Abstract
Recent studies have indicated that chemoautotrophic Epsilonproteobacteria might play an important role, especially as anaerobic or microaerophilic dark CO2-fixing organisms, in marine pelagic redoxclines. However, knowledge of their distribution and abundance as actively CO2-fixing microorganisms in pelagic redoxclines is still deficient. We determined the contribution of Epsilonproteobacteria to dark CO2 fixation in the sulfidic areas of central Baltic Sea and Black Sea redoxclines by combining catalyzed reporter deposition-fluorescence in situ hybridization with microautoradiography using [14C]bicarbonate and compared it to the total prokaryotic chemoautotrophic activity. In absolute numbers, up to 3 × 105 14CO2-fixing prokaryotic cells ml−1 were enumerated in the redoxcline of the central Baltic Sea and up to 9 × 104 14CO2-fixing cells ml−1 were enumerated in the Black Sea redoxcline, corresponding to 29% and 12%, respectively, of total cell abundance. 14CO2-incorporating cells belonged exclusively to the domain Bacteria. Among these, members of the Epsilonproteobacteria were approximately 70% of the cells in the central Baltic Sea and up to 100% in the Black Sea. For the Baltic Sea, the Sulfurimonas subgroup GD17, previously assumed to be involved in autotrophic denitrification, was the most dominant CO2-fixing group. In conclusion, Epsilonproteobacteria were found to be mainly responsible for chemoautotrophic activity in the dark CO2 fixation maxima of the Black Sea and central Baltic Sea redoxclines. These Epsilonproteobacteria might be relevant in similar habitats of the world's oceans, where high dark CO2 fixation rates have been measured.
Pelagic redoxclines represent the transition zones between the oxic and anoxic realms. Extensive pelagic redoxclines are reported for the Black Sea (18, 34, 36), the Cariaco Basin (40, 42), the Framvaren Fjord (27), the Mariager Fjord (45), the Baltic Sea (19), and freshwater lakes (6, 9, 14) and are often characterized by high dark CO2 fixation rates. Characteristically, the peak of dark CO2 fixation within the water column is often located below the chemocline, which we define as the shallowest appearance of sulfide (13). Epsilonproteobacteria have already been suggested to be involved in chemoautotrophic production at several marine pelagic redoxclines (21, 23, 26, 43), and their prevalence at oxic-anoxic transition zones, especially in areas with previously detected high chemoautotrophic activity, has been demonstrated for the Black Sea and the Cariaco Basin (23, 24, 26, 43). Whether Epsilonproteobacteria are indeed responsible for chemoautotrophic production, however, has not been shown for any of these habitats.
For the Baltic Sea, Jost et al. (19) determined the abundance of chemoautotrophic cells in waters below the chemocline by combining dark CO2 fixation measurements and flow-cytometric cell sorting. Two main prokaryotic cell clusters responsible for total dark CO2 fixation were detected, and 20 to 40% of the total prokaryotic community was estimated to be chemoautotrophic, but the phylogenetic identity of these clusters remained unknown. Recently, Glaubitz et al. (10) determined the diversity of chemoautotrophs using stable-isotope probing (rRNA-SIP) combined with fingerprint techniques in samples from the dark CO2 fixation maximum in the central Baltic Sea. Autotrophic activity of Gammaproteobacteria and Epsilonproteobacteria, mainly represented by the Sulfurimonas subgroup GD17, which previously has been found to be abundant in Baltic Sea redoxclines (4, 13, 21), was detected. However, these studies did not address the quantitative impact of Epsilonproteobacteria for chemoautotrophic activity in the central Baltic Sea. Consequently, the objective of the present work was to determine the contribution of Epsilonproteobacteria to dark CO2 fixation in sulfidic areas of marine redoxclines of the central Baltic Sea and the Black Sea.
For identification and quantification of metabolically active cells in environmental samples, the combination of fluorescence in situ hybridization (FISH) and microautoradiography has often been used (7, 12, 22). Here we used the more-sensitive catalyzed reporter deposition (CARD)-FISH protocol combined with microautoradiography (MICRO-CARD-FISH) to assess the specific uptake of radiolabeled bicarbonate by prokaryotic cells (41). Our results demonstrate, for the first time, with special emphasis on Epsilonproteobacteria, the quantitative distribution of CO2-fixing cells in the sulfidic areas of two marine redoxclines.
MATERIALS AND METHODS
Sampling.
The samples were obtained from the central Baltic Sea during a research cruise onboard the RV Professor Albrecht Penck in April 2007 (station 271; 57°19.2′N, 20°03′E; Gotland Deep) and from the Black Sea during a research cruise onboard the RV Meteor in May 2007 (station 21; 42°45′N, 37°30′E). Water samples from the redoxclines were collected with free-flow bottles (Hydrobios) attached to a conductivity, temperature, and depth rosette (SBE 911+; Seabird). Concentrations of inorganic nutrients, oxygen, and hydrogen sulfide were analyzed as described by Grasshoff et al. (11).
Microautoradiographic incubations and dark CO2 fixation measurements.
Incubations for MICRO-CARD-FISH and the determination of dark CO2 fixation rates started within one hour after collecting the samples from the free-flow bottles. Special care was taken to avoid oxygen contamination during sample collection. Therefore, samples were directly filled from free-flow bottles into the test tubes with an overflow of at least five tube volumes. In the Baltic Sea, samples were taken from three depths, and in the Black Sea, from six depths. One hundred μCi of [14C]bicarbonate in anoxic solution (specific activity, 53.0 mCi mmol−1; Hartmann Analytic GmbH, Braunschweig, Germany) was added to 9-ml glass test tubes containing the water samples. Thereafter, the tubes were sealed without headspace with glass stoppers. Controls were fixed with paraformaldehyde (2% final concentration) before the addition of the radiolabeled solution. After incubation at in situ temperatures (4°C for the Baltic Sea samples and 8°C for the Black Sea samples) for 24 h in the dark, 1-ml subsamples were filtered through 0.2-μm-pore-size cellulose nitrate filters (diameter, 25 mm; Purabind 02; Whatman) and exposed to HCl fumes, and the radioactivity of the individual filters was counted in a liquid scintillation counter (Packard). The activity ranged between 99 and 6,707 dpm ml−1 of filtered sample. For MICRO-CARD-FISH, the remaining volume was immediately fixed with particle-free paraformaldehyde (2% final concentration) for 12 to 18 h at 4°C. Portions of 1.5 to 4 ml were filtered onto white polycarbonate membrane filters (type GTTP; pore size, 0.2 μm; diameter, 25 mm; Millipore). The filters were then carefully rinsed with sterile Milli-Q water, air dried, and stored at −80°C until further processing. The total inorganic carbon content of both habitats was determined by a coulometric single-operator multimetabolic analyzer (SOMMA) system (17). For samples from the Black Sea, the mean inorganic carbon concentration accounted for 3,300 μmol/kg; for those from the Baltic Sea, the concentration was 1,950 μmol/kg. Dark CO2 fixation rates were calculated from the total inorganic carbon concentration, the initial amount of [14C]bicarbonate added, and the amount of 14CO2 fixed in biomass during the incubation time.
CARD-FISH.
CARD-FISH was carried out according to the protocols of Pernthaler et al. (33) and Sekar et al. (35), modified as described previously (13). For enumeration of Bacteria, a mix of probes EUB338 (GCTGCCTCCCGTAGGAGT) (3), EUB338-II (GCAGCCACCCGTAGGTGT), and EUB338-III (GCTGCCACCCGTAGGTGT) (8) was used. Epsilonproteobacteria were detected with probe EPS914 (25), and the specific Sulfurimonas subgroup GD17 was detected with probe SUL90 (CGTGCGCCACTAATCATA) (13). Probe EPS914 (GGTCCCCGTCTATTCCTT) was successfully tested for its specificity as described previously (13). Nonspecific binding was determined using the NonEUB probe (ACTCCTACGGGAGGCAGC) (44). Negative control counts with probe NonEUB averaged 0.08 to 0.6% for both the Baltic Sea and the Black Sea samples, implying that cell counts in the same order of magnitude could be considered negligible. For hybridization, 400 μl of hybridization buffer (55% formamide for all probes) and 2 μl of probe working solution (50 pmol μl−1) were mixed. For probe mix EUB338I-III, 600 μl of hybridization buffer and 3 μl of probe working solution were used. Hybridization was carried out at 35°C for 8 to 12 h on a rotary shaker in the dark. After the filter sections had been washed in a prewarmed washing buffer (37°C) for at least 10 min, the tyramide signal amplification with 5- and 6-carboxyfluorescein-labeled tyramides was carried out for 15 min in the dark on a rotary shaker. The filters were then washed first in phosphate-buffered saline and afterwards in ethanol and then air dried.
Microautoradiography.
The autoradiographic procedure followed the protocol of Teira et al. (41), modified according to Alonso and Pernthaler (1). Hybridized filter sections were glued onto glass slides (UHU Plus Sofortfest; UHU GmbH, Germany), which were then dipped into the photographic emulsion (Kodak; type NTB-2; melted at 43°C for 20 min) and placed in a light-tight box with silica gel as a drying agent. Optimal exposure times at 4°C were 2 days for the Baltic Sea samples and 3 days for the Black Sea samples. The slides were developed (Dektol developer) and fixed (Kodak fixer) according to the specifications of Kodak. The completely dry filter sections were counterstained with the previously described mixture of 4′,6′-diamidino-2-phenylindole (DAPI), Citifluor, and VectaShield (33).
Microscopy.
Filter sections were examined with an epifluorescence microscope (Axioskop 2 MOT Plus; Zeiss) equipped with a 100× Plan Apochromat oil objective lens (Zeiss) and appropriate filter sets for DAPI and fluorescein isothiocyanate (FITC). The transmission mode of the microscope allowed the detection of silver grains attached to cells. Cells associated with two or more silver grains were defined as 14CO2 positive. In negative control samples, the percentage of cells attached to at least two silver grains accounted, on average, for only 1% of all DAPI-stained cells for the Baltic Sea redoxcline and 0.6% for the Black Sea redoxcline. Switching between fluorescence and transmission modes allowed probe-hybridized 14CO2-positive and inactive cells to be counted directly, followed by the determination of DAPI-stained cells to estimate the total prokaryotic abundance. Between 500 and 1,000 DAPI-stained cells in randomly distributed microscopic fields were counted for each filter section. Bacterial counting of DAPI-stained samples and hybridized samples is usually done with a standard deviation of less than 7.5% for replicates of the central Baltic Sea redoxcline (13).
RESULTS
Bacterial abundance and activity in the Baltic Sea.
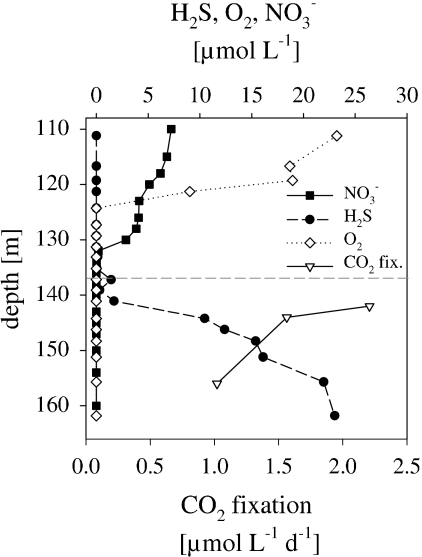
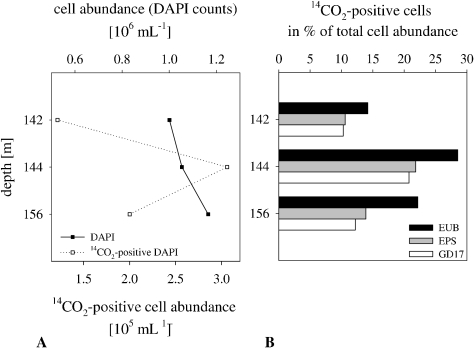
The chemocline, defined as the shallowest appearance of hydrogen sulfide, was located at a depth of 136 m in the Gotland Deep in April 2007 (Fig. 1). Oxygen was not detected below a depth of 121 m; nitrate was below the detection limit at a depth below 132 m. Dark CO2 fixation rates below the chemocline ranged from 1.0 to 2.2 μmol of C liter−1 day−1; the highest rate was measured at a depth of 142 m. Prokaryotic abundance, determined by DAPI, remained constant across the three depth layers, at around 1 × 106 cells ml−1 (Fig. 2A). MICRO-CARD-FISH was applied to visualize the 14CO2-fixing activity of individual prokaryotic cells, and 14CO2-positive cells were enumerated for the central Baltic Sea. 14CO2-positive cells, ranging between 1.2 × 105 and 3.1 × 105 cells ml−1, accounted for 12.2 to 29.0% of the total cell abundance (Fig. 2A). Nearly all 14CO2-positive cells were positive for probe EUB338I-III and were consequently assigned to Bacteria. The use of probe EPS914, specific for Epsilonproteobacteria, revealed that 14CO2-positive Epsilonproteobacteria contributed between 63 and 77% of the total number of 14CO2-fixing Bacteria at all depths examined. Hence, in the Gotland Deep, Bacteria taking up 14CO2 belonged mainly to Epsilonproteobacteria and, more specifically, to the Sulfurimonas subgroup GD17 (Fig. 2B). Generally, the abundance of GD17 cells with respect to total cell abundance was in the same order of magnitude as for Epsilonproteobacteria; thus, members of group GD17 predominated within this group (Table 1). Concerning the proportion of 14CO2-positive GD17 cells, no more than 65% of total GD17 cells were 14CO2 fixing at all depths examined (Table 1).
FIG. 1.
Concentrations of H2S, O2, and NO3− and dark CO2 fixation rates in the central Baltic Sea redoxcline in April 2007. The dashed horizontal line indicates the chemocline.
FIG. 2.
Abundances of total and 14CO2-assimilating prokaryotic cells (DAPI) (A) and percentages of 14CO2-assimilating Bacteria (EUB), Epsilonproteobacteria (EPS), and group GD17 cells (GD17) with respect to total cell abundance, determined by MICRO-CARD-FISH (B), in the sulfidic area of the central Baltic Sea in April 2007.
TABLE 1.
Proportion of Bacteria, Epsilonproteobacteria, and group GD17 cells as percentages of total cell abundance (DAPI counts) in the Baltic Sea and Black Sea redoxclinesa
| Location | CARD-FISH
|
MICRO-CARD-FISH
|
||||
|---|---|---|---|---|---|---|
| Depth (m) | EUB (%) | EPS (%) | GD17 (%) | 14CO2-positive EPS (%) | 14CO2-positive GD17 (%) | |
| Baltic Sea | 142 | 70.8 | 16.9 | 18.1 | 62.8 | 56.8 |
| 144 | 59.9 | 27.9 | 31.8 | 78.2 | 65.4 | |
| 156 | 64.3 | 21.7 | 19.7 | 64.0 | 62.0 | |
| Black Sea | 110 | 54.1 | 21.0 | 1.9 | 33.0 | 3.5 |
| 115 | 58.5 | 12.2 | 1.6 | 33.6 | 10.7 | |
| 120 | 52.1 | 35.1 | 4.9 | 41.9 | 32.7 | |
| 125 | 54.7 | 10.9 | — | 61.0 | — | |
| 130 | 56.8 | 2.9 | — | 41.9 | — | |
| 135 | 26.6 | 3.0 | — | 9.3 | — | |
The percentages of 14CO2-positive Epsilonproteobacteria with respect to total epsilonproteobacterial cell numbers and the percentages of 14CO2-positive GD17 cells with respect to total GD17 abundance are also given. Results are based, respectively, on CARD-FISH and MICRO-CARD-FISH analyses. EUB, Bacteria; EPS, Epsilonproteobacteria; —, below detection limit.
Bacterial abundance and activity in the Black Sea.
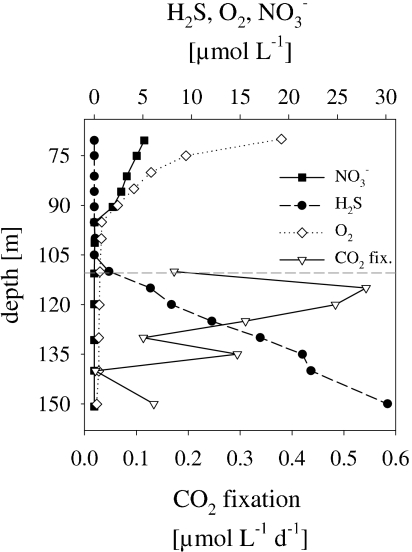
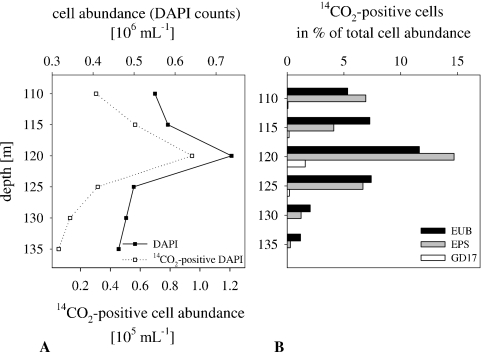
In the Black Sea redoxcline, the chemocline was located at a depth of 110 m (Fig. 3). Nitrate and oxygen were below the detection limit at a depth of more than 90 m. The pronounced maximum of dark CO2 fixation rates was detected at a depth of 115 m (Fig. 3). Total prokaryotic abundance ranged between 0.45 × 106 and 0.72 × 106 cells ml−1; between 0.1 × 105 and 0.95 × 105 cells ml−1 were identified as 14CO2 positive, constituting up to 12.8% of total DAPI counts (Fig. 4A). As in the Baltic Sea, MICRO-CARD-FISH of Black Sea samples revealed that 14CO2-fixing activity was exclusively due to Bacteria, i.e., EUB338I-III-positive cells. Epsilonproteobacteria taking up 14CO2 constituted 24 to 100% of all 14CO2-positive cells, indicating their dominance in dark CO2 fixation among Bacteria (Fig. 4B; also see Fig. S1 in the supplemental material). 14CO2-positive GD17 cells were only detected at a depth of 120 m and amounted to only 1.6% of all DAPI-stained cells. The contribution of group GD17 cells to total cell abundance was low and below the detection limit for deeper layers (Table 1) but accounted for, on average, 12% of total epsilonproteobacterial abundance in the upper layers. 14CO2-assimilating cells within the Epsilonproteobacteria ranged from 33.0 to 61.0% of the total Epsilonproteobacteria in the zone characterized by the highest dark CO2 fixation rates (110 to 125 m) (Table 1).
FIG. 3.
Concentrations of H2S, O2, and NO3− and dark CO2 fixation rates in the Black Sea redoxcline in May 2007. The dashed horizontal line indicates the chemocline.
FIG. 4.
Abundances of total and 14CO2-assimilating prokaryotic cells (DAPI) (A) and percentages of 14CO2-assimilating Bacteria (EUB), Epsilonproteobacteria (EPS), and group GD17 cells (GD17) compared to total cell abundance, determined by MICRO-CARD-FISH (B), in the sulfidic area of the Black Sea in May 2007.
DISCUSSION
The results of this study provide direct evidence for the quantitative significance of Epsilonproteobacteria in dark CO2 fixation in sulfidic areas of two marine redoxclines. In the sulfidic area of the Baltic Sea as well as the Black Sea redoxcline, cells taking up 14CO2 were identified by MICRO-CARD-FISH as Bacteria; more specifically, they were mainly composed of Epsilonproteobacteria. In this study, the fraction of 14CO2-assimilating Bacteria in the Baltic Sea was two times higher than the highest corresponding percentage obtained for the Black Sea. Similarly, dark CO2 fixation rates for the Baltic Sea were substantially higher than those determined for the Black Sea, albeit comparable to previously reported rates (13, 18, 21, 37). The total abundance of Epsilonproteobacteria in the Black Sea and, more specifically of group GD17 cells in the Gotland Deep, was consistent with previous CARD-FISH results, which showed high abundance and a wide depth distribution (13, 24). Likewise, the layer with the highest abundance of CO2-fixing cells was located in the sulfidic area, with a sulfide concentration of 8 to 10 μmol liter−1 for both habitats. For the Baltic Sea, the number of cells taking up 14CO2 generally agreed with the calculations of Jost et al. (19), who estimated between 20 and 40% chemoautotrophic cells.
The importance of bacterial chemoautotrophic production, measured as dark CO2 fixation, has been discussed for different marine redoxclines in relation to phototrophic primary production (34, 39). Combining dark CO2 fixation measurements with the number of 14CO2-positive cells assessed by MICRO-CARD-FISH allows cell-specific dark CO2 fixation rates to be estimated for chemoautotrophic cells, assuming CO2 as the sole carbon source. The anaplerotic uptake of CO2 by heterotrophic bacteria was considered to be insignificant for chemoautotrophic CO2 fixation, as outlined by Jost et al. (19) and Taylor et al. (40). Based on these assumptions, cell-specific dark CO2 fixation rates would amount to 61 to 217 fg of C cell−1 day−1 for the Baltic Sea redoxcline and 61 to 115 fg of C cell−1 day−1 for the Black Sea redoxcline. These rates are unrealistically high and also are not in line with the cell-specific CO2 uptake rates of 10 to 25 fg of C cell−1 day−1 calculated for a Baltic Sea redoxcline by Jost et al. (19) after flow-cytometric sorting of 14CO2-labeled cell clusters. However, those authors based their calculation on a higher abundance of CO2-fixing cells than was found in the present study by using MICRO-CARD-FISH. It is possible that the amount of 14CO2-positive cells reported here was underestimated due to the detection limit of microautoradiography and to possible leakage of incorporated radioactivity during fixation and storage (31). Furthermore, protists that were grazing on chemoautotrophic bacteria during incubation or were harboring chemoautotrophic ecto- and endosymbiotic bacteria could have reduced the amount of 14CO2-positive cells visible after MICRO-CARD-FISH. It has been reported previously that the standard CARD-FISH procedure is destructive to protist cells (29).
Chemoautotrophic Archaea have recently been identified in mesopelagic waters of the North Atlantic (15) and elsewhere (16, 20). Almost all 14CO2-positive cells in the dark CO2 fixation maxima of the Baltic Sea and Black Sea redoxclines belonged to Bacteria, precluding a major archaeal contribution to the chemoautotrophic community in the sulfidic areas of these redoxclines. As mentioned above, for the Baltic Sea, Glaubitz et al. (10) investigated the incorporation of 13C into chemoautotrophic cells by rRNA-SIP. The authors could not identify autotrophic Archaea; however, autotrophic activity of Gammaproteobacteria in addition to that of Epsilonproteobacteria, mostly belonging to group GD17, was shown. Consequently, for the Baltic Sea, it is likely that the remaining proportion of unidentified 14CO2-fixing cells in the present study consisted, at least partly, of Gammaproteobacteria. However, hybridizations with probe GAM42a (28) did not detect chemoautotrophic cells in samples from the central Baltic Sea; however, this may have been due to sequence mismatch (our unpublished results) (2).
Campbell et al. (5) emphasized the potential role of Epsilonproteobacteria for biogeochemical cycles, especially at the oxic-anoxic interfaces. For deep-sea hydrothermal fields, chemoautotrophic activity by Epsilonproteobacteria has been demonstrated by different authors (30, 32, 38). Lin et al. (23, 24) showed elevated epsilonproteobacterial abundances but low epsilonproteobacterial 3H-leucine assimilation activity for the Cariaco Basin and proposed chemoautotrophic activity of Epsilonproteobacteria there. Epsilonproteobacteria constitute 75 to 100% of 14CO2-assimilating Bacteria in the layers of highest dark CO2 fixation in this study, and they apparently contributed substantially to chemoautotrophic production in both the Black Sea and Baltic Sea redoxclines and can be regarded as key organisms for chemoautotrophic production. Therefore, the question arises as to whether or not Epsilonproteobacteria are globally important chemoautotrophs in marine habitats where high dark CO2 fixation rates around redoxclines have been measured.
Among 14CO2-positive Epsilonproteobacteria, members of the Sulfurimonas subgroup GD17 were the dominant representatives in the sulfidic area of the Baltic Sea, in contrast to the rather low contribution of group GD17 to Epsilonproteobacteria in the Black Sea. Grote et al. (13) discussed the possible contribution of group GD17 to autotrophic activity, even though quantitative PCR data suggested a restricted high-activity zone of this group around the chemocline several meters above the dark CO2 fixation maximum. However, the results of this study evidence the major contribution of group GD17 to dark CO2 fixation in the sulfidic area of the Baltic Sea. Still, the prevailing metabolism for chemoautotrophy in marine sulfidic areas is unknown, and the availability of electron acceptors for chemoautotrophy has been discussed by several authors previously (13, 18, 40). Dark CO2 fixation driven by autotrophic denitrification with reduced sulfur compounds as electron donors is unlikely, since there was no evidence for a clear overlap between nitrate and hydrogen sulfide. The oxidation of reduced sulfur species combined with the reduction of particulate metal oxides might be possible in the Baltic Sea and in the Black Sea (18, 19). Members of group GD17 were initially regarded as sulfur-oxidizing denitrifiers, but the remaining high cell numbers in sulfidic nitrate-deprived waters suggested a possible metabolic versatility for this group (4, 13). Notably, the percentage of 14CO2-assimilating epsilonproteobacterial cells in layers with the highest dark CO2 fixation rates never exceeded 65% of total Epsilonproteobacteria, leaving 35% of the cells either metabolically inactive or heterotrophic. As mentioned above, Lin et al. (23) detected a significant number of heterotrophic Epsilonproteobacteria in the Cariaco Basin. These findings stress the potential for heterotrophic as well as autotrophic activity within the Epsilonproteobacteria at marine redoxclines. Moreover, considering the Baltic Sea redoxcline, with group GD17 as the dominant epsilonproteobacterial representative, it is likely that group GD17 is metabolically versatile, exhibiting chemoautotrophy and, potentially, heterotrophy. The ability of members of group GD17 to switch between heterotrophic and autotrophic metabolism could also explain its wide depth distribution, from the suboxic to the sulfidic layers, as reported by Grote et al. (13).
In conclusion, even though the entire ecophysiological capacity and metabolism of these Epsilonproteobacteria are not fully understood yet, this study demonstrates the major role of these bacteria in chemoautotrophic production in the sulfidic areas of redoxclines of the central Baltic Sea and Black Sea. Together with the results of Glaubitz et al. (10), who reported the transfer of chemoautotrophic production to the microeukaryotic community, our findings underline the importance of Epsilonproteobacteria for these habitats. Further studies in other sulfidic areas exhibiting high dark CO2 fixation rates will show whether Epsilonproteobacteria are also key players in inorganic carbon fixation in similar specific aquatic habitats worldwide.
Supplementary Material
Acknowledgments
We are very grateful to the captains and crews of the RV Professor Albrecht Penck and RV Meteor for their excellent support during sampling cruises. Data for the nitrate concentration in the Black Sea were provided by G. Lavik. We thank A. Loy for making available probe EPS914. The excellent technical assistance of H. Brockmöller, B. Sadkowiak, D. Setzkorn, and N. Schreiber is greatly appreciated.
This work was funded by the Leibniz-Institut für Ostseeforschung Warnemünde and by a DFG grant (LA 1466/4-1) to M.L.
Footnotes
Published ahead of print on 24 October 2008.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Alonso, C., and J. Pernthaler. 2005. Incorporation of glucose under anoxic conditions by bacterioplankton from coastal North Sea surface waters. Appl. Environ. Microbiol. 71:1709-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amann, R., and B. M. Fuchs. 2008. Single-cell identification in microbial communities by improved fluorescence in situ hybridization techniques. Nat. Rev. Microbiol. 6:339-348. [DOI] [PubMed] [Google Scholar]
- 3.Amann, R. I., B. J. Binder, R. J. Olson, S. W. Chisholm, R. Devereux, and D. A. Stahl. 1990. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl. Environ. Microbiol. 56:1919-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brettar, I., M. Labrenz, S. Flavier, J. Bötel, H. Kuosa, R. Christen, and M. G. Höfle. 2006. Identification of a Thiomicrospira denitrificans-like epsilonproteobacterium as a catalyst for autotrophic denitrification in the central Baltic Sea. Appl. Environ. Microbiol. 72:1364-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell, B. J., A. S. Engel, M. L. Porter, and K. Takai. 2006. The versatile ɛ-proteobacteria: key players in sulphidic habitats. Nat. Rev. Microbiol. 4:458-468. [DOI] [PubMed] [Google Scholar]
- 6.Casamayor, E. O., J. García-Cantizano, and C. Pedrós-Alió. 2008. Carbon dioxide fixation in the dark by photosynthetic bacteria in sulfide-rich stratified lakes with oxic-anoxic interfaces. Limnol. Oceanogr. 53:1193-1203. [Google Scholar]
- 7.Cottrell, M. T., and D. L. Kirchman. 2000. Natural assemblages of marine proteobacteria and members of the Cytophaga-Flavobacter cluster consuming low- and high-molecular-weight dissolved organic matter. Appl. Environ. Microbiol. 66:1692-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daims, H., A. Bruhl, R. Amann, K.-H. Schleifer, and M. Wagner. 1999. The domain-specific probe EUB338 is insufficient for the detection of all bacteria: development and evaluation of a more comprehensive probe set. Syst. Appl. Microbiol. 22:434-444. [DOI] [PubMed] [Google Scholar]
- 9.García-Cantizano, J., E. O. Casamayor, J. M. Gasol, R. Guerrero, and C. Pedrós-Alió. 2005. Partitioning of CO2 incorporation among planktonic microbial guilds and estimation of in situ specific growth rates. Microb. Ecol. 50:230-241. [DOI] [PubMed] [Google Scholar]
- 10.Glaubitz, S., T. Lüders, W.-R. Abraham, G. Jost, K. Jürgens, and M. Labrenz. 2008. 13C-isotope analyses reveal that chemolithoautotrophic Gamma- and Epsilonproteobacteria feed a microbial food web in a pelagic redoxcline of the central Baltic Sea. Environ. Microbiol. doi: 10.1111/j.1462-2920.2008.01770.x. [DOI] [PubMed]
- 11.Grasshoff, K., M. Erhardt, and K. Kremling. 1983. Methods of seawater analysis, vol. 2. Verlag Chemie, Weinheim, Germany.
- 12.Gray, N. D., R. Howarth, R. W. Pickup, J. G. Jones, and I. M. Head. 2000. Use of combined microautoradiography and fluorescence in situ hybridization to determine carbon metabolism in mixed natural communities of uncultured bacteria from the genus Achromatium. Appl. Environ. Microbiol. 66:4518-4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grote, J., M. Labrenz, B. Pfeiffer, G. Jost, and K. Jürgens. 2007. Quantitative distributions of Epsilonproteobacteria and a Sulfurimonas subgroup in pelagic redoxclines of the central Baltic Sea. Appl. Environ. Microbiol. 73:7155-7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hadas, O., R. Pinkas, and J. Erez. 2001. High chemoautotrophic primary production in Lake Kinneret, Israel: a neglected link in the carbon cycle of the lake. Limnol. Oceanogr. 46:1968-1976. [Google Scholar]
- 15.Herndl, G. J., T. Reinthaler, E. Teira, H. van Aken, C. Veth, A. Pernthaler, and J. Pernthaler. 2005. Contribution of Archaea to total prokaryotic production in the deep Atlantic Ocean. Appl. Environ. Microbiol. 71:2303-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ingalls, A. E., S. R. Shah, R. L. Hansman, L. I. Aluwihare, G. M. Santos, E. R. M. Druffel, and A. Pearson. 2006. Quantifying archaeal community autotrophy in the mesopelagic ocean using natural radiocarbon. Proc. Natl. Acad. Sci. USA 103:6442-6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson, K. M., K. D. Wills, D. B. Butler, W. K. Johnson, and C. S. Wong. 1993. Coulometric total carbon dioxide analysis for marine studies: maximizing the performance of an automated gas extraction system and coulometric detector. Mar. Chem. 44:167-187. [Google Scholar]
- 18.Jørgensen, B. B., H. Fossing, C. O. Wirsen, and H. W. Jannasch. 1991. Sulfide oxidation in the anoxic Black Sea chemocline. Deep-Sea Res. 38:S1083-S1103. [Google Scholar]
- 19.Jost, G., M. V. Zubkov, E. Yakushev, M. Labrenz, and K. Jürgens. 2008. High abundance and dark CO2 fixation of chemolithoautotrophic prokaryotes in anoxic waters of the Baltic Sea. Limnol. Oceanogr. 53:14-22. [Google Scholar]
- 20.Kirchman, D. L., H. Elifantz, A. I. Dittel, R. R. Malmstrom, and M. T. Cottrell. 2007. Standing stocks and activity of archaea and bacteria in the western Arctic Ocean. Limnol. Oceanogr. 52:495-507. [Google Scholar]
- 21.Labrenz, M., G. Jost, C. Pohl, S. Beckmann, W. Martens-Habbena, and K. Jürgens. 2005. Impact of different in vitro electron donor/acceptor conditions on potential chemolithoautotrophic communities from marine pelagic redoxclines. Appl. Environ. Microbiol. 71:6664-6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee, N., P. H. Nielsen, K. H. Andreasen, S. Juretschko, J. L. Nielsen, K. H. Schleifer, and M. Wagner. 1999. Combination of fluorescent in situ hybridization and microautoradiography—a new tool for structure-function analyses in microbial ecology. Appl. Environ. Microbiol. 65:1289-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin, X., M. I. Scranton, R. Varela, A. Chistoserdov, and G. T. Taylor. 2007. Compositional responses of bacterial communities to redox gradients and grazing in the anoxic Cariaco Basin. Aquat. Microb. Ecol. 47:57-72. [Google Scholar]
- 24.Lin, X., S. G. Wakeham, I. F. Putnam, Y. M. Astor, M. I. Scranton, A. Y. Chistoserdov, and G. T. Taylor. 2006. Comparison of vertical distributions of prokaryotic assemblages in the anoxic Cariaco Basin and Black Sea by use of fluorescence in situ hybridization. Appl. Environ. Microbiol. 72:2679-2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loy, A., F. Maixner, M. Wagner, and M. Horn. 2007. probeBase—an online resource for rRNA-targeted oligonucleotide probes: new features 2007. Nucleic Acids Res. 35:D800-D804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madrid, V. M., G. T. Taylor, M. I. Scranton, and A. Y. Chistoserdov. 2001. Phylogenetic diversity of bacterial and archaeal communities in the anoxic zone of the Cariaco Basin. Appl. Environ. Microbiol. 67:1663-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mandernack, K. W., and B. M. Tebo. 1999. In situ sulfide removal and CO2 fixation rates at deep-sea hydrothermal vents and the oxic/anoxic interface in Framvaren Fjord, Norway. Mar. Chem. 66:201-213. [Google Scholar]
- 28.Manz, W., R. Amann, W. Ludwig, M. Wagner, and K.-H. Schleifer. 1992. Phylogenetic oligodeoxynucleotide probes for the major subclasses of Proteobacteria: problems and solutions. Syst. Appl. Microbiol. 15:593-600. [Google Scholar]
- 29.Medina-Sánchez, J. M., M. Felip, and E. O. Casamayor. 2005. Catalyzed reported deposition-fluorescence in situ hybridization protocol to evaluate phagotrophy in mixotrophic protists. Appl. Environ. Microbiol. 71:7321-7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakagawa, S., K. Takai, F. Inagaki, H. Hirayama, T. Nunoura, K. Horikoshi, and Y. Sako. 2005. Distribution, phylogenetic diversity and physiological characteristics of epsilon-Proteobacteria in a deep-sea hydrothermal field. Environ. Microbiol. 7:1619-1632. [DOI] [PubMed] [Google Scholar]
- 31.Nielsen, J. L., D. Christensen, M. Kloppenborg, and P. H. Nielsen. 2003. Quantification of cell-specific substrate uptake by probe-defined bacteria under in situ conditions by microautoradiography and fluorescence in situ hybridization. Environ. Microbiol. 5:202-211. [DOI] [PubMed] [Google Scholar]
- 32.Perner, M., R. Seifert, S. Weber, A. Koschinsky, K. Schmidt, H. Strauss, M. Peters, K. Haase, and J. F. Imhoff. 2007. Microbial CO2 fixation and sulfur cycling associated with low-temperature emissions at the Lilliput hydrothermal field, southern Mid-Atlantic Ridge (9°S). Environ. Microbiol. 9:1186-1201. [DOI] [PubMed] [Google Scholar]
- 33.Pernthaler, A., J. Pernthaler, and R. Amann. 2002. Fluorescence in situ hybridization and catalyzed reporter deposition for the identification of marine bacteria. Appl. Environ. Microbiol. 68:3094-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pimenov, N. V., and L. N. Neretin. 2006. Composition and activities of microbial communities involved in carbon, sulfur, nitrogen and manganese cycling in the oxic/anoxic interface of the Black Sea, p. 501-521. In L. N. Neretin (ed.), Past and present marine water column anoxia. Springer, Dordrecht, The Netherlands.
- 35.Sekar, R., A. Pernthaler, J. Pernthaler, F. Warnecke, T. Posch, and R. Amann. 2003. An improved protocol for quantification of freshwater Actinobacteria by fluorescence in situ hybridization. Appl. Environ. Microbiol. 69:2928-2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sorokin, J. I. 1964. On the primary production and bacterial activities in the Black Sea. J. Council Explor. Mer. 29:41-60. [Google Scholar]
- 37.Sorokin, Y. I. 1972. The bacterial population and the processes of hydrogen sulphide oxidation in the Black Sea. J. Council Explor. Mer. 34:423-454. [Google Scholar]
- 38.Takai, K., F. Inagaki, S. Nakagawa, H. Hirayama, T. Nunoura, Y. Sako, K. H. Nealson, and K. Horikoshi. 2003. Isolation and phylogenetic diversity of members of previously uncultivated ɛ-proteobacteria in deep-sea hydrothermal fields. FEMS Microbiol. Lett. 218:167-174. [DOI] [PubMed] [Google Scholar]
- 39.Taylor, G. T., M. Iabichella-Armas, R. Varela, F. Müller-Karger, X. Lin, and M. I. Scranton. 2006. Microbial ecology of the Cariaco Basin's redoxcline: the U.S.-Venezuela Cariaco times series program, p. 473-499. In L. N. Neretin (ed.), Past and present marine water column anoxia. Springer, Dordrecht, The Netherlands.
- 40.Taylor, G. T., M. Iabichella, T.-Y. Ho, M. I. Scranton, R. C. Thunell, F. Müller-Karger, and R. Varela. 2001. Chemoautotrophy in the redox transition zone of the Cariaco Basin: a significant midwater source of organic carbon production. Limnol. Oceanogr. 46:148-163. [Google Scholar]
- 41.Teira, E., T. Reinthaler, A. Pernthaler, J. Pernthaler, and G. J. Herndl. 2004. Combining catalyzed reporter deposition-fluorescence in situ hybridization and microautoradiography to detect substrate utilization by bacteria and archaea in the deep ocean. Appl. Environ. Microbiol. 70:4411-4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tuttle, J. H., and H. W. Jannasch. 1979. Microbial dark assimilation of CO2 in the Cariaco Trench. Limnol. Oceanogr. 24:746-753. [Google Scholar]
- 43.Vetriani, C., H. V. Tran, and L. J. Kerkhof. 2003. Fingerprinting microbial assemblages from the oxic/anoxic chemocline of the Black Sea. Appl. Environ. Microbiol. 69:6481-6488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wallner, G., R. Amann, and W. Beisker. 1993. Optimizing fluorescent in situ hybridization with rRNA-targeted oligonucleotide probes for flow cytometric identification of microorganisms. Cytometry 14:136-143. [DOI] [PubMed] [Google Scholar]
- 45.Zopfi, J., T. G. Ferdelman, B. B. Jørgensen, A. Teske, and B. Thamdrup. 2001. Influence of water column dynamics on sulfide oxidation and other major biogeochemical processes in the chemocline of Mariager Fjord (Denmark). Mar. Chem. 74:29-51. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.