2423. doi: 10.1126/science.1057415. [DOI] [PubMed] [Google Scholar]
- Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci. 1995;92:8856–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte M, Griesbeck O, Gravel C, Carroll P, Staiger V, Thoenen H, Bonhoeffer T. Virus-mediated gene transfer into hippocampal CA1 region restores long-term potentiation in brain-derived neurotrophic factor mutant mice. Proc Natl Acad Sci. 1996;93:12547–12552. doi: 10.1073/pnas.93.22.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte M, Kang H, Bonhoeffer T, Schuman E. A role for BDNF in the late-phase of hippocampal long-term potentiation. Neuropharmacology. 1998;37:553–559. doi: 10.1016/s0028-3908(98)00035-5. [DOI] [PubMed] [Google Scholar]
- Kovalchuk Y, Hanse E, Kafitz KW, Konnerth A. Postsynaptic induction of BDNF-mediated long-term potentiation. Science. 2002;295:1729–1734. doi: 10.1126/science.1067766. [DOI] [PubMed] [Google Scholar]
- Levine ES, Crozier RA, Black IB, Plummer MR. Brain-derived neurotrophic factor modulates hippocampal synaptic transmission by increasing N-methyl-d-aspartic acid receptor activity. Proc Natl Acad Sci. 1998;95:10235–10239. doi: 10.1073/pnas.95.17.10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SY, Wu K, Levine ES, Mount HT, Suen PC, Black IB. BDNF acutely increase tyrosine phosphorylation of the NMDA receptor subunit 2B in cortical and hippocampal postsynaptic densities. Brain Res Mol Brain Res. 1998;55:20–27. doi: 10.1016/s0169-328x(97)00349-5. [DOI] [PubMed] [Google Scholar]
- Lu Y, Christian K, Lu B. BDNF: A key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiol Learn Mem. 2008;89:312–323. doi: 10.1016/j.nlm.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Reis G, Parada LF, Schuman EM. Neuronal NT-3 is not required for synaptic transmission or long-term potentiation in area CA1 of the adult rat hippocampus. Learn Mem. 1999;6:267–275. [PMC free article] [PubMed] [Google Scholar]
- Ma YL, Wang HL, Wu HC, Wei CL, Lee HH. Brain-derived neurotrophic factor antisense oligonucleotide impairs memory retention and inhibits long-term potentiation in rats. Neuroscience. 1998;82:957–967. doi: 10.1016/s0306-4522(97)00325-4. [DOI] [PubMed] [Google Scholar]
- Magby JP, Bi C, Chen ZY, Lee FS, Plummer MR. Single-cell characterization of retrograde signaling by brain-derived neurotrophic factor. J Neurosci. 2006;26:13531–13536. doi: 10.1523/JNEUROSCI.4576-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoudi E, Bardsen K, Srebro B, Bramham CR. Acute intrahippocampal infusion of BDNF induces lasting potentiation of synaptic transmission in the rat dentate gyrus. J Neurophysiol. 1998;79:496–499. doi: 10.1152/jn.1998.79.1.496. [DOI] [PubMed] [Google Scholar]
- Messaoudi E, Ying SW, Kanhema T, Croll SD, Bramham CR. Brain-derived neurotrophic factor triggers transcription-dependent, late phase long-term potentiation in vivo. J Neurosci. 2002;22:7453–7461. doi: 10.1523/JNEUROSCI.22-17-07453.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokin M, Lindahl JS, Keifer J. Immediate-early gene-encoded protein Arc is associated with synaptic delivery of GluR4-containing AMPA receptors during in vitro classical conditioning. J Neurophysiol. 2006;95:215–224. doi: 10.1152/jn.00737.2005. [DOI] [PubMed] [Google Scholar]
- Mokin M, Keifer J. Targeting of GluR4-containing AMPA receptors to synaptic sites during in vitro classical conditioning. Neuroscience. 2004;128:219–228. doi: 10.1016/j.neuroscience.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Mokin M, Keifer J. Quantitative analysis of immunofluorescent punctate staining of synaptically localized proteins using confocal microscopy and stereology. J Neurosci Methods. 2006;157:218–224. doi: 10.1016/j.jneumeth.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Mokin M, Zheng Z, Keifer J. Conversion of silent synapses into the active pool by selective GluR1-3 and GluR4 AMPA trafficking during in vitro classical conditioning. J Neurophysiol. 2007;98:1178–1286. doi: 10.1152/jn.00212.2007. [DOI] [PubMed] [Google Scholar]
- Nakata H, Nakamura S. Brain-derived neurotrophic factor regulates AMPA receptor trafficking to post-synaptic densities via IP3R and TRPC calcium signaling. FEBS Lett. 2007;58:2047–2054. doi: 10.1016/j.febslet.2007.04.041. [DOI] [PubMed] [Google Scholar]
- Patterson SL, Abel T, Deuel TA, Martin KC, Rose JC, Kandel ER. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- Perrett SP, Ruiz BP, Mauk MD. Cerebellar cortex lesions disrupt learning-dependent timing of conditioned eyelid responses. J Neurosci. 1993;13:1708–1718. doi: 10.1523/JNEUROSCI.13-04-01708.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzo-Miller LD, Gottschalk W, Zhang L, McDermott K, Du J, Gopalakrishnan R, Oho C, Sheng ZH, Lu B. Impairments in high-frequency transmission, synaptic vesicle docking, and synaptic protein distribution in the hippocampus of BDNF knockout mice. J Neurosci. 1999;19:4972–4983. doi: 10.1523/JNEUROSCI.19-12-04972.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prast H, Philippu A. Nitric oxide as modulator of neuronal function. Prog Neurobiol. 2001;64:51–68. doi: 10.1016/s0301-0082(00)00044-7. [DOI] [PubMed] [Google Scholar]
- Rattiner LM, Davis M, French CT, Ressler KJ. Brain-derived neurotrophic factor and tyrosine kinase receptor B involvement in amygdala-dependent fear conditioning. J Neurosci. 2004;24:4796–4806. doi: 10.1523/JNEUROSCI.5654-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahun I, Delgado-Garcia JM, Amador-Arjona A, Giralt A, Alberch J, Dierssen M, Gruart A. Dissociation between CA3-CA1 synaptic plasticity and associative learning in TgNTRK3 transgenic mice. J Neurosci. 2007;27:2253–2260. doi: 10.1523/JNEUROSCI.4055-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SK, Sherff CM, Stough S, Hsuan V, Carew TJ. A tropomyosin-related kinase B ligand is required for ERK activation, long-term synaptic facilitation, and long-term memory in Aplysia. Proc Natl Acad Sci. 2006;103:14206–14210. doi: 10.1073/pnas.0603412103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuki K, Kimura S. Dynamic properties of nitric oxide release from parallel fibers in rat cerebellar slices. J Physiol. 1997;498:443–452. doi: 10.1113/jphysiol.1997.sp021870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao HW, Poo M. Retrograde signaling at central synapses. Proc Natl Acad Sci. 2001;98:11009–11015. doi: 10.1073/pnas.191351698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WJ, Zhang XL, Hartman K, Winterer J, Muller W, Stanton PK, Pozzo-Miller L. BDNF increases release probability and the size of a rapidly recycling vesicle pool within rat hippocampal excitatory synapses. J Physiol. 2006;574:787–803. doi: 10.1113/jphysiol.2006.111310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Gottschalk W, Chow A, Wilson RI, Schnell E, Zang K, Wang D, Nicoll RA, Lu B, Reichardt LF. The role of brain-derived neurotrophic factor receptors in the mature hippocampus: Modulation of long-term potentiation through a presynaptic mechanisms involving TrkB. J Neurosci. 2000;20:6888–6897. doi: 10.1523/JNEUROSCI.20-18-06888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying S-W, Futter M, Rosenblum K, Webber MJ, Hunt SP, Bliss TVP, Bramham CR. Brain-derived neurotrophic factor induces long-term potentiation in intact adult hippocampus: Requirement for ERK activation coupled to CREB and upregulation of Arc synthesis. J Neurosci. 2002;22:1532–1540. doi: 10.1523/JNEUROSCI.22-05-01532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharenko SS, Patterson SL, Dragatsis I, Zeitlin SO, Siegelbaum SA, Kandel ER, Morozov A. Presynaptic BDNF required for a presynaptic but not postsynaptic component of LTP at hippocampal CA1–CA3 synapses. Neuron. 2003;39:975–990. doi: 10.1016/s0896-6273(03)00543-9. [DOI] [PubMed] [Google Scholar]
- Zhu D, Keifer J. Pathways controlling trigeminal and auditory nerve-evoked abducens eyeblink reflexes in pond turtles. Brain Behav Evol. 2004;64:207–222. doi: 10.1159/000080242. [DOI] [PubMed] [Google Scholar]