Abstract
Background and purpose:
We have previously demonstrated antinociceptive effects of fatty acid amide hydrolase (FAAH) inhibition that were accompanied by increases in the levels of endocannabinoids (ECs) in the hind paw. Here, the effects of the FAAH inhibitor URB597 (3′-carbamoyl-biphenyl-3-yl-cyclohexylcarbamate) on responses of spinal neurons were studied.
Experimental approach:
Extracellular single-unit recordings of dorsal horn neurons were made in anaesthetized rats with hind paw inflammation induced by λ-carrageenan. Effects of intraplantar pre-administration of URB597, or vehicle, on carrageenan-evoked expansion of peripheral receptive fields of spinal neurons and mechanically evoked responses of neurons were studied. The cannabinoid receptor type 1 (CB1) antagonist AM251 (N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide) and the peroxisome proliferator-activated receptor (PPAR)-α antagonist GW6471 ([(2S)-2-[[(1Z)-1-methyl-3-oxo-3-[4-(trifluoromethyl)phenyl]-1-propenyl]amino]-3-[4-[2-(5-methyl-2-phenyl-4-oxa zolyl)ethoxy]phenyl]propyl]-carbamic acid ethyl ester) were used to investigate the roles of these receptors in mediating the effects of URB597.
Key results:
URB597 (25 μg in 50 μL) pretreatment significantly inhibited carrageenan-evoked receptive field expansion and this was significantly reversed by co-administration of the PPAR-α antagonist but not the CB1 antagonist. Pretreatment with the PPAR-α receptor agonist WY14643 ([[4-chloro-6-[(2,3-dimethylphenyl)amino]-2-pyrimidinyl]thio]acetic acid) also significantly inhibited receptive field expansion. URB597 (25 or 100 μg in 50 μL) had no significant effect on mechanically evoked responses of spinal neurons.
Conclusions and implications:
URB597 inhibited receptive field expansions but not mechanically evoked responses of spinal neurons in rats with hind paw inflammation. These effects were blocked by PPAR-α receptor antagonism. These data support the contention that URB597 exerts its antinociceptive effects by indirect inhibition of sensitization of neuronal responses at least partly through PPAR-α activation due to enhanced EC levels.
Keywords: inflammation, pain, endocannabinoid, spinal electrophysiology, PPAR
Introduction
The antinociceptive and anti-inflammatory effects of cannabinoids have been well described; however, their clinical usefulness is often limited by the occurrence of unwanted psychotropic side effects.
The endocannabinoids (ECs) anandamide (N-arachidonoylethanolamine (AEA); Devane et al., 1992) and 2-arachidonoylglycerol (2-AG; Mechoulam et al., 1995; Sugiura et al., 1995) activate cannabinoid receptor types 1 (CB1) and 2 (CB2) and have antinociceptive effects in models of acute and chronic pain (Mechoulam et al., 1995; Calignano et al., 1998; Richardson et al., 1998; Walker et al., 1999; Sokal et al., 2003; Guindon et al., 2007). Recent work has focused on targeting ECs and their metabolism, in preference to the use of receptor agonists, as an approach to achieving analgesia in the absence of side effects (for review, see Jhaveri et al., 2007). One such approach is to inhibit EC breakdown by targeting their metabolizing enzymes. Two of the main enzymes responsible for their metabolism are fatty acid amide hydrolase (FAAH) and monoacyl glycerol lipase. FAAH is the enzyme responsible for the breakdown of ECs such as AEA and N-acyl ethanolamines such as N-palmitoylethanolamide (PEA) and oleoylethanolamide with monoacyl glycerol lipase primarily metabolizing 2-AG. In addition to these two main enzymes, N-acylethanolamine-hydrolysing acid amidase also contributes to the metabolism of AEA and PEA (Tsuboi et al., 2007). Inhibition, or genetic deletion, of FAAH produces antinociceptive effects in animal models of inflammatory pain (Cravatt et al., 2004; Lichtman et al., 2004; Chang et al., 2006; Jayamanne et al., 2006). The generation of an FAAH knockout mouse with specific deletion of gene coding for FAAH in peripheral tissues has indicated a function of peripheral FAAH in the modulation of nociceptive processing in the carrageenan model of inflammatory pain (Cravatt et al., 2004). In addition to this, augmented inhibitory effects of exogenous ECs on paw oedema have been reported in mice lacking FAAH (Wise et al., 2008). Previous studies in our group have demonstrated an attenuation of the inflammation-induced hyperalgesia following peripheral administration of the FAAH inhibitor URB597 (3′-carbamoyl-biphenyl-3-yl-cyclohexylcarbamate) (Kathuria et al., 2003) in the carrageenan model of inflammatory pain, which was accompanied by significantly increased levels of AEA and 2-AG in the paw (Jhaveri et al., 2008). In addition to the established function of the CB1 and CB2 receptors in peripheral nociceptive processing (Richardson et al., 1998; Kelly et al., 2003; Quartilho et al., 2003; Sokal et al., 2003; Elmes et al., 2004; Nackley et al., 2004), some ECs such as AEA (Sun et al., 2007) and PEA (Rockwell and Kaminski, 2004; LoVerme et al., 2006; Rockwell et al., 2006) are also ligands for the peroxisome proliferator-activated receptor-α (PPAR-α), which could mediate their well-described anti-inflammatory effects. PPAR-α-mediated anti-inflammatory and antinociceptive effects of PEA have been demonstrated in animals with adjuvant-induced arthritis, formalin-evoked pain and carrageenan inflammation (Calignano et al., 1998; Lo Verme et al., 2005; LoVerme et al., 2006; D'Agostino et al., 2007; Wise et al., 2008).
The aim of this study was to investigate the functions of CB1 receptors and PPAR-α in mediating the inhibitory effects of FAAH inhibition in a model of inflammatory hyperalgesia that is characterized by primary and secondary sensitization resulting in a lowering of thresholds to noxious stimuli and an expansion of peripheral receptive fields of spinal neurons. Herein, we have determined the effects of inhibition of FAAH by URB597 on receptive field size and evoked responses of spinal neurons in rats with carrageenan-inflamed paws. We have also investigated the function of PPAR-α in mediating these effects.
Methods
In vivo electrophysiology
All animal procedures were in accordance with the Animals (Scientific Procedures) Act 1986 and International Association for the Study of Pain (IASP) guidelines. Experiments were carried out with adult male Sprague–Dawley rats (250–300 g; n=49 rats), purchased from Charles River, Margate, UK, and group housed in a light-controlled room with ad libitum access to food and water. The experimental methods are similar to those previously described by Elmes et al. (2004). Briefly, anaesthesia was induced with 2–3% isoflurane in 66% N2O/33% O2, and a tracheal cannula was inserted. Rats were placed in a stereotaxic frame and a laminectomy was performed, exposing segments L4–L5 of the spinal cord. The exposed segment of spinal cord was held rigid by the use of clamps applied rostral and caudal to the exposed area. On completion of surgery, the level of isoflurane was reduced to 1–1.5%, which maintained a state of complete areflexia. Core body temperature was monitored and maintained at 36–37 °C.
Extracellular single-unit recordings of deep (500–1000 μm, laminae V–VI) wide dynamic range (WDR) dorsal horn neurons were made with glass-coated tungsten microelectrodes (Merrill and Ainsworth, 1972). Electrodes were lowered through the cord in 10-μm steps with a SCAT-01 microdrive (Digitimer, Welwyn Garden City, UK) and the depth of neurons from the spinal cord surface were recorded and verified at the end of the experiment. Action potentials were digitized and analysed using a CED micro1401 interface and Spike 2 data acquisition software (Cambridge Electronic Design, Cambridge, UK). Receptive fields of neurons covering one or two toes were identified with brush and pinch stimuli. The responses of neurons following transcutaneous electrical stimulation (fine stimulating needles, 2 ms wide pulses) of the centre of the receptive field were recorded. All neurons selected had a clear short latency Aβ-fibre-evoked response (0–20 ms post-stimulus) followed by Aδ-fibre-evoked response (20–90 ms post-stimulus) and a longer latency C-fibre-evoked response (90–300 ms post-stimulus).
Mechanically evoked responses of spinal neurons to mechanical punctate stimulation with von Frey hairs were characterized. Individual von Frey hairs (Semmes-Weinstein Monofilaments; North Coast Medical Inc., USA, via Linton Instrumentation, Norfolk, UK) of differing bending forces (8, 10, 15, 26, 60 and 100 g) were applied to the centre of the receptive field of the neuron, for 10 s, in ascending order. Mechanically evoked firing of neurons was recorded and stable (<10% variation) mechanically (8–100 g) evoked neuronal responses were measured at 10-min intervals. The range of von Frey hairs studied included non-noxious and noxious mechanical punctate stimuli, as the noxious withdrawal threshold in conscious animals is 15 g (Chaplan et al., 1994).
Receptive field mapping
In separate experiments, innocuous (8 g) and noxious (26 g) mechanical punctate stimuli were applied to the paw to map peripheral receptive fields of WDR dorsal horn neurons using a similar method to that previously described (Torsney and Fitzgerald, 2003; Elmes et al., 2004). Receptive field sizes of WDR neurons on the hind paw were plotted at 10-min intervals alternating between 8 and 26 g stimuli to ascertain control receptive field size prior to intervention (n=30 neurons in 30 rats).
Carrageenan inflammation
Following identification of a single WDR neuron and recording of control mechanically evoked responses or receptive field mapping, λ-carrageenan (100 μL 2% in saline; Sigma, Poole, UK) was injected into the plantar surface of the hind paw. Mechanical stimuli (8–100 g) were applied (in ascending order as described above) to the peripheral receptive field, or receptive field mapping was carried out as described as above at 10-min intervals, for 180 min, following injection of carrageenan. Using a method similar to Morris (2003), hind paw circumference was measured using suture looped around the paw at metatarsal level and gently tightened. The thread was then opened out and measured to the nearest millimetre (Morris, 2003). Measurements were taken prior to carrageenan injection and then at 60-min intervals thereafter (n=19 neurons in 19 rats).
The 180 min end time point for pharmacological studies was selected on the basis of previous behavioural studies reporting maximal hyperalgesia at this time point (Hargreaves et al., 1988).
Drug administration
All treatments were given as intraplantar injections, unless otherwise noted. Effects of pre-administration (30 min prior to carrageenan injection) of the FAAH inhibitor URB597 (25 μg in 50 μL; n=6 neurons in six rats), or vehicle (3% Tween-80 in saline; n=6 neurons in six rats), on carrageenan-induced expansion of peripheral receptive field size were determined at 10-min intervals (alternating between 8 and 26 g) for 210 min post-injection.
To investigate the function of the CB1 receptor in URB597 (25 μg in 50 μL)-mediated effects on receptive field expansion, the CB1 receptor-selective antagonist AM251 (N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide) (30 μg in 50 μL) was co-injected with URB597 30 min prior to carrageenan injection (n=6 neurons in six rats). To investigate the function of the PPAR-α in URB597-mediated effects, the PPAR-α antagonist GW6741 (30 μg in 50 μL) was co-injected with URB597 30 min prior to carrageenan injection (n=6 neurons in six rats). To investigate potential pro- or antinociceptive effects resulting from blockade of the PPAR-α receptor, the effects of GW6471 ([(2S)-2-[[(1Z)-1-methyl-3-oxo-3-[4-(trifluoromethyl)phenyl]-1-propenyl]amino]-3-[4-[2-(5-methyl-2-phenyl-4-oxa zolyl)ethoxy]phenyl]propyl]-carbamic acid ethyl ester) alone 30 min prior to carrageenan injection was studied in a separate group of rats (n=6 neurons in six rats). Effects of direct activation of PPAR-α on receptive field expansion were investigated in a separate group of rats using the PPAR-α-selective agonist WY14643 ([[4-chloro-6-[(2,3-dimethylphenyl)amino]-2-pyrimidinyl]thio]acetic acid) (100 μg in 50 μL), which was injected 30 min prior to carrageenan (n=6 neurons in six rats). This dose of WY14643 was selected on the basis of an earlier study reporting an ED50 of 100 μg in 50 μL in the formalin test (LoVerme et al., 2006).
To investigate the effects of inhibition of FAAH on mechanically evoked responses of WDR neurons, effects of an intraplantar pre-administration (30 min prior to carrageenan injection) of URB597 (25 or 100 μg in 50 μL; n=6 neurons in six rats and n=7 neurons in seven rats, respectively) or vehicle (3% Tween-80 in saline; n=6 neurons in six rats) on mechanically (8–100 g) evoked responses of dorsal horn neurons were studied in a separate group of rats. Effects of an intraplantar injection of URB597 (25 μg in 50 μL; n=7 neurons in seven rats) or vehicle (3% Tween-80 in saline; n=6 neurons in six rats) were studied in a separate group of naïve rats at 10-min intervals for 60 min.
Statistical analysis
Statistical analyses comparing carrageenan-induced expansion of peripheral receptive fields of WDR neurons following drug treatment were performed using area under the curve analysis and the Mann–Whitney test.
Statistical analyses comparing the effects of intraplantar URB597 and vehicle on mechanically evoked responses of WDR neurons in carrageenan-inflamed rats were performed using a two-way ANOVA with Bonferroni post-test.
Statistical analyses comparing paw circumference values 3 h following carrageenan injection of all drug treatment groups to pre-drug values were performed using one-way ANOVA with Dunnett's post-test.
Statistical analyses comparing control electrically evoked neuronal characteristics of WDR neurons between drug treatment groups were performed using one-way ANOVA with Bonferroni post-test.
Statistical analyses comparing mean maximal inhibitory effects of URB597 and mechanically evoked responses of WDR neurons in naïve rats were performed using the Mann–Whitney test.
Materials
Isoflurane was obtained from Abbott Laboratories Ltd (Maidenhead, UK). URB597 was purchased from Alexis Biochemicals (via Axxora, Nottingham, UK). AM251, GW6471 and WY14643 were all purchased from Tocris Bioscience (Bristol, UK).
Results
The mean depth at which recordings of WDR neurons were made (500–1000 μm corresponding to laminae V–VI) was similar for the groups of WDR neurons used in each pharmacological study group (Table 1).
Table 1.
Depth of neurons and injection-evoked firing of WDR neurons following intraplantar injection of vehicle, WY14643 or URB597 alone or in combination with AM251 or GW6471
| Number of rats per drug treatment group | Mean depth of neurons (μm) | Mean duration of firing (s) | Mean frequency of firing (Hz) |
|---|---|---|---|
| URB597 (25 μg) Carrageenan-inflamed paws n=12 |
663±43 | 96±17 | 28±3 |
| URB597 (100 μg) Carrageenan-inflamed paws n=7 |
785±62 | 69±28 | 39±10 |
| Vehicle Carrageenan-inflamed paws n=12 |
793±42 | 137±24 | 35±5 |
| URB597 (25 μg)+AM251 (30 μg) Carrageenan-inflamed paws n=6 |
635±40 | 102±25 | 25±4 |
| URB597 (25 μg)+GW6471 (30 μg) Carrageenan-inflamed paws n=6 |
718±27 | 131±33 | 44±11 |
| GW6471 (30 μg) Carrageenan-inflamed paws n=6 |
740±33 | 114±26 | 27±4 |
| WY14643 (100 μg) Carrageenan-inflamed paws n=5 |
808±87 | 109±31 | 39±13 |
Abbreviations: AM251, N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide; GW6471, [(2S)-2-[[(1Z)-1-methyl-3-oxo-3-[4-(trifluoromethyl)phenyl]-1-propenyl]amino]-3-[4-[2-(5-methyl-2-phenyl-4-oxa zolyl)ethoxy]phenyl]propyl]-carbamic acid ethyl ester; URB597, 3′-carbamoyl-biphenyl-3-yl-cyclohexylcarbamate; WDR, wide dynamic range; WY14643, [[4-chloro-6-[(2,3-dimethylphenyl)amino]-2-pyrimidinyl]thio]acetic acid.
Data are expressed as mean±s.e.mean; n=number of rats. All treatments were given as intraplantar injections in a volume of 50 μl.
Effects of peripheral pre-administration of URB597 on receptive field expansion in carrageenan-inflamed paws
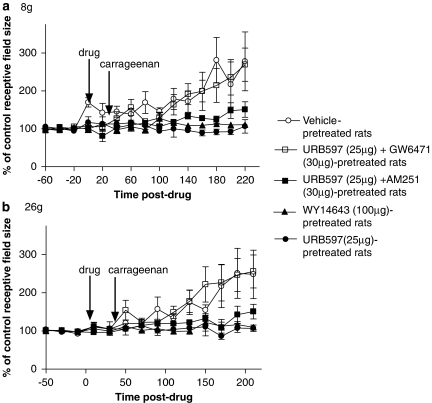
Prior to drug administration, all neurons exhibited negligible or no spontaneous activity. Intraplantar injection of carrageenan produced a robust and significant increase in the size of the peripheral receptive fields of WDR neurons in response to 8 g (pre-carrageenan area: 86±19 mm2; post-carrageenan area: 191±35 mm2) and 26 g (pre-carrageenan area: 119±23 mm2; post-carrageenan area: 241±41 mm2) mechanical stimuli in vehicle-treated rats compared with pre-drug baseline values; Figures 1a and b, and 2a and b). Carrageenan-induced increases in peripheral receptive fields of WDR neurons were accompanied by an increase in the circumference of the ipsilateral hind paw 3 h after carrageenan injection, which was significant compared with pre-drug baseline values (Figure 3).
Figure 1.
Intraplantar pre-administration of URB597 (3′-carbamoyl-biphenyl-3-yl-cyclohexylcarbamate; 25 μg in 50 μL; n=6) or WY14643 ([[4-chloro-6-[(2,3-dimethylphenyl)amino]-2-pyrimidinyl]thio]acetic acid; 100 μg in 50 μL; n=5) attenuated the expansion of peripheral receptive fields of wide dynamic range (WDR) neurons following intraplantar injection of carrageenan (2%) compared with vehicle (3% Tween-80; n=6) as determined by 8 (a) and 26 g (b) mechanical stimuli. Inhibitory effects of URB597 were blocked by the peroxisome proliferator-activated receptor-α (PPAR-α) antagonist GW6471 ([(2S)-2-[[(1Z)-1-methyl-3-oxo-3-[4-(trifluoromethyl)phenyl]-1-propenyl]amino]-3-[4-[2-(5-methyl-2-phenyl-4-oxa zolyl)ethoxy]phenyl]propyl]-carbamic acid ethyl ester; 30 μg in 50 μL; n=6), but not by the cannabinoid receptor type 1 (CB1) antagonist AM251 (N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide; 30 μg in 50 μL; n=6). Data are expressed as mean % of control receptive field size±s.e.mean.
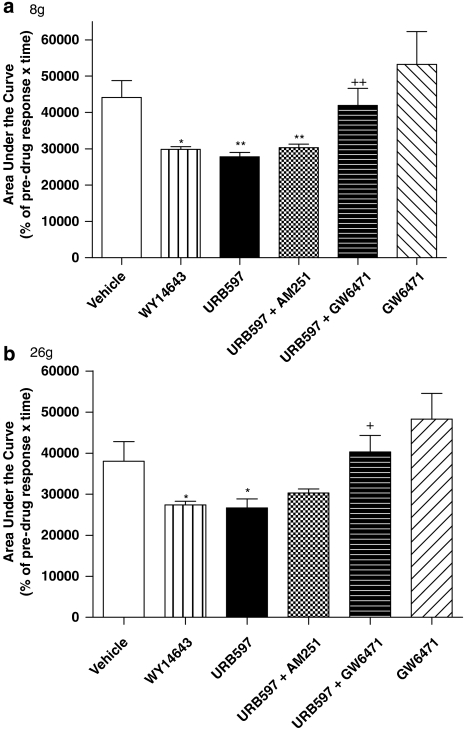
Figure 2.
Carrageenan-evoked changes in receptive field size of wide dynamic range (WDR) neurons to 8 (a) and 26 g (b) stimulation following intraplantar administration of vehicle, WY14643 ([[4-chloro-6-[(2,3-dimethylphenyl)amino]-2-pyrimidinyl]thio]acetic acid; 100 μg in 50 μL) or URB597 (3′-carbamoyl-biphenyl-3-yl-cyclohexylcarbamate; 25 μg in 50 μL) alone or in the presence of AM251 (N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide; 30 μg in 50 μL) or GW6471 (30 μg in 50 μL). Statistical analyses comparing area under the curve (AUC) values were performed using the Mann–Whitney test. *P<0.05, compared with vehicle. +P<0.05, **P<0.01 compared with URB597 alone. Data are expressed as mean AUC±s.e.mean.
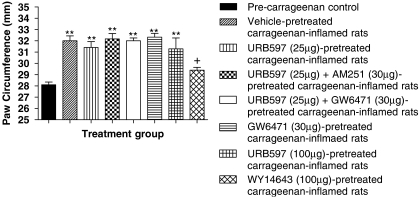
Figure 3.
Intraplantar pretreatment with URB597 (3′-carbamoyl-biphenyl-3-yl-cyclohexylcarbamate; 25 or 100 μg in 50 μL; n=12 and 7, respectively) alone or in the presence of either the cannabinoid receptor type 1 (CB1)-selective antagonist AM251 (N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide; 30 μg in 50 μL; n=6) or the peroxisome proliferator-activated receptor-α (PPAR-α)-selective antagonist GW6471 ([(2S)-2-[[(1Z)-1-methyl-3-oxo-3-[4-(trifluoromethyl)phenyl]-1-propenyl]amino]-3-[4-[2-(5-methyl-2-phenyl-4-oxa zolyl)ethoxy]phenyl]propyl]-carbamic acid ethyl ester; 30 μg in 50 μL; n=6) did not significantly alter increases in paw circumference following intraplantar injection of carrageenan (100 μL; 2% in saline) compared with vehicle (3% Tween-80 in saline; n=12). Intraplantar pretreatment of the PPAR-α-selective agonist WY14643 ([[4-chloro-6-[(2,3-dimethylphenyl)amino]-2-pyrimidinyl]thio]acetic acid; 100 μg in 50 μL; n=5) significantly attenuated the carrageenan-evoked increase in paw circumference compared with vehicle. Data are expressed as mean paw circumference ±s.e.mean. Statistical analyses comparing all groups to pre-drug values were performed using one-way ANOVA with Dunnett's post-test; **P<0.01. Statistical analyses comparing all groups to vehicle were performed using one-way ANOVA with Dunnett's post-test; +P<0.05.
Intraplantar injection of URB597 (25 μg in 50 μL) into the peripheral receptive field of dorsal horn neurons tended to increase the firing frequency of WDR neurons, although this effect was not significantly different from the effect of vehicle (Table 1).
Intraplantar injection of URB597 (25 μg in 50 μL) 30 min prior to carrageenan injection significantly blocked the carrageenan-induced expansion of peripheral receptive fields (8 g: pre-carrageenan area: 124±14 mm2; post-carrageenan area: 129±21 mm2 and 26 g: pre-carrageenan area: 132±14 mm2; post-carrageenan area: 178±33 mm2) compared with vehicle (Figures 1a and b, and 2a and b).
Previously, inhibitory effects of URB597 on oedema and inflammatory pain behaviour have been attributed to activation of the CB1 receptor, presumably by enhanced levels of ECs (Holt et al., 2005; Jayamanne et al., 2006); we, therefore, investigated the function of the CB1 receptor in modulating the effects of URB597 on peripheral receptive field expansion. The inhibitory effects of URB597 on carrageenan-induced expansion of 8 and 26 g receptive fields of WDR neurons tended to be reduced in the presence of the CB1 receptor antagonist AM251 (30 μg in 50 μL), although significance was not reached Figures 1a and b, and 2a and b).
As intraplantar injection of URB597 has been shown to increase the levels of AEA and PEA in carrageenan-inflamed rats, both of which are PPAR-α ligands (Jhaveri et al., 2008), we investigated the role of this receptor in the inhibitory effects of URB597. Co-administration of the PPAR-α antagonist GW6471 completely abolished the inhibitory effects of URB597 on the carrageenan-evoked expansion of receptive fields (8 g) of WDR neurons in carrageenan-inflamed rats (Figures 1a and b, and 2a and b). Indeed, there was no significant difference between the peripheral receptive field size in vehicle-pretreated carrageenan-inflamed rats and rats pretreated with URB957 and GW6471. Pre-administration of GW6471 alone, 30 min prior to carrageenan injection, did not significantly alter the carrageenan-induced expansion of peripheral receptive fields of WDR neurons compared with vehicle pretreatment (Figures 1a and b, and 2a and b).
To evaluate further the potential function of PPAR-α in receptive field expansion, we compared the effects of URB597 to those of a selective PPAR-α agonist, WY14643, in this paradigm. Intraplantar pre-administration of WY14643 (100 μg in 50 μL) 30 min prior to carrageenan injection significantly attenuated carrageenan-evoked expansion of peripheral receptive fields of WDR neurons to both 8 and 26 g stimuli compared with vehicle (Figures 1a and b, and 2a and b). In parallel, WY14643 pretreatment significantly inhibited carrageenan-induced hind paw oedema 3 h post-carrageenan injection compared with paw circumferences in both pre-carrageenan and vehicle-treated rats (Figure 3).
Effects of peripheral URB597 on mechanically evoked responses of dorsal horn neurons in carrageenan-inflamed and naïve rat paws
Prior to injection of drug or carrageenan, stimulation of the peripheral receptive field of WDR neurons with von Frey hairs (8–100 g) produced an increase in the frequency of firing that was stimulus intensity dependent (Table 2)
Table 2.
Mechanically evoked (8–100 g) responses of WDR neurons before (pre-drug/carrageenan) and 3 h following intraplantar carrageenan administration
| von Frey weight (g) |
Vehicle |
URB597 (25 μg in 50 μl) |
URB597 (100 μg in 50 μl) |
|||
|---|---|---|---|---|---|---|
| Pre-drug/carrageenan | 3 h post- carrageenan | Pre-drug/carrageenan | 3 h post-carrageenan | Pre-drug/carrageenan | 3 h post-carrageenan | |
| 8 | 13±3 | 8±2 | 14±4 | 28±8 | 16±9 | 10±4 |
| 10 | 27±9 | 9±3 | 27±10 | 30±9 | 17±9 | 10±4 |
| 15 | 42±9 | 20±7 | 41±12 | 43±16 | 29±15 | 18±6 |
| 26 | 66±12 | 42±8 | 58±12 | 49±11 | 42±14 | 29±9 |
| 60 | 88±11 | 75±13 | 90±10 | 78±11 | 57±14 | 51±14 |
| 100 | 100±11 | 87±15 | 98±11 | 83±7 | 74±18 | 61±15 |
Abbreviations: URB597, 3′-carbamoyl-biphenyl-3-yl-cyclohexylcarbamate; WDR, wide dynamic range.
Rats were given either an intraplantar injection of vehicle (n=6 neurons in six rats), URB597 (25 μg in 50 μl; n=6 neurons in six rats) or URB597 (100 μg in 50 μl; n=7 neurons in seven rats). Data are expressed as mean frequency of firing±s.e.mean.
Statistical analyses comparing mechanically evoked responses before and 3 h following carrageenan administration were carried out using a one-way ANOVA with the Bonferroni post-test. There was no statistical difference between drug treatment groups.
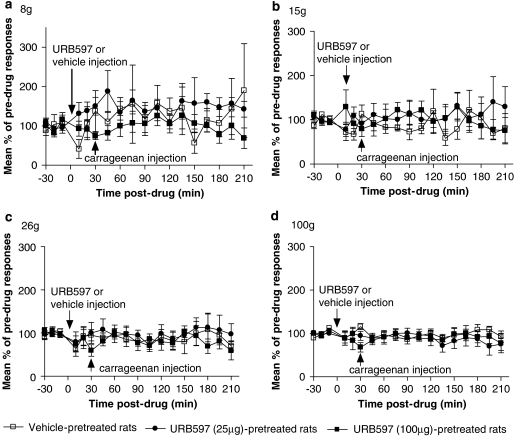
Pre-administration of vehicle 30 min prior to carrageenan injection tended to facilitate low- and intermediate-weight (8–15 g) mechanically evoked responses of WDR neurons; however, this did not reach significance compared with pre-drug control responses (Figures 4a and b). Higher weight (26–100 g) evoked responses of WDR neurons were not altered by intraplantar injection of vehicle (Figures 4c and d). Intraplantar injection of URB597 (25 μg in 50 μL) did not alter mechanically evoked responses (8–100 g) of dorsal horn neurons, compared with pre-drug control values or to vehicle-pretreated, carrageenan-inflamed paws (Figures 4a–d). In the light of the lack of effect of 25 μg URB597, effects of a higher dose, 100 μg also in 50 μL of URB597, were studied. Injection-evoked firing of WDR neurons following intraplantar injection of URB597 (100 μg in 50 μL) did not differ from that seen in vehicle-treated rats, and paw circumferences were not different from any other treatment group 3 h after carrageenan administration (Figure 3). Pre-administration of the higher dose did not alter mechanically evoked (8–100 g) responses of WDR neurons compared with pre-drug control values or to vehicle-pretreated carrageenan-inflamed rats (Figures 4a–d).
Figure 4.
Intraplantar pre-administration of URB597 (3′-carbamoyl-biphenyl-3-yl-cyclohexylcarbamate; 25 μg in 50 μL; n=6) or (100 μg in 50 μL; n=7) did not alter 8 (a), 15 (b), 26 (c) and 100 g (d) mechanically evoked responses of wide dynamic range (WDR) neurons following intraplantar injection of carrageenan (2%) in anesthetized rats compared with vehicle (3% Tween-80 in saline; n=6). Data are presented as mean % of pre-drug response±s.e.mean.
Neither URB597 pretreatment alone, nor in the presence of either antagonist, had any significant effect on paw oedema as indicated by changes in paw circumference (Figure 3).
As intraplantar administration of URB597 produced negligible effects on mechanically evoked responses of WDR neurons in carrageenan-inflamed rats, we investigated whether URB597 was able to modify similar neuronal responses in naïve rats.
Intraplantar injection of URB597 (25 μg in 50 μL; n=7 neurons in seven rats) produced an increase in the frequency of firing of WDR neurons; however, this did not significantly differ from injection of vehicle (n=6 neurons in six rats; Table 1). Intraplantar injection of URB597 (25 μg in 50 μL) attenuated lower weight (8–15 g) mechanically evoked responses (45±8, 45±11 and 41±9% of pre-drug controls, respectively), but did not alter higher weight (26–100 g) evoked responses compared with pre-drug control values (53±11, 65±7 and 76±9% of pre-drug controls, respectively). Effects of URB597 (25 μg in 50 μL) were significantly (P<0.05 and P<0.01, respectively) different from vehicle-treated rats for 10 and 15 g mechanically evoked responses (110±23 and 115±21% of pre-drug controls, respectively). Mean maximal inhibitory effects of URB597 on mechanically evoked responses of WDR neurons occurred at 20–30 min post-drug administration.
Discussion
In this study, the FAAH inhibitor, URB597, reduced carrageenan-induced expansion of peripheral receptive fields of WDR neurons, and this effect was blocked by a PPAR-α antagonist. Previously, we have shown that the levels of AEA and PEA are decreased in the carrageenan-inflamed hind paws of rats and that intraplantar injection of URB597, at the same dose as used in this study, significantly elevated the levels of AEA and 2-AG in the hind paw (Jhaveri et al., 2008). Collectively, these data suggest that decreased hind paw levels of ECs may contribute to neuronal sensitization during inflammation in vivo and the associated expansion of neuronal receptive fields. The ability of URB597 to block the expansion of neuronal receptive fields in parallel with the elevation of ECs implicates ECs in mediating this inhibitory effect. It has previously been shown that URB597 does not directly bind to CB1 or CB2 receptors, ruling out a direct effect of URB597 on these receptors (Kathuria et al., 2003).
Our data are consistent with the well-described antinociceptive effects of FAAH inhibitors in behavioural models of inflammatory (Lichtman et al., 2004; Jayamanne et al., 2006; Jhaveri et al., 2008) and neuropathic pain (Chang et al., 2006; Jhaveri et al., 2006; Russo et al., 2007). Our novel electrophysiological data demonstrating that inhibition of FAAH prevents neuronal receptive field expansion, a key element of hyperalgesia, indicate a mechanism by which elevation of ECs produces the antinociceptive effects. These data are wholly consistent with our recent report that intraplantar injection of URB597 produces a PPAR-α-mediated attenuation of carrageenan-induced behavioural hyperalgesia (Jhaveri et al., 2008). Other PPAR receptor subtypes do not appear to be involved in these effects of URB597 as they were not sensitive to blockade of the PPAR-γ receptor (Jhaveri et al., 2008). There is no evidence for the function of PPAR-β/δ in mediating these effects, with the lack of selective antagonists precluding the investigation of these receptors in this model.
The profound inhibitory effects of URB597 on receptive field expansion in carrageenan-inflamed rats were abolished by co-administration of a PPAR-α antagonist. As the inhibitory effects of URB597 appear to be mediated by PPAR-α, but not by CB1 receptors, and URB597 does not bind directly to PPAR-α (Jhaveri et al., 2008), these data suggest that URB597 elevates the levels of endogenous ligands for PPAR-α, which can modulate the expansion of neuronal receptive fields. Previously, we have shown that AEA, which is a ligand both at cannabinoid receptors and PPAR-α, is elevated following intraplantar injection of URB597 in the hind paw of carrageenan-treated rats (Jhaveri et al., 2008). Thus, endogenous AEA may activate PPAR-α to drive the observed inhibitory effects. To provide further evidence for the modulation of nociceptive processing by PPAR-α, we also investigated the effects of a selective PPAR-α agonist in this model. Intraplantar pre-administration of the PPAR-α-selective agonist WY14643 inhibited carrageenan-evoked receptive field expansion to a similar extent to that produced by the FAAH inhibitor URB597. Thus, activation of PPAR-α directly with a synthetic ligand and indirectly, presumably, through the elevation of endogenous PPAR-α ligands, both inhibited the expansion of neuronal receptive fields in the carrageenan model of inflammatory pain. Our data are consistent with the previously described analgesic effects of PPAR-α agonists in the formalin model of inflammatory pain in rats and mice (LoVerme et al., 2006) and the anti-inflammatory effects demonstrated in the carrageenan model of inflammation (Lo Verme et al., 2005). As URB597 does not act as a PPAR-α ligand (Jhaveri et al., 2008), it is likely that inhibition of FAAH results in the generation of endogenous PPAR-α ligands, possibly including AEA, and that activation of this receptor system produces the observed inhibition of receptive field expansion, a correlate of hyperalgesia. As PPAR-αs are nuclear receptors, it is unlikely that the effects of hind paw administration of URB597 are due to acute alteration of peripheral nerve function. Modulation of immune cells, such as neutrophils, associated with inflammatory responses is more likely (Delayre-Orthez et al., 2005), although rapid changes in gene transcription in sensory neurons cannot be ruled out.
In this study, neither dose of URB597 employed altered the carrageenan-induced hind paw oedema, which is consistent with our previous findings (Jhaveri et al., 2008). As URB597 inhibited carrageenan-evoked expansion of WDR-receptive fields, it can be assumed that the dose used was biologically active. Previous studies have demonstrated that systemic administration of URB597 can inhibit inflammation-evoked hind paw oedema (Holt et al., 2005). However, no measures of antinociception were carried out in the latter study, which, when compounded with the differences in the route of administration between that study and this one, make comparisons difficult. In contrast to the lack of effect of URB597 on oedema formation, the PPAR-α ligand WY14643 significantly inhibited carrageenan-induced oedema formation, which is consistent with the previously reported anti-inflammatory effects of PPAR-α agonists (Lo Verme et al., 2005; D'Agostino et al., 2007). Differences in the effects of WY14643 and URB597 on oedema formation may reflect the differences in the efficacy between a high-potency PPAR-α ligand and multiple putative endogenous ligands elevated by URB597.
In contrast to the involvement of the PPAR-α system, we found no evidence for a contribution of the CB1 receptor to the inhibitory effects of URB597 on receptive field expansion. Although it could be argued that the dose of AM251 employed was too low, it has previously been found to be effective at blocking an equivalent intraplantar dose of URB597 on mechanically evoked responses in sham-operated rats (Jhaveri et al., 2006). Furthermore, the effects of systemic administration of URB597 were significantly attenuated by systemic administration of AM251 in adjuvant-induced inflammation (Jayamanne et al., 2006). Our data are, however, supported by the report that reduced carrageenan-induced inflammatory responses in FAAH-null mice are not affected by CB1 or CB2 receptor antagonists (Cravatt et al., 2004).
In this study, intraplantar injection of URB597 did not alter mechanically evoked responses of WDR neurons in carrageenan-inflamed rats, despite inhibiting mechanically evoked responses in naïve rats. The inhibitory effects of URB597 in naïve rats are consistent with our earlier report that intraplantar injection of URB597 (at an equivalent dose) was antinociceptive in sham-operated rats (Jhaveri et al., 2006). Thus, in the carrageenan model of inflammatory pain, URB597 does not alter mechanically evoked responses of spinal neurons, an effect which is observed in naïve rats and mediated by CB1 receptors. By contrast, in the same animals, URB597 attenuated the expansion of neuronal receptive fields, an effect that was mediated by PPAR-α. Although it is possible that the attenuation of carrageenan-induced receptive field expansion may occur due to direct effects of activation of PPAR-α on peripheral neurons, the nuclear location of the receptor, combined with the lack of inhibitory effects of URB597 on mechanically evoked responses of WDR neurons in this study, indicates that this explanation is unlikely in this instance. Our data support the suggestion that events associated with inflammation may be critical to the inhibitory role of PPAR-α, which may include peripheral sensitization mechanisms, such as neurogenic inflammation and immune cell responses.
Carrageenan-induced inflammation is associated with increased hind paw levels of cytokines such as tumour necrosis factor-α (TNF-α), interleukin 1β (IL-1β), IL-6 (Cunha et al., 2000; Loram et al., 2007) and prostaglandins such as PGE2 (Guay et al., 2004), which contribute to neuronal sensitization. PPAR-α has a well-established function in inflammation (for review, see Moraes et al., 2006), which indeed modulates nociceptive responses. For example, PPAR-α activation modulates the duration of inflammation induced by leukotriene B4 (LTB4)/arachidonic acid and a direct interaction between PPAR-α and LTB4 increases the catabolism of LTB4, thus providing feedback control of inflammation (Devchand et al., 1996). Furthermore, recent studies have revealed impaired production of inflammatory cytokines interferon-γ, IL-6 and TNF-α in vitro (Cunard et al., 2002; Murakami et al., 2007) following exposure to the PPAR-α ligand WY14643, with similar inhibitory effects on IL-6 and TNF secretion observed in lipopolysaccharide-stimulated macrophages following treatment with the PPAR-α agonist K-111 (2,2-dichloro-12-(4-chlorophenyl)dodecanoic acid) (Murakami et al., 2007). Similarly, the PPAR-α agonist WY14643 has anti-inflammatory effects in arachidonic acid-evoked oedema in the murine ear-swelling test (Colville-Nash et al., 2005). Mice lacking the PPAR-α receptor have significantly elevated levels of neutrophils, macrophages and TNF-α following intranasal administration of lipopolysaccharide compared with wild-type littermates (Delayre-Orthez et al., 2005). The mechanism by which PPAR-α inhibits the expansion of neuronal receptive fields is unclear but may arise as a result of the attenuation of carrageenan-evoked increases in the levels of cytokines and/or PGEs. In support of this concept, URB597 produced a CB1/CB2 receptor-independent downregulation of the lipopolysaccharide-induced enzymes, cyclooxygenase-2 (COX-2) and inducible NO synthase, which was accompanied by a concomitant decreased release of PGE2 and NO from rat microglia (Tham et al., 2007). Increased levels of IL-1 receptor antagonist (IL-1ra), an endogenous inhibitor of TNF-α and IL-1β that stimulate eicosanoid production, have been shown to limit carrageenan-induced inflammatory hyperalgesia (Cunha et al., 2000). As IL-1ra is a direct target gene of PPAR-α (Stienstra et al., 2007) and overexpression of PPAR-α increases the IL-1ra promoter activity (Francois et al., 2006), PPAR-α-mediated control of IL-1ra may contribute to the inhibitory effects reported herein.
This study demonstrated that intraplantar pre-administration of URB597 prevented carrageenan-evoked expansion of peripheral receptive fields of WDR neurons, which was mediated, at least partly, through the activation of PPAR-α, possibly resulting from locally raised EC levels. These data support previous studies demonstrating both the antinociceptive effects of FAAH inhibition and PPAR-α activation following inflammation and indicate that these peripheral effects may arise indirectly through the attenuation of neuronal sensitization.
Acknowledgments
This study was supported by the Wellcome Trust. We thank Dr AJ Bennett for useful discussions.
Abbreviations
- AEA
N-arachidonoylethanolamine or anandamide
- AM251
N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide
- CB1
cannabinoid receptor type 1
- CB2
cannabinoid receptor type 2
- EC
endocannabinoid
- FAAH
fatty acid amide hydrolase
- GW6471
[(2S)-2-[[(1Z)-1-methyl-3-oxo-3-[4-(trifluoromethyl)phenyl]-1-propenyl]amino]-3-[4-[2-(5-methyl-2-phenyl-4-oxa zolyl)ethoxy]phenyl]propyl]-carbamic acid ethyl ester
- IL
interleukin
- IL-1ra
interleukin-1 receptor antagonist
- PEA
N-palmitoylethanolamide
- PPAR-α
peroxisome proliferator-activated receptor-α
- TNF-α
tumour necrosis factor-α
- URB597
3′-carbamoyl-biphenyl-3-yl-cyclohexylcarbamate
- WDR
wide dynamic range
- WY14643
[[4-chloro-6-[(2,3-dimethylphenyl)amino]-2-pyrimidinyl]thio]acetic acid
Conflict of interest
The authors state no conflict of interest.
References
- Calignano A, La Rana G, Giuffrida A, Piomelli D. Control of pain initiation by endogenous cannabinoids. Nature. 1998;394:277–281. doi: 10.1038/28393. [DOI] [PubMed] [Google Scholar]
- Chang L, Luo L, Palmer JA, Sutton S, Wilson SJ, Barbier AJ, et al. Inhibition of fatty acid amide hydrolase produces analgesia by multiple mechanisms. Br J Pharmacol. 2006;148:102–113. doi: 10.1038/sj.bjp.0706699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Colville-Nash P, Willis D, Papworth J, Freemantle C, Lam C, Andrews G, et al. The peroxisome proliferator-activated receptor alpha activator, Wy14 643, is anti-inflammatory in vivo. Inflammopharmacology. 2005;12:493–504. doi: 10.1163/156856005774382724. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Saghatelian A, Hawkins EG, Clement AB, Bracey MH, Lichtman AH. Functional disassociation of the central and peripheral fatty acid amide signaling systems. Proc Natl Acad Sci USA. 2004;101:10821–10826. doi: 10.1073/pnas.0401292101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunard R, DiCampli D, Archer DC, Stevenson JL, Ricote M, Glass CK, et al. WY14,643, a PPAR alpha ligand, has profound effects on immune responses in vivo. J Immunol. 2002;169:6806–6812. doi: 10.4049/jimmunol.169.12.6806. [DOI] [PubMed] [Google Scholar]
- Cunha JM, Cunha FQ, Poole S, Ferreira SH. Cytokine-mediated inflammatory hyperalgesia limited by interleukin-1 receptor antagonist. Br J Pharmacol. 2000;130:1418–1424. doi: 10.1038/sj.bjp.0703434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Agostino G, La Rana G, Russo R, Sasso O, Iacono A, Esposito E, et al. Acute intracerebroventricular administration of palmitoylethanolamide, an endogenous peroxisome proliferator-activated receptor-alpha agonist, modulates carrageenan-induced paw edema in mice. J Pharmacol Exp Ther. 2007;322:1137–1143. doi: 10.1124/jpet.107.123265. [DOI] [PubMed] [Google Scholar]
- Delayre-Orthez C, Becker J, Guenon I, Lagente V, Auwerx J, Frossard N, et al. PPARalpha downregulates airway inflammation induced by lipopolysaccharide in the mouse. Respir Res. 2005;6:91. doi: 10.1186/1465-9921-6-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Devchand PR, Keller H, Peters JM, Vazquez M, Gonzalez FJ, Wahli W. The PPARalpha-leukotriene B4 pathway to inflammation control. Nature. 1996;384:39–43. doi: 10.1038/384039a0. [DOI] [PubMed] [Google Scholar]
- Elmes SJ, Jhaveri MD, Smart D, Kendall DA, Chapman V. Cannabinoid CB2 receptor activation inhibits mechanically evoked responses of wide dynamic range dorsal horn neurons in naive rats and in rat models of inflammatory and neuropathic pain. Eur J Neurosci. 2004;20:2311–2320. doi: 10.1111/j.1460-9568.2004.03690.x. [DOI] [PubMed] [Google Scholar]
- Francois M, Richette P, Tsagris L, Fitting C, Lemay C, Benallaoua M, et al. Activation of the peroxisome proliferator-activated receptor alpha pathway potentiates interleukin-1 receptor antagonist production in cytokine-treated chondrocytes. Arthritis Rheum. 2006;54:1233–1245. doi: 10.1002/art.21728. [DOI] [PubMed] [Google Scholar]
- Guay J, Bateman K, Gordon R, Mancini J, Riendeau D. Carrageenan-induced paw edema in rat elicits a predominant prostaglandin e2 (pge2) response in the central nervous system associated with the induction of microsomal PGE2 synthase-1. J Biol Chem. 2004;279:24866–24872. doi: 10.1074/jbc.M403106200. [DOI] [PubMed] [Google Scholar]
- Guindon J, Desroches J, Beaulieu P. The antinociceptive effects of intraplantar injections of 2-arachidonoyl glycerol are mediated by cannabinoid CB2 receptors. Br J Pharmacol. 2007;150:693–701. doi: 10.1038/sj.bjp.0706990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Holt S, Comelli F, Costa B, Fowler CJ. Inhibitors of fatty acid amide hydrolase reduce carrageenan-induced hind paw inflammation in pentobarbital-treated mice: comparison with indomethacin and possible involvement of cannabinoid receptors. Br J Pharmacol. 2005;146:467–476. doi: 10.1038/sj.bjp.0706348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayamanne A, Greenwood R, Mitchell VA, Aslan S, Piomelli D, Vaughan CW. Actions of the FAAH inhibitor URB597 in neuropathic and inflammatory chronic pain models. Br J Pharmacol. 2006;147:281–288. doi: 10.1038/sj.bjp.0706510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhaveri MD, Richardson D, Chapman V. Endocannabinoid metabolism and uptake: novel targets for neuropathic and inflammatory pain. Br J Pharmacol. 2007;152:624–632. doi: 10.1038/sj.bjp.0707433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhaveri MD, Richardson D, Kendall DA, Barrett DA, Chapman V. Analgesic effects of fatty acid amide hydrolase inhibition in a rat model of neuropathic pain. J Neurosci. 2006;26:13318–13327. doi: 10.1523/JNEUROSCI.3326-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhaveri MD, Richardson D, Robinson I, Garle MJ, Patel A, Sun Y, et al. Inhibition of fatty acid amide hydrolase and cyclooxygenase-2 increases levels of endocannabinoid related molecules and produces analgesia via peroxisome proliferator-activated receptor-alpha in a model of inflammatory pain. Neuropharmacology. 2008;55:85–93. doi: 10.1016/j.neuropharm.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- Kelly S, Jhaveri MD, Sagar DR, Kendall DA, Chapman V. Activation of peripheral cannabinoid CB1 receptors inhibits mechanically evoked responses of spinal neurons in noninflamed rats and rats with hindpaw inflammation. Eur J Neurosci. 2003;18:2239–2243. doi: 10.1046/j.1460-9568.2003.02957.x. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Shelton CC, Advani T, Cravatt BF. Mice lacking fatty acid amide hydrolase exhibit a cannabinoid receptor-mediated phenotypic hypoalgesia. Pain. 2004;109:319–327. doi: 10.1016/j.pain.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Lo Verme J, Fu J, Astarita G, La Rana G, Russo R, Calignano A, et al. The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol Pharmacol. 2005;67:15–19. doi: 10.1124/mol.104.006353. [DOI] [PubMed] [Google Scholar]
- Loram LC, Fuller A, Fick LG, Cartmell T, Poole S, Mitchell D. Cytokine profiles during carrageenan-induced inflammatory hyperalgesia in rat muscle and hind paw. J Pain. 2007;8:127–136. doi: 10.1016/j.jpain.2006.06.010. [DOI] [PubMed] [Google Scholar]
- LoVerme J, Russo R, La Rana G, Fu J, Farthing J, Mattace-Raso G, et al. Rapid broad-spectrum analgesia through activation of peroxisome proliferator-activated receptor-alpha. J Pharmacol Exp Ther. 2006;319:1051–1061. doi: 10.1124/jpet.106.111385. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- Merrill EG, Ainsworth A. Glass-coated platinum-plated tungsten microelectrodes. Med Biol Eng. 1972;10:662–672. doi: 10.1007/BF02476084. [DOI] [PubMed] [Google Scholar]
- Moraes LA, Piqueras L, Bishop-Bailey D. Peroxisome proliferator-activated receptors and inflammation. Pharmacol Ther. 2006;110:371–385. doi: 10.1016/j.pharmthera.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Morris CJ.Carrageenan-induced paw edema in the rat and mouse Inflammation Protocols 2003Humana Press: Totowa, New Jersey; 115–121.In: Winyard PG, Willoughby D (eds).vol. 225 [DOI] [PubMed] [Google Scholar]
- Murakami K, Bujo H, Unoki H, Saito Y. Effect of PPARalpha activation of macrophages on the secretion of inflammatory cytokines in cultured adipocytes. Eur J Pharmacol. 2007;561:206–213. doi: 10.1016/j.ejphar.2006.12.037. [DOI] [PubMed] [Google Scholar]
- Nackley AG, Zvonok AM, Makriyannis A, Hohmann AG. Activation of cannabinoid CB2 receptors suppresses C-fiber responses and windup in spinal wide dynamic range neurons in the absence and presence of inflammation. J Neurophysiol. 2004;92:3562–3574. doi: 10.1152/jn.00886.2003. [DOI] [PubMed] [Google Scholar]
- Quartilho A, Mata HP, Ibrahim MM, Vanderah TW, Porreca F, Makriyannis A, et al. Inhibition of inflammatory hyperalgesia by activation of peripheral CB2 cannabinoid receptors. Anesthesiology. 2003;99:955–960. doi: 10.1097/00000542-200310000-00031. [DOI] [PubMed] [Google Scholar]
- Richardson JD, Kilo S, Hargreaves KM. Cannabinoids reduce hyperalgesia and inflammation via interaction with peripheral CB1 receptors. Pain. 1998;75:111–119. doi: 10.1016/S0304-3959(97)00213-3. [DOI] [PubMed] [Google Scholar]
- Rockwell CE, Kaminski NE. A cyclooxygenase metabolite of anandamide causes inhibition of interleukin-2 secretion in murine splenocytes. J Pharmacol Exp Ther. 2004;311:683–690. doi: 10.1124/jpet.104.065524. [DOI] [PubMed] [Google Scholar]
- Rockwell CE, Snider NT, Thompson JT, Vanden Heuvel JP, Kaminski NE. Interleukin-2 suppression by 2-arachidonyl glycerol is mediated through peroxisome proliferator-activated receptor gamma independently of cannabinoid receptors 1 and 2. Mol Pharmacol. 2006;70:101–111. doi: 10.1124/mol.105.019117. [DOI] [PubMed] [Google Scholar]
- Russo R, Loverme J, La Rana G, Compton TR, Parrott J, Duranti A, et al. The fatty acid amide hydrolase inhibitor URB597 (cyclohexylcarbamic acid 3′-carbamoylbiphenyl-3-yl ester) reduces neuropathic pain after oral administration in mice. J Pharmacol Exp Ther. 2007;322:236–242. doi: 10.1124/jpet.107.119941. [DOI] [PubMed] [Google Scholar]
- Sokal DM, Elmes SJ, Kendall DA, Chapman V. Intraplantar injection of anandamide inhibits mechanically-evoked responses of spinal neurones via activation of CB2 receptors in anaesthetised rats. Neuropharmacology. 2003;45:404–411. doi: 10.1016/s0028-3908(03)00195-3. [DOI] [PubMed] [Google Scholar]
- Stienstra R, Mandard S, Tan NS, Wahli W, Trautwein C, Richardson TA, et al. The interleukin-1 receptor antagonist is a direct target gene of PPARalpha in liver. J Hepatol. 2007;46:869–877. doi: 10.1016/j.jhep.2006.11.019. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, et al. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- Sun Y, Alexander SPH, Garle MJ, Gibson CL, Hewitt K, Murphy SP, et al. Cannabinoid activation of PPAR[alpha]; a novel neuroprotective mechanism. Br J Pharmacol. 2007;152:734–743. doi: 10.1038/sj.bjp.0707478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tham CS, Whitaker J, Luo L, Webb M. Inhibition of microglial fatty acid amide hydrolase modulates LPS stimulated release of inflammatory mediators. FEBS Lett. 2007;581:2899–2904. doi: 10.1016/j.febslet.2007.05.037. [DOI] [PubMed] [Google Scholar]
- Torsney C, Fitzgerald M. Spinal dorsal horn cell receptive field size is increased in adult rats following neonatal hindpaw skin injury. J Physiol. 2003;550:255–261. doi: 10.1113/jphysiol.2003.043661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi K, Takezaki N, Ueda N. The N-acylethanolamine-hydrolyzing acid amidase (NAAA) Chem Biodivers. 2007;4:1914–1925. doi: 10.1002/cbdv.200790159. [DOI] [PubMed] [Google Scholar]
- Walker JM, Huang SM, Strangman NM, Tsou K, Sanudo-Pena MC. Pain modulation by release of the endogenous cannabinoid anandamide. Proc Natl Acad Sci USA. 1999;96:12198–12203. doi: 10.1073/pnas.96.21.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise LE, Cannavacciulo R, Cravatt BF, Martin BF, Lichtman AH. Evaluation of fatty acid amides in the carrageenan-induced paw edema model. Neuropharmacology. 2008;54:181–188. doi: 10.1016/j.neuropharm.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]