Abstract
The islands of French Polynesia cover an area the size of Europe, though total land area is smaller than Rhode Island. Each hot spot archipelago (Societies, Marquesas, Australs) is chronologically arranged. With the advent of molecular techniques, relatively precise estimations of timing and source of colonization have become feasible. We compile data for the region, first examining colonization (some lineages dispersed from the west, others from the east). Within archipelagos, blackflies (Simulium) provide the best example of adaptive radiation in the Societies, though a similar radiation occurs in weevils (Rhyncogonus). Both lineages indicate that Tahiti hosts the highest diversity. The more remote Marquesas show clear examples of adaptive radiation in birds, arthropods and snails. The Austral Islands, though generally depauperate, host astonishing diversity on the single island of Rapa, while lineages on other islands are generally widespread but with large genetic distances between islands. More recent human colonization has changed the face of Polynesian biogeography. Molecular markers highlight the rapidity of Polynesian human (plus commensal) migrations and the importance of admixture from other populations during the period of prehistoric human voyages. However, recent increase in traffic has brought many new, invasive species to the region, with the future of the indigenous biota uncertain.
Keywords: Society Islands, Tahiti, Marquesas Islands, Austral Islands, adaptive radiation, dispersal
1. Introduction
The Pacific Ocean contains approximately 30 000 islands (more than the rest of the world's oceans combined; Spiess 2007). The most remote islands are those of Polynesia, notably the Hawaiian Islands, and the five archipelagos of French Polynesia: the Society, Marquesas, Austral, Gambier, and Tuamotu Islands. French Polynesia has a total land area of approximately 3660 km2 (less than one-quarter of the size of the Hawaiian Islands) and is spread over 5 million km2 (similar to the size of Europe) in the South Pacific. For most terrestrial species, these islands represent tiny specks of suitable habitat separated by vast distances, conditions that can provide successful colonists the opportunity for adaptive radiation.
Biologists have been attracted to the region since Sir Joseph Banks, as naturalist on the Endeavour (1768–1771), first returned with an intriguing collection of specimens (Whitehead 1969; Diment et al. 1984). The Hawaiian Islands, in particular, have been the focus of intensive scientific research, which has led to insights into patterns of diversification and processes of community and ecosystem development. French Polynesia, by comparison, has received much less attention. Even Darwin (1859), during his voyage on the Beagle, had little to say about the terrestrial fauna of Tahiti, being more concerned by the process of coral reef formation. His experience aptly reflects the general situation; scientific research has mostly focused on marine environments. This is partly due to the inaccessibility of the mountainous regions, but also the general perception that, overall, the region is not species rich, lacking many groups that are common to continental faunas. However, French Polynesia exhibits levels of diversity comparable to those of the Hawaiian Islands when total island area is taken into account.
Until recently, information on arthropods was largely confined to a series of articles published by the Bishop Museum (Honolulu), as a result of the Pacific Entomological Survey in the 1920s and 1930s (Adamson 1936, 1939). Information from this survey, while important in laying a foundation for future research, is both limited and dated for the following reasons. (i) In archipelagos other than the Marquesas, collecting localities were limited to a few islands and sites owing to logistical difficulties (the Marquesas were relatively well known owing to the efforts of a local entomologist, G. LeBronnec, although for any one group collections are patchy). (ii) Native and non-native elements were mixed together in most of the publications, often without precise locality information. Accordingly, useful biogeographic information is limited. For most taxa, there is much more information on cosmopolitan species, which are found in lowland areas and probably represent recent introductions. The native fauna generally appears to be confined to higher elevations, and for many taxonomic groups it remains largely unknown and undescribed.
For terrestrial molluscs, work has been largely limited to one group, the partulids of the Society and Marquesas Islands, with early studies over a century ago (Pilsbry 1900). This early work, which showed remarkable similarities among species at a large geographical scale, led to the widespread acceptance of a hypothesis that the remote islands of Polynesia were remnants of a Late Palaeozoic or Early Mesozoic mid-Pacific continent (Gregory 1928). Although this idea was debunked as geological understanding of the area increased, it dominated the literature for much of the early part of the last century. Additional influential malacological research in the South Pacific was that of Crampton (1925, 1932), who demonstrated the Mendelian inheritance of visible polymorphisms, such as shell colour and chirality, and attempted to calculate the rate of evolution from the observed changes in morph frequency, that had occurred in the time between two field trips. This work, which showed the value of these Polynesian organisms in studying evolutionary processes, arguably had a wider effect through its influence on other evolutionary biologists such as Dobzhansky and Mayr.
While overall understanding of the South Pacific is still in its infancy relative to that of the Hawaiian Islands, considerable advances have been made in recent years. Here, we compile information generated to date, focusing on molecular phylogenetic studies of terrestrial taxa, and attempt to elucidate some general biogeographic patterns in the region.
2. Colonization of the islands
Once the idea of a mid-Pacific continent had been dismissed, with geological evidence showing that the islands were formed de novo from different hot spots (Nunn 1994), the prevailing paradigm in Pacific biogeography was that the biota of the central Pacific is predominantly derived from sources on the western Pacific Rim, either from continental islands, such as New Guinea, or from continental regions, namely Australia and southeast Asia (Miller 1996). Accordingly, lineages that occur in the eastern Pacific are generally thought to have used intervening archipelagos as stepping stones for eastward dispersal (e.g. Zimmerman 1948). As a result, lineages occurring on increasingly isolated eastern Pacific islands are a subset of those found on islands to the west, resulting in lineage ‘attenuation’ (Gillespie & Roderick 2002). Recent molecular evidence has shown the pattern in some lineages of plants, in which some diversification has occurred on the different islands of Polynesia, right out to Hawaii (Wright et al. 2000; Gemmill et al. 2001), and in arthropods such as broad-nosed Rhyncogonus weevils, which have sister genera occurring on the western Pacific Rim and which appear to have colonized island chains in a conservative stepping stone pattern (Claridge et al. submitted). Other molecular studies indicate that the blackfly genus Simulium (Simuliidae) originated in Australasia and has colonized the more remote islands of the South Pacific in a stepping stone manner, diversifying prolifically in the Societies and Marquesas (Craig et al. 2001; Craig 2003). Partula land snails also appear to have colonized from west to east, with large radiations in the Society and Marquesas Islands, although the apparent retention of ancestral lineages within species complicates the interpretation of colonization history from molecular data (Goodacre 2002). Many other groups have not been the subjects of molecular phylogenetic studies, but taxonomic affiliations strongly suggest that they may have western origins and colonized the more remote islands of the Pacific progressively from the less isolated western islands.
Overall, however, few lineages have been shown to demonstrate a stepping stone model and an increasing number of molecular phylogenies indicate that several eastern Polynesian lineages colonized remote Oceania from the east (the Americas). This has been demonstrated for numerous plant lineages in the Hawaiian Islands (Eggens et al. 2007), with some of these sharing affinities with the Marquesas (Ganders et al. 2000) and the Societies (Cuenoud et al. 2000). All Hawaiian spider radiations studied show affinities with the Americas, with two of these—jumping spiders (Salticidae; Arnedo & Gillespie 2006) and crab spiders (Thomisidae; Garb & Gillespie 2006)—including the Society and Marquesas Islands in a large central Pacific lineage. This American element in the eastern Polynesian fauna should not be surprising, especially as both air and ocean currents provide opportunities for colonization of the central Pacific from either direction (Reverdin et al. 1994; Jokiel & Cox 2003). We might expect to see a continental American element on central Pacific islands proportional to the distance of island groups from the continental source.
The pertinent question may then be: why does the French Polynesian biota not show as much affinity with the Americas as does that of the Hawaiian Islands? An explanation may be the relative distances from the American continent: although North America (Mexico) is the closest mainland to the Marquesas, it is approximately 5000 km to the northeast, while only approximately 3200 km east of the Hawaiian chain (the Societies and Australs are slightly closer to Australia than South America). There may also be geological reasons that colonization from the east is more unlikely: the central Pacific is moving westward, away from the active spreading centre. Therefore, older landmasses lie to the west, making them more likely sources of colonization for younger islands to the east. Evidence of biogeographic affinities with the eastern Pacific may also have been obscured by the subduction of the eastern Pacific plate and any island chains at the eastern plate margin, beneath the Americas. Because land masses in the western Pacific are drifting westwards, colonization will generally be from older hot spot islands west of the younger islands.
Irrespective of exactly where the original colonists come from, the islands of French Polynesia, as other remote islands, are characterized by a ‘disharmonic’ biota: many entire groups, notably mammals, are absent from the native biota. There are no frogs. Among lizards, their status in the Pacific is unclear. There are five species of geckos in French Polynesia, which are almost uniform genetically and hence almost certainly of very recent origin in the area (Fisher 1997). Also, three species of widespread Pacific geckos are considered native in French Polynesia: Nactus pelagicus, Lepidodactylus sp. and Gehyra oceanica. Evidence that G. oceanica is native is based largely on a north–south oceanic divide in genetic structure; however, human-mediated dispersal cannot be ruled out. At the same time, groups, such as many birds, arthropods and snails, can be diverse with high levels of endemism.
3. Affinities within the central Pacific
Biological similarities across the islands of the central Pacific, recognized among snails (Pilsbry 1900), certain insects (Meyrick 1935a,b), spiders (Berland 1942) and plants (Guillaumin 1928; Campbell 1933), were initially explained as the result of a former land connection across the Pacific Ocean. However, it is now clear that colonization of the islands occurred through transoceanic dispersal. Similarities among the biotas of different archipelagos can be attributed to two types of dispersal (Gillespie 2002): (i) jumping from one island group to the next, as described above, or (ii) long-distance colonization by only the most dispersive organisms that can readily colonize repeatedly and independently from a mainland source, with similarity across archipelagos explained by convergent adaptation to similar ecological opportunities. For example, reed warblers have colonized nearly all the islands of the Marquesas and look remarkably similar across the islands. However, molecular data indicate that the Marquesas reed warbler includes two independent lineages: the northern Marquesas reed warbler, closely related to the Tuamotu reed warbler, and the southern Marquesas reed warbler, sister to that of Kiribati (Cibois et al. 2007). Both colonizations occurred ca 0.6 Ma, more recently than the formation of the islands, and suggest that the bird is a ‘supertramp’, colonizing remote islands easily and repeatedly.
Although a few lineages have colonized all the islands of the central Pacific from either the east or the west, others include elements that have colonized part of the Pacific from the west, and part from the east. The questions are: where do they meet and how do they interact where they meet? This has been examined in crab spiders (Thomisidae; Garb & Gillespie 2006). Two of the common genera in the central Pacific are Misumenops and Diaea. Both genera are worldwide in distribution but Misumenops has its highest diversity in the Americas (65% of species), though with some representation in Asia (15%) and none in Australia/New Guinea; Diaea has its highest diversity in Australia/New Guinea (77% species) and very few species in the Americas (2%). A third genus, Mecaphesa, has four endemic Hawaiian species (Simon 1900). Misumenops is widely distributed in eastern Polynesia (Hawaiian, Society, Marquesas and Austral Islands), while representatives of Diaea occupy parts of western Polynesia (Samoa, Tonga and New Zealand) and Melanesia (Fiji, New Caledonia, New Guinea, Solomon Islands and Vanuatu; Platnick 2008). Molecular data have shown that the Austral Islands species, Misumenops rapaensis, is far more closely related to Diaea spp. from western Polynesian and Melanesia than to other species of Misumenops from eastern Polynesia. The narrow and well-defined break between fauna originating from the east and west is interesting because the eastern fauna covers 4500 km from Hawaii to the Societies and extends to North America, yet does not reach the Australs, which are only 600 km south of Tahiti. Moreover, the ranges of the two lineages abut, but do not overlap. The ability of either group to become successfully established in any of these islands may depend on the order of their arrival. Indeed, it is likely that many taxa that have colonized any of the remote islands successfully have the potential for further colonization, with this potential seldom being realized owing to historical precedence of earlier colonists.
4. Biogeographic patterns within archipelagos
Whatever their origin, much of the fauna of the remote French Polynesian islands, just as the Hawaiian fauna, may be endemic not only to an archipelago but often to a single island and even a single area within an island. Moreover, there can be multiple closely related species in an archipelago (usually attributed to adaptive radiation; Craig et al. 2001). However, patterns may differ considerably between islands.
(a) Society Islands
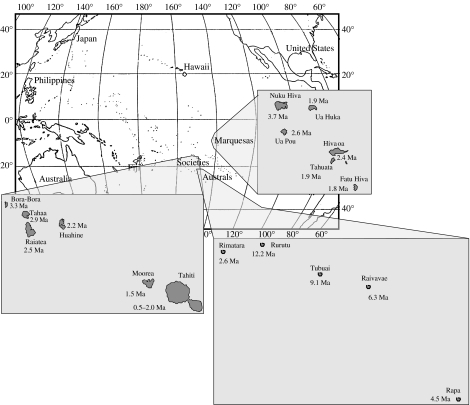
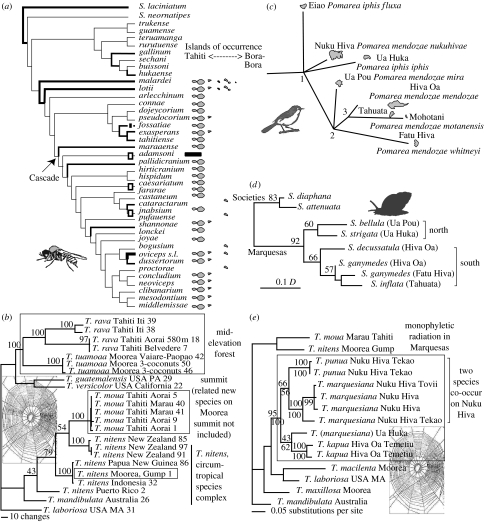
Age progression in the Society Islands is in good agreement with the fixed hot spot hypothesis (figure 1; Clouard & Bonneville 2005). The islands extend from Maupiti, the oldest of the current islands in the north at 4.3 Ma (Guillou et al. 2005), to the largest and youngest island of Tahiti at 2.0–0.5 Ma and the islet of Mehetia (0–0.26 Ma) in the south (Clouard & Bonneville 2005). One of the best-studied groups of arthropods in the Society Islands—and indeed in all of French Polynesia—is the blackfly genus Simulium (Joy & Conn 2001; Craig 2003). In the Society Islands, there are 31 described species of Simulium; Tahiti has the most (29) with fewer on the older and smaller islands of Moorea (10), Huahine (2) and Bora-Bora (1). The two widespread species, Simulium malardei and Simulium lotii, appear to be basal, and all species appear to have arisen on the youngest island, Tahiti, with back-dispersal of the highly modified cascade-dwelling species northwest to Moorea and Raiatea (figure 2a). The phylogenetically basal position of the widespread species coupled with the molecular clock calibration suggest that the Tahiti specialist species Simulium oviceps is 1.8–2.0 Ma old, which fits the geological framework (Clouard & Bonneville 2005), although it is also possible that some of the species on Tahiti arose on older islands from which they have subsequently gone extinct (Craig 2003). Extinctions of habitat specialist species, and others, on the older islands might be expected owing to island erosion and concomitant loss of running water habitats. Tahiti is the largest island at present, with an abundance of running water habitats; however, its diversity may simply be a temporary result of the latest intra-island species radiation. As Tahiti ages and erodes away, most of these species will no doubt become extinct.
Figure 1.
The main high archipelagos in French Polynesia, with geological ages indicated (Clouard & Bonneville 2005).
Figure 2.
Phylogenetic hypotheses for different lineages on the (a,b) Society and (c–e) Marquesas Islands. (a) Phylogenetic hypothesis for blackflies, Simulium (Simuliidae), from the Society Islands showing distribution and shifts in larval habitat (redrawn from Craig et al. 2001). Islands within the Society chain where each species occurs are shown (Simuliun adamsoni is the only species in the Society Island clade that does not occur in the Societies, as indicated by the black bars). Habitats range from streams, of various types, to rivers, cascades and madicolous flow (thin films of water). Line width is proportional to the number of different habitats used. The phylogeny suggests a single shift to the specialized cascade habitat with three losses (white bars on branches) and one independent gain earlier in the phylogeny (black bar to Simulium fossatiae). (b) Phylogenetic hypothesis of spiders, genus Tetragnatha (Araneae, Tetragnathidae), in the Society Islands. The data are based on sequences (approx. 750 bp) of mitochondrial COI DNA (GenBank accession numbers EU796899–EU796932). Details of methods are provided by Gillespie (2002). Analysis was by parsimony, maximum likelihood and Bayesian estimates of likelihood, and values beside nodes indicate bootstrap support (above node) and posterior probabilities (below node). (c) Phylogenetic tree for the Pomarea monarch flycatchers mapped on the Marquesas Islands (branch lengths not proportional to sequence evolution; redrawn from Cibois et al. 2004). Taxa endemic to other Polynesian archipelagos are connected to the Marquesan topology with a dashed line. Three taxa, Pomarea iphis fluxa, Pomarea mendozae nukuhivae and Pomarea iphis iphis, are basal in the tree (relative positions uncertain). All other taxa form a tight clade consistent with the age and proximity of the islands. For estimating the age of lineages, the three best-supported nodes were used, labelled as 1 (separation of the basal Marquesan monarchs), 2 (divergence between the basal Marquesan taxa and the remaining taxa) and 3 (divergence between species on Hiva Oa versus Tahuata and Fatu Hiva). Node 1 was estimated at 3.0–3.3 Ma, node 2 at 1.6–1.8 Ma and node 3 at 0.41–0.45 Ma. (d) Fitch tree based on genetic distances (allozymes) among samples and species of Samoana snails from the Marquesas and Society Islands. Numbers at each node indicate the number of times that clade appeared in 100 iterations in the bootstrap analysis (redrawn from Johnson et al. 2000). (e) Phylogenetic hypothesis of Tetragnatha spiders in the Marquesas. Data and analysis as in (b).
Larval ecology has played a major role in the diversification of blackflies in the Society Islands, and fine-scale partitioning of feeding niches may have facilitated the repeated colonizations of rivers (Joy et al. 2007). Cascade populations exhibit higher levels of genetic subdivision than river populations, perhaps because cascades are intrinsically more isolated from each other than rivers. Genetic assimilation, in which moderate levels of phenotypic plasticity promote establishment in novel habitats, and subsequent selection for extreme phenotypes leading to genetic differentiation, and perhaps speciation, may have played a major role in the radiation of blackflies in the Society Islands by enhancing their ability to successfully colonize novel niches (Joy et al. 2007).
The broad-nosed weevil genus Rhyncogonus also occurs across French Polynesia. There are 16 described species (Van Dyke 1937) and 11 recently collected undescribed species in the Society Islands. As in the blackflies, Tahiti hosts the highest number of species (14) and the smaller and older islands host fewer; each species is endemic to a single island. Unlike the blackflies, in which the Society Islands appear to have been colonized once, the closest out-groups being a lineage that includes Fiji, New Caledonia and Micronesia/Marquesas (Craig et al. 2001), in Rhyncogonus there appear to have been multiple independent colonizations of Tahiti from neighbouring island chains (Claridge 2006).
There are two lineages of long-jawed spiders (Tetragnatha, Tetragnathidae; Gillespie 2003b), representing independent colonizations to the islands (Gillespie 2002). Tetragnatha moua is limited to the highest elevations of Tahiti, with a close relative (undescribed) on the summit of Moorea (R. G. Gillespie 2007, unpublished data), while Tetragnatha rava and Tetragnatha tuamoaa are sister taxa that occur lower down on both islands (figure 2b). A similar pattern, in which species from the cloud forest (limited to the summits of Raiatea, Moorea and Tahiti) are most closely related to taxa in other adjacent cloud forests rather than species farther down the mountain on the same island (Gillespie et al. 2008; R. G. Gillespie 2007, unpublished data), has been shown for Polynesian plants (Meyer 2004). Likewise, taxa lower down the mountain are related to others lower down on adjacent islands. This is in contrast to the genetic similarity between Partula snail species found in the same geographical location rather than between those in similar habitat types at different geographical locations, a factor that is believed to be strongly associated with historic (and/or contemporary) hybridization between sympatric species (Goodacre 2002).
Like the arthropods, the terrestrial snails of the genus Partula in French Polynesia also show what is probably an important feature of Pacific Island colonization, i.e. occasional long-distance migration may play an important part in determining the current distributions, since indirect estimators of gene flow have much higher values than those predicted directly from observed migration distances (Murray & Clarke 1984). One explanation for the discrepancy is that direct measures fail to account for the effects of occasional long-distance migrants. Like the blackflies, there are fewer species of Partula on older islands (Johnson et al. 1993).
Pacific islands such as Tahiti and Moorea have high ridges and steep-sided valleys, characteristic features of volcanic islands, but the ridges are no significant barrier to the movement of snails, which are often observed at high altitude. Despite the absence of the current movement barriers, marked differences are observed between conspecific Partula populations, which appear highly structured in terms of shell shape, shell colour and banding patterns (Clarke & Murray 1969; Johnson et al. 1986) and mitochondrial DNA haplotypes (Goodacre 2002). A leptokurtic pattern of dispersal may explain this patchiness, which is the expected consequence of long-distance migration, that is predicted to remain for many generations in the absence of selection (Ibrahim et al. 1996).
(b) Marquesas Islands
The Marquesas extend from Nuku Hiva, the oldest of the current high islands in the north at 3.7 Ma (plus some lower islands, including Eiao, 5.5 Ma, and Hatutaa, 4.8 Ma, farther north), to Hiva Oa (2.4 Ma), Tahuata (1.9 Ma) and Fatu Hiva (1.8 Ma), the youngest island, in the south (figure 1; Clouard & Bonneville 2005). The arrangement of islands in a chronological series is not strictly regular, with Ua Huka (1.9 Ma) adjacent to the much older island of Nuku Hiva.
The best-known radiation of birds in the Marquesas is the monarch genus Pomarea (Monarchidae), which is endemic to the Cook, Society and Marquesas archipelagos, with the most extensive diversification in the Marquesas (figure 2c). The genus has suffered extensive recent extinction but molecular studies have used specimens at the American Museum of Natural History collected during the Whitney South Sea Expedition in the 1920s, which allowed development of a phylogeny of the entire genus, including extinct taxa (Cibois et al. 2004). The phylogeny is consistent with the sequential appearance of the Marquesas Islands. Differences between the ages of the islands and the estimated ages of the nodes indicated colonization 1–2 Ma after the islands emerged.
Among Tetragnatha spiders, sampling is incomplete, but data from Nuku Hiva, Ua Huka and Hiva Oa indicate that the genus has undergone a small radiation, with two sympatric species on Nuku Hiva and one species on each of the other islands (Gillespie 2003a). The species on Nuku Hiva are sisters and the sister relationship between the species on Ua Huka and Hiva Oa accords with the youth of these islands relative to Nuku Hiva (figure 2e).
There are 22 described species of Rhyncogonus weevils in the archipelago, three of which inhabit the oldest islands of Eiao and Hatutaa. There was a single colonization of the island chain. There has also been considerable diversification within the southern island group, particularly on Hiva Oa (seven species) and Fatu Hiva (five), but there are fewer species in the northern group, with Nuku Hiva, of size comparable to Hiva Oa, having just a single species (Claridge 2006).
There is less information on the diversity and history of blackflies in the Marquesas, with the southern islands poorly sampled. However, the flies have not diversified into specialized habitats, as in the Society Islands, and the larvae of most species are habitat generalists.
Partulid land snails of the genus Samoana are monophyletic in the Marquesas, based on allozyme variation, with multiple co-occurring species (Johnson et al. 2000; figure 2d). Several Marquesan Samoana species display a suite of characteristics (thick shells, short tentacles and non-sticky mucus) that were thought to be restricted to the partulid genus Partula, drawing into question the classification of these species. But molecular data (Johnson et al. 2000; Goodacre & Wade 2001) showed the similarity to be the result of independent evolution of ‘thick-shelled’ and ‘thin-shelled’ species in Samoana. The recent discovery of a thin-shelled Partula on Raiatea demonstrates that such independent evolution has occurred several times and in both directions (Burch 2007).
(c) Austral Islands
The Austral archipelago is approximately 500 km southwest of the Society Islands (at their closest points: between Tahiti and Rurutu) and extends over 1500 km from the southernmost Marotiri Isles to the atoll Maria. The Austral Islands are geologically continuous with the Cook Islands (to the northwest), which together were formed from repeated episodes of vulcanism at several sites (Dickinson 1998; Bonneville et al. 2002). Potassium–Argon (K–Ar) dating indicates that the Cook–Austral chain began forming ca 20–30 Ma (Keating 1987; Munschy et al. 1998) with ages of the Australs ranging from 4.3 Ma (Marotiri Isles) to 15.7 Ma (Maria), and the main islands ranging from 4.5 to 12.2 Ma (Clouard & Bonneville 2005; figure 1). The Austral Islands, like the Societies and Marquesas, are sequentially ordered from southeast to northwest by increasing age, as a result of the north-westward movement of the Pacific tectonic plate over stationary volcanic plumes, decreasing in age from northwest to southeast. However, there was secondary volcanic activity beneath Rurutu ca 1–2 Ma, which is also associated with secondary uplift of the island and the neighbouring island of Rimatara. It is possible that both islands were subaerial prior to their recent uplift. Thus, the progression rule here would predict colonization from west to east down the chain, but with possible secondary recolonization of Rurutu and Rimatara.
Although generally taxonomically depauperate, the Austral Islands host a surprising number of endemic species, in particular on Rapa (Claridge et al. in press). For example, among beetles, the genus Miocalles, a group of tiny flightless weevils, has undergone an astonishing radiation on Rapa with 67 described species in an area of just 40 km2, with diversification accompanied by striking morphological and ecological differentiation (Paulay 1985). The weevil genus Rhyncogonus is also particularly diverse in the Australs, with 22 described species, half of these on Rapa, though three previously undescribed species have been collected recently on Raivavae, and the oldest high island, Rimatara, just 8 km2 and 83 m high, supports an endemic species (Van Dyke 1937; Claridge 2006). Studies by Clarke (1971) on the Lepidoptera Rapa resulted in the description of a number of diverse groups. Recent phylogenetic studies (Craig et al. 2001; Wright et al. 2001; Mitchell & Heenan 2002) have incorporated a few species from some of these islands in the context of broader biogeographic analyses of relationships among different sets of islands in the Pacific Basin. However, there have been no thorough phylogenetic treatments of these groups and much work remains to elucidate the enigmatic diversity of Rapa's insects.
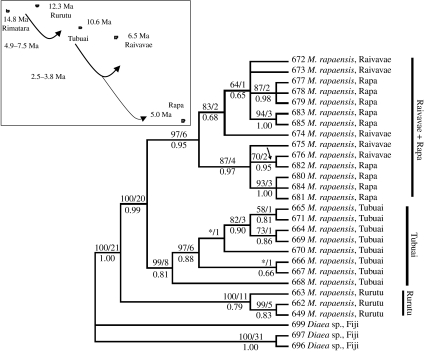
Similar stories are emerging of sequential colonization of islands by spiders, with large genetic distances between island populations. The crab spider M. rapaensis occurs throughout the Austral Islands, with large genetic distances between islands: the uncorrected distance between Rurutu and Tubuai is 8.4 per cent, nearly as much as the maximal distance across all 16 included Hawaiian taxa (Garb & Gillespie 2006; figure 3). Molecular clock estimates indicate that the split between the Tubuai clade and the clade comprising individuals from Raivavae and Rapa occurred more recently (ca 2.5–3.8 Ma) than the divergence of M. rapaensis from Rurutu and Tubuai (estimated at 4.9–7.5 Ma), suggesting that M. rapaensis initially colonized the Australs several million years after the formation of Rurutu (12.7 Ma) and Tubuai (10.4 Ma) but possibly before the emergence of Raivavae (6.8 Ma) and Rapa (5.0 Ma). The estimated divergences further suggest that from the initial point of colonization (Rurutu or Tubuai), M. rapaensis did not reach Raivavae or Rapa until the emergence of both islands. The orb web spider Tangaroa tahitiensis (Uloboridae) occurs throughout both the Society and Austral Islands, again with large genetic distances between islands (R. G. Gillespie 2007, unpublished data), although, as with M. rapaensis, the data suggest a more recent colonization than the geological age would predict.
Figure 3.
Phylogenetic hypotheses for crab spiders, M. rapaensis (Thomisidae), in the Austral Islands inferred from molecular genetic analysis (Garb & Gillespie 2006). Numbers above branches refer to parsimony bootstrap values from 1000 replicates (asterisks indicate less than 50% bootstrap support) followed by decay indices. Numbers below the branch indicate posterior probability values.
The land snail fauna of the Australs is particularly impressive, with more than 100 species on Rapa alone (Solem 1982, 1984), but their relationship to species elsewhere in Polynesia has not been studied in depth using molecular methods, with one exception. The rather atypically widely distributed Partula hyalina is shared among the geographically distant Cook, Society and Austral archipelagos, a distribution suggested as being the legacy of prehistoric human transport (Lee et al. 2007a).
5. Human colonization
The human history of Polynesia is one of the most fascinating and tractable systems in which to examine the interactions between people and biodiversity. The best-known scenarios for the peopling of the Pacific are the ‘express train’ and the ‘entangled bank’ (Hurles et al. 2003). The express train is built on the notion of a simple spread, with a strong phylogenetic signal, of Polynesian ancestors (Austronesians), first into near Oceania and then into remote Oceania. The entangled bank is a reticulate model that highlights the importance of ongoing interaction among populations. Many intermediate models have been proposed, such as the ‘slow train’ (more genetic mixing between Austronesians and the original inhabitants before moving into remote Oceania) and ‘slow boat’ (ultimate origin Asia, and proximate origin within Wallacea). Mitochondrial DNA studies have detected characteristic haplotypes, such as a 9 bp deletion at high frequency, that predominate in Polynesia. The mutations imply an ultimate origin in Asia (‘out of Asia’ model), with subsequent movement following either the express-train, slow-train or slow-boat models (Melton et al. 1995). Y-chromosome studies also show male-biased European admixture among some Polynesian populations and raise the possibility of sex-specific differences in prehistoric demography, including levels of endogamy and/or migration patterns (Su et al. 2000).
Polynesians carried a range of plants and animals with them as they moved across the Pacific, and genetic evidence from these has also been examined. Mitochondrial DNA lineages shared between Pacific rat (Rattus exulans) populations identify homeland regions, and disjunct variation within island rat populations suggests separate introductions from several sources, with origins in the Lapita and a spread into remote Oceania. The express train to Polynesia model is rejected, while a ‘voyaging corridor’ aspect of the slow-boat model is supported, but with most of the evidence for a ‘voyaging corridor triple’, a model allowing various components of the Lapita cultural complex to be the result of intrusion of new components (Matisoo-Smith & Robins 2004). Other animals examined include pigs, dogs and chickens, although evidence from these is complicated by more recent introduction of the same species by European settlers. However, recent data from the pig, Sus scrofa, suggest that a Pacific clade originated in peninsular Southeast Asia, where they were first domesticated. Polynesian dispersals into Oceania appear to be exclusively associated with Pacific clade pigs (Larson et al. 2007).
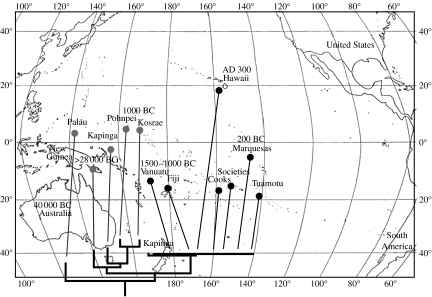
Other organisms were introduced accidentally by early Polynesians. For example, genetic analysis of populations across the Pacific of Lipinia noctua, a lizard native to New Guinea, which has been transported from near to remote Oceania, support the express-train model of human colonization (figure 4; Austin 1999).
Figure 4.
Evidence of the ‘express-train’ model of Polynesian island colonization by lizards (redrawn from Austin 1999). Maximum-parsimony phylogram for Lipinia noctua; localities denoted by grey circles are all genetically distinct and represent natural prehuman dispersal. Localities denoted by black circles are genetically similar (mean sequence divergence 0.008%) and represent human-mediated dispersal within the past 4000 years. Dates represent approximate time of first human settlement.
In addition to the standard migration routes of Polynesians and associated organisms, recent molecular work has revealed ‘trading’ of species between archipelagos. In particular, the white-shelled tree snail, P. hyalina, has been found in the Society, Austral and southern Cook Islands. Recent molecular work has shown that P. hyalina was originally restricted to Tahiti, but trading between archipelagos resulted in multiple founder populations in the Australs and southern Cooks (Lee et al. 2007a). With the recent arrival of the alien carnivorous land snail Euglandina rosea and subsequent devastation of island snails on Tahiti (see below), P. hyalina is now restricted to the southern Cooks and Australs; on Tahiti it still persists but is threatened.
6. Recent impacts
Since the arrival of Europeans in Polynesia (late 1700s), the rate of influx of non-native species has increased tremendously. Native plants are now largely confined to montane forests above approximately 300 m (cloud forests and subalpine forests), while coastal, dry lowland and low and middle elevation valley forests have been seriously disrupted by human activities, introduced mammals (feral goats, sheep, cattle, horses, pigs) and invasive plants (approx. 375 alien plant species are naturalized on Tahiti, and approx. 220 on Nuku Hiva (Florence 1993)). The many bird extinctions on these islands are now well known (Steadman & Rolett 1996).
The origins of more recent arrivals to French Polynesia can best be predicted by tracing international trade and transport routes. Most invasive species arrive in French Polynesia via the international airport or port on Tahiti and then gradually spread across the region, again following transport routes (Grandgirard et al. 2006; Petit et al. in press). Recent introductions to French Polynesia include a series of predators. The sac spider Cheiracanthium mordax (Miturgidae), native to Australia, is invasive in the Pacific, with almost identical haplotypes from Micronesia through all of French Polynesia to Hawaii (R. G. Gillespie 2007, unpublished data). Likewise, the spider Pholcus ancoralis, also native to Australia, has almost identical haplotypes in Fiji, Micronesia and all of French Polynesia; it has also recently been reported in Hawaii (R. G. Gillespie 2007, unpublished data). All these introductions appear to have been accidental, mediated through transportation routes.
An exception to the main route of accidental introduction is the arrival of the rosy wolf snail, E. rosea, which was deliberately introduced to French Polynesia as part of a biological control programme directed towards eliminating the giant African land snail, Achatina fulica. Documentation of the spread of this non-native species illustrates the speed at which invasive species may become established and influence the distribution of native species (Clarke et al. 1984). Indeed, snails in the genus Partula epitomize rapid extinction caused by an introduced predator, with 56 of 61 Society Island species now extinct in the wild (Cowie 1992; Coote & Loeve 2003). However, recent molecular work paints a more positive conservation picture, with montane populations of Partula otaheitana and valley populations of Partula clara/Partula hyalina persisting, and these populations include genetic representation of all major mitochondrial clades that occurred historically on Tahiti (Lee et al. 2007b).
Other non-native taxa that have been studied in the Pacific include parasites and their associated vectors. Among parasites, the Hawaiian form of malaria is the only lineage of malaria parasite that appears to be common among passerines of French Polynesia (Beadell et al. 2006). In a survey of birds on Moorea, Society Islands, this parasite lineage was seen at low frequency in several introduced species (no data on native species owing to their rarity). However, in the Marquesas, the parasite is common in a small sample of endemic Marquesan reed warblers (Acrocephalus mendanae), suggesting that these birds, which are relatively recent (1–2 Ma) colonists of the islands, may be resistant to the parasites. On the other hand, the Pomarea flycatchers may have succumbed to the disease.
Marked genetic structure has been demonstrated among Tahiti and Moorea populations of mosquitoes, in particular Aedes aegypti, which is associated with dengue fever in humans. Here, the structure appears to be dictated by human population density, intensity of insecticidal control and ecological characteristics of mosquito ecotopes (Paupy et al. 2000).
The glassy-winged sharpshooter, Homalodisca coagulata, is notorious in North America for transmitting Pierce's disease, caused by the bacterium Xylella fastidiosa, in grapevines. The bacterium can also infect many other plants. The sharpshooter's arrival in French Polynesia (Tahiti) in July 1999 caused considerable concern, both owing to its abundance, which results in almost constant ‘rain’ from the excreta in some areas, and its potential role as a vector of X. fastidiosa, which could affect species of Metrosideros, Weinmannia, Dodonaea, Glochidion, Hibiscus and Gardenia in the Society Islands (Grandgirard et al. 2006). Populations of H. coagulata are geographically structured into two groups of COI haplotypes in North America, a group of populations from east of the Mississippi River, and a group comprising populations from west of the Mississippi River (Texas and California): haplotypes from Tahiti fall in the latter group (Smith 2005).
7. Conclusions
Several general remarks can be made from this overview of studies of such a wide range of taxa throughout French Polynesia. First, patterns of biodiversity differ among archipelagos within the area. This may reflect environmental heterogeneity across the region and differences in the degree to which island populations are influenced by immigration, natural selection and drift. For example, the Society Islands are characterized by high numbers of endemic species on the youngest island, with independent colonization from either neighbouring islands or archipelagos playing a prominent role in shaping biodiversity. By contrast, the Australs show a somewhat different pattern, with little evidence of ecological differentiation on the low islands but extensive genetic divergence among islands. This pattern might be predicted on the basis of the small size of these islands, if size limits diversification. If so, Rapa, the southernmost of the Australs, is an exception, with its high level of endemism and striking examples of adaptive radiation, despite being similar in size to others in the Austral group.
The Marquesas show a strong signal of adaptive radiation within the archipelago, although to a lesser extent than in the Hawaiian Islands. Invertebrates are characterized by a large number of monophyletic lineages with evidence for at least some degree of adaptive radiation and (in snails) for parallel evolution of similar morphs on different islands.
Phylogenies for the Australs and Marquesas show some support for the progression rule, a pattern seen in the Hawaiian Islands, where lineage formation reflects successive colonization of islands in the order of their formation (Wagner & Funk 1995). However, at least for spiders in the Australs, the age of the lineages is considerably younger than the geological age of the islands. There is little evidence as yet for the progression rule in the Societies.
Another general trend is that the non-native biota appears to be similar across much of French Polynesia. It is interesting to observe that native communities appear to be more intact on the Australs than on the other higher archipelagos, at least at lower elevations. This is somewhat surprising, given that native communities are smaller and apparently more depauperate on the Australs; it could provide insights into factors underlying successful biological invasion. Further work is required in order to understand the interaction between native and non-native elements of the biota. In summary, results to date highlight the value of the fauna of French Polynesia for studying the different evolutionary processes that drive adaptation, speciation and community assembly, and the forces that govern how species invade and become established over both evolutionary and ecological time.
Acknowledgments
We thank Rob Cowie and Steve Trewick for organizing this special issue. The manuscript was greatly improved by the comments of three anonymous reviewers. This work was supported by the Territorial Government of French Polynesia, with funds from the National Science Foundation (DEB 0451971 to R.G.G.), the Schlinger Foundation, University of California Berkeley and the Gordon and Betty Moore Foundation.
Footnotes
One contribution of 15 to a Theme Issue ‘Evolution on Pacific islands: Darwin's legacy’.
References
- Adamson A.M. Marquesan insects: environment. Bernice P. Bishop Mus. Bull. 1936;139:1–73. [Google Scholar]
- Adamson A.M. Review of the fauna of the Marquesas Islands and discussion of its origin. Bernice P. Bishop Mus. Bull. 1939;159:1–93. [Google Scholar]
- Arnedo M.A, Gillespie R.G. Species diversification patterns in the Polynesian jumping spider genus Havaika Proszynski 2001 (Araneae, Salticidae) Mol. Phylogenet. Evol. 2006;41:472–495. doi: 10.1016/j.ympev.2006.05.012. doi:10.1016/j.ympev.2006.05.012 [DOI] [PubMed] [Google Scholar]
- Austin C.C. Lizards took express train to Polynesia. Nature. 1999;397:113–114. doi:10.1038/16365 [Google Scholar]
- Beadell J.S, et al. Global phylogeographic limits of Hawaii's avian malaria. Proc. R. Soc. B. 2006;273:2935–2944. doi: 10.1098/rspb.2006.3671. doi:10.1098/rspb.2006.3671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berland L. Polynesian spiders. Occas. Pap. Bernice P. Bishop Mus. 1942;17:1–24. [Google Scholar]
- Bonneville A, LeSuave R, Audin L, Clouard V, Dosso L, Gillot P, Janney P, Jordahl K, Maamaatuaiahutapu K. Arago Seamount: the missing hotspot found in the Austral Islands. Geology. 2002;30:1023–1026. doi:10.1130/0091-7613(2002)030<1023:ASTMHF>2.0.CO;2 [Google Scholar]
- Burch J.B. A new species of land snail (Stylommatophora, Partulidae) from Raiatea, French Polynesia, Oceania. Occas. Pap. Mus. Zool. Univ. Michigan. 2007;740:1–8. [Google Scholar]
- Campbell D.H. The flora of the Hawaiian Islands. Quart. Rev. Biol. 1933;8:164–184. doi:10.1086/394432 [Google Scholar]
- Cibois A, Thibault J.-C, Pasquet E. Biogeography of eastern Polynesian monarchs (Pomarea): an endemic genus close to extinction. Condor. 2004;106:837–851. doi:10.1650/7491 [Google Scholar]
- Cibois A, Thibault J.C, Pasquet E. Uniform phenotype conceals double colonization by reed-warblers of a remote Pacific archipelago. J. Biogeogr. 2007;34:1150–1166. doi:10.1111/j.1365-2699.2007.01703.x [Google Scholar]
- Claridge, E. M. 2006 The Systematics and diversification of Rhyncogonus (Entiminae: Curculionidae: Coleoptera) in the central Pacific. PhD thesis, University of California, Berkeley.
- Claridge, E. M., Garb, J. E., Gillespie, R. G. & Percy, D. M. In press. Insects and spiders of the Austral Islands. In The terrestrial biodiversity of the Austral Islands (French Polynesia) (eds J.-Y. Meyer, P. Bouchet & A. Allison). Paris & Honolulu: Service des Publications du Muséum national d'Histoire naturelle.
- Claridge, E. M., Gillespie, R. G. & Roderick, G. K. Submitted. Repeated radiation: diversification of South Pacific weevils (Coleoptera: Curculionidae: Entiminae: Rhyncogonus).
- Clarke J.F.G. The Lepidoptera of Rapa Island. Smithson. Contrib. Zool. 1971;56:1–282. [Google Scholar]
- Clarke B, Murray J. Ecological genetics and speciation in land snails of the genus Partula. Biol. J. Linn. Soc. 1969;1:31–42. doi:10.1111/j.1095-8312.1969.tb01810.x [Google Scholar]
- Clarke B, Murray J, Johnson M.S. The extinction of endemic species by a programme of biological control. Pac. Sci. 1984;38:97–104. [Google Scholar]
- Clouard V, Bonneville A. Ages of seamounts, islands, and plateaus on the Pacific plate. Geol. Soc. Am. Bull. 2005;388:71–90. [Google Scholar]
- Coote T, Loève E. From 61 species to five: endemic tree snails of the Society Islands fall prey to an ill-judged biological control programme. Oryx. 2003;37:91–96. doi:10.1017/S0030605303000176 [Google Scholar]
- Cowie R.H. Evolution and extinction of Partulidae, endemic Pacific Island land snails. Phil. Trans. R. Soc. B. 1992;335:167–191. doi:10.1098/rstb.1992.0017 [Google Scholar]
- Craig D.A. Geomorphology, development of running water habitats, and evolution of black flies on Polynesian islands. BioScience. 2003;53:1079–1093. doi:10.1641/0006-3568(2003)053[1079:GDORWH]2.0.CO;2 [Google Scholar]
- Craig D.A, Currie D.C, Joy D.A. Geographical history of the central-western Pacific black fly subgenus Inseliellum (Diptera: Simuliidae: Simulium) based on a reconstructed phylogeny of the species, hot spot archipelagoes and hydrogeological considerations. J. Biogeogr. 2001;28:1101–1127. doi:10.1046/j.1365-2699.2001.00619.x [Google Scholar]
- Crampton H.E. Studies on the variation, distribution and evolution of the genus Partula. The species of the Mariana Islands, Guam and Saipan. Carnegie Inst. Wash. Pub. 1925;228A:1–116. [Google Scholar]
- Crampton H.E. Studies on the variation, distribution, and evolution of the genus Partula. The species inhabiting Moorea. Carnegie Inst. Wash. Pub. 1932;410:1–335. [Google Scholar]
- Cuenoud P, Del Pero Martinez M.A, Loizeau P.-A, Spichiger R, Andrews S, Manen J.-F. Molecular phylogeny and biogeography of the genus Ilex L. (Aquifoliaceae) Ann. Bot. 2000;85:111–122. doi:10.1006/anbo.1999.1003 [Google Scholar]
- Darwin C. Penguin Classics; Harmondsworth, UK: 1859. The Origin of species by means of natural selection. [Google Scholar]
- Dickinson W.R. Geomorphology and geodynamics of the Cook-Austral Island seamount chain in the South Pacific Ocean: implications for hotspots and plumes. Int. Geol. Rev. 1998;40:1039–1075. [Google Scholar]
- Diment J.A, Humphries C.J, Newington L, Shaughnessy E. Catalogue of the natural history drawings commissioned by Joseph Banks on the Endeavour voyage 1768–1771 held in the British Museum (Natural History) Bull. Br. Mus. Nat. Hist. 1984;11:1–183. [Google Scholar]
- Eggens F, Popp M, Nepokroeff M, Wagner W.L, Oxelman B. The origin and number of introductions of the Hawaiian endemic Silene species (Caryophyllaceae) Am. J. Bot. 2007;94:210–218. doi: 10.3732/ajb.94.2.210. doi:10.3732/ajb.94.2.210 [DOI] [PubMed] [Google Scholar]
- Fisher R.N. Dispersal and evolution of the Pacific basin gekkonid lizards Gehyra oceanica and Gehyra mutilata. Evolution. 1997;51:906–921. doi: 10.1111/j.1558-5646.1997.tb03672.x. doi:10.2307/2411165 [DOI] [PubMed] [Google Scholar]
- Florence, J. 1993 La végétation de quelques îles de Polynésie française. In Atlas de la Polynésie Française (ed. ORSTOM), pp. 54–55. Paris, France: ORSTOM.
- Ganders F.R, Berbee M, Pirseyedi M. ITS base sequence phylogeny in Bidens (Asteraceae): evidence for the continental relatives of Hawaiian and Marquesan Bidens. Syst. Bot. 2000;25:122–133. doi:10.2307/2666678 [Google Scholar]
- Garb J.E, Gillespie R.G. Island hopping across the central Pacific: mitochondrial DNA detects sequential colonization of the Austral Islands by crab spiders (Araneae: Thomisidae) J. Biogeogr. 2006;33:201–220. doi:10.1111/j.1365-2699.2005.01398.x [Google Scholar]
- Gemmill C.E.C, Allan G.J, Wagner W.L, Zimmer E.A. Evolution of insular Pacific Pittosporum (Pittosporaceae): origin of the Hawaiian radiation. Mol. Phylogenet. Evol. 2001;22:31–42. doi: 10.1006/mpev.2001.1019. doi:10.1006/mpev.2001.1019 [DOI] [PubMed] [Google Scholar]
- Gillespie R.G. Colonization of remote oceanic islands of the Pacific: archipelagos as stepping stones? J. Biogeogr. 2002;29:655–662. doi:10.1046/j.1365-2699.2002.00714.x [Google Scholar]
- Gillespie R.G. Marquesan spiders of the genus Tetragnatha. J. Arachnol. 2003a;31:62–77. doi:10.1636/0161-8202(2003)031[0062:MSOTGT]2.0.CO;2 [Google Scholar]
- Gillespie R.G. Spiders of the genus Tetragnatha in the Society Islands. J. Arachnol. 2003b;31:157–172. doi:10.1636/0161-8202(2003)031[0157:SOTGTA]2.0.CO;2 [Google Scholar]
- Gillespie R.G, Roderick G.K. Arthropods on islands: evolution and conservation. Annu. Rev. Entomol. 2002;47:595–632. doi: 10.1146/annurev.ento.47.091201.145244. doi:10.1146/annurev.ento.47.091201.145244 [DOI] [PubMed] [Google Scholar]
- Gillespie R.G, Claridge E.M, Roderick G.K. Biodiversity dynamics in isolated island communities: interaction between natural and human-mediated processes. Mol. Ecol. 2008;17:45–57. doi: 10.1111/j.1365-294X.2007.03466.x. doi:10.1111/j.1365-294x.2007.03466.x [DOI] [PubMed] [Google Scholar]
- Goodacre S.L. Population structure, history and gene flow in a group of closely-related land snails: genetic variation in Partula from the Society Islands of the Pacific. Mol. Ecol. 2002;11:55–68. doi: 10.1046/j.0962-1083.2001.01422.x. doi:10.1046/j.0962-1083.2001.01422.x [DOI] [PubMed] [Google Scholar]
- Goodacre S.L, Wade C.M. Molecular evolutionary relationships between partulid land snails of the Pacific. Proc. R. Soc. B. 2001;268:1–7. doi: 10.1098/rspb.2000.1322. doi:10.1098/rspb.2000.1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandgirard J, Hoddle M.S, Roderick G.K, Petit J.N, Percy D, Putoa R, Garnier C, Davies N. Invasion of French Polynesia by the glassy-winged sharpshooter, Homalodisca coagulata (Hemiptera: Cicadellidae): a new threat to the South Pacific. Pac. Sci. 2006;60:429–438. doi:10.1353/psc.2006.0028 [Google Scholar]
- Gregory H.E. Types of Pacific islands. Proc. Third Pan-Pac. Sci. Congr. 1928;2:1662. [Google Scholar]
- Guillaumin A. Les regions floristiques du Pacifique d'apres leur endemisme et la repartition de quelques plantes phanerogames. Proc. Third Pan-Pac. Sci. Congr. 1928;1:920–928. [Google Scholar]
- Guillou H, Maury R.C, Blais S, Cotten J, Legendre C, Guille G, Caroff M. Age progression along the Society hotspot chain (French Polynesia) based on new unspiked K–Ar ages. Bull. Soc. Geol. Fr. 2005;176:135–150. doi:10.2113/176.2.135 [Google Scholar]
- Hurles M.E, Matisoo-Smith E, Gray R.D, Penny D. Untangling Oceanic settlement: the edge of the knowable. Trends Ecol. Evol. 2003;18:531–540. doi:10.1016/S0169-5347(03)00245-3 [Google Scholar]
- Ibrahim K.M, Nichols R.A, Hewitt G.M. Spatial patterns of genetic variation generated by different forms of dispersal during range expansion. Heredity. 1996;77:282–291. doi:10.1038/hdy.1996.142 [Google Scholar]
- Johnson M.S, Murray J, Clarke B.C. An electrophoretic analysis of phylogeny and evolutionary rates in the genus Partula from the Society Islands. Proc. R. Soc. B. 1986;227:161–177. doi:10.1098/rspb.1986.0017 [Google Scholar]
- Johnson M.S, Murray J, Clarke B. The ecological genetics and adaptive radiation of Partula on Moorea. Oxford Surv. Evol. Biol. 1993;9:167–238. [Google Scholar]
- Johnson M.S, Murray J, Clarke B. Parallel evolution in Marquesan partulid land snails. Biol. J. Linn. Soc. 2000;69:577–598. doi:10.1006/bijl.1999.0386 [Google Scholar]
- Jokiel P.L, Cox E.F. Drift pumice at Christmas Island and Hawaii: evidence of oceanic dispersal patterns. Mar. Geol. 2003;202:121–133. doi:10.1016/S0025-3227(03)00288-3 [Google Scholar]
- Joy D.A, Conn J.E. Molecular and morphological phylogenetic analysis of an insular radiation in Pacific black flies (Simulium) Syst. Biol. 2001;50:18–38. doi:10.1080/106351501750107431 [PubMed] [Google Scholar]
- Joy D.A, Craig D.A, Conn J.E. Genetic variation tracks ecological segregation in Pacific Island black flies. Heredity. 2007;99:452–459. doi: 10.1038/sj.hdy.6801023. doi:10.1038/sj.hdy.6801023 [DOI] [PubMed] [Google Scholar]
- Keating B. Summary of radiometric ages from the Pacific. Intergovernmental Oceanogr. Commiss. Tech. Ser., UNESCO. 1987;32:1–67. [Google Scholar]
- Larson G, et al. Phylogeny and ancient DNA of Sus provides insights into Neolithic expansion in island Southeast Asia and Oceania. Proc. Acad. Nat. Sci. Phila. 2007;104:4834–4839. doi: 10.1073/pnas.0607753104. doi:10.1073/pnas.0607753104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T, Burch J.B, Coote T, Fontaine B, Gargominy O, Pearce-Kelly P, Ó Foighil D. Prehistoric inter-archipelago trading of Polynesian tree snails leaves a conservation legacy. Proc. R. Soc. B. 2007a;274:2907–2914. doi: 10.1098/rspb.2007.1009. doi:10.1098/rspb.2007.1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T, Burch J.B, Jung Y, Coote T, Pearce-Kelly P, Ó Foighil D. Tahitian tree snail mitochondrial clades survived recent mass extirpation. Curr. Biol. 2007b;17:R502–R503. doi: 10.1016/j.cub.2007.05.006. doi:10.1016/j.cub.2007.05.006 [DOI] [PubMed] [Google Scholar]
- Matisoo-Smith E, Robins J.H. Origins and dispersals of Pacific peoples: evidence from mtDNA phylogenies of the Pacific rat. Proc. Acad. Nat. Sci. Phila. 2004;101:9167–9172. doi: 10.1073/pnas.0403120101. doi:10.1073/pnas.0403120101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton T, Peterson R, Redd A.J, Saha N, Sofro A.S, Martinson J, Stoneking M. Polynesian genetic affinities with Southeast Asian populations as identified by mtDNA analysis. Am. J. Hum. Genet. 1995;57:403–414. [PMC free article] [PubMed] [Google Scholar]
- Meyer J.-Y. Threat of invasive alien plants to native flora and forest vegetation of eastern Polynesia. Pac. Sci. 2004;58:357–375. doi:10.1353/psc.2004.0032 [Google Scholar]
- Meyrick E. Pyrales and Microlepidoptera of the Marquesas Islands. Bernice P. Bishop Mus. Bull. 1935a;114:333–355. [Google Scholar]
- Meyrick E. Pyrales and Microlepidoptera of the Society Islands. Bernice P. Bishop Mus. Bull. 1935b;113:109–110. [Google Scholar]
- Miller S.E. Biogeography of Pacific insects and other terrestrial invertebrates: a status report. In: Keast J.A, Miller S.E, editors. The origin and evolution of Pacific Island biotas, New Guinea to Eastern Polynesia: patterns and processes. SPB Academic Publishing; Amsterdam, The Netherlands: 1996. pp. 463–475. [Google Scholar]
- Mitchell A.D, Heenan P.B. Sophora sect. Edwardsia (Fabaceae): further evidence from nrDNA sequence data of a recent and rapid radiation around the Southern Oceans. Bot. J. Linn. Soc. 2002;140:435–441. doi:10.1046/j.1095-8339.2002.00101.x [Google Scholar]
- Munschy M, Antoine C, Guille G, Guillou H. La croûte océanique et les points chauds de la Polynésie Française (océan Pacifique central) Géol. Fr. 1998;3:5–13. [Google Scholar]
- Murray J, Clarke B. Movement and gene flow in Partula taeniata. Malacologia. 1984;25:343–348. [Google Scholar]
- Nunn P.D. Blackwell; Oxford, UK: 1994. Oceanic islands. [Google Scholar]
- Paulay G. Adaptive radiation on an isolated oceanic island: the Cryptorhynchinae (Curculionidae) of Rapa revisited. Biol. J. Linn. Soc. 1985;26:95–187. doi:10.1111/j.1095-8312.1985.tb01554.x [Google Scholar]
- Paupy C, Vazeille-Falcoz M, Mousson L, Rodhain F, Failloux A.B. Aedes aegypti in Tahiti and Moorea (French Polynesia): isoenzyme differentiation in the mosquito population according to human population density. Am. J. Trop. Med. Hyg. 2000;62:217–224. doi: 10.4269/ajtmh.2000.62.217. [DOI] [PubMed] [Google Scholar]
- Petit, J. N., Hoddle, M. S., Grandgirard, J., Roderick, G. K. & Davies, N. In press. Invasion dynamics of the glassy-winged sharpshooter Homalodisca vitripennis (Germar) (Hemiptera: Cicadellidae) in French Polynesia. Biol. Inv.
- Pilsbry H.E. The genesis of mid Pacific faunas. Proc. Acad. Nat. Sci. Phila. 1900;52:568–581. [Google Scholar]
- Platnick N.I. In: The world spider catalog, version 8.5. Merrett P, Cameron H.D, editors. American Museum of Natural History; New York, NY: 2008. [Google Scholar]
- Reverdin G, Frankignoul C, Kestenare E. Seasonal variability in the surface currents of the equatorial Pacific. J. Geophys. Res. Oceans. 1994;99:20 323–20 344. doi:10.1029/94JC01477 [Google Scholar]
- Simon, E. 1900 Arachnida. In Fauna Hawaiiensis, vol. 2 (ed. D. Sharp), pp. 443–519. Cambridge, UK: Cambridge University Press.
- Smith P.T. Mitochondrial DNA variation among populations of the glassy-winged sharpshooter, Homalodisca coagulata. J. Insect Sci. 2005;5:41. doi: 10.1093/jis/5.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solem A. Field Museum of Natural History; Chicago, IL: 1982. Endodontoid land snails from Pacific islands (Mollusca: Pulmonata: Sigmurethra). Part II. Families Punctidae and Charopidae, zoogeography. [Google Scholar]
- Solem A. A world model of land snail diversity and abundance. In: Solem A, van Bruggen A.C, editors. World-wide snails: biogeographical studies on non-marine Mollusca. E.J. Brill/W. Backhuys; Leiden, The Netherlands: 1984. pp. 6–22. [Google Scholar]
- Spiess, F. N. 2007 Pacific Ocean. In Microsoft Encarta online encyclopedia Redmond, WA: Microsoft Corporation. See http://www.msnencarta.com/encyclopedia_761564220/Pacific_Ocean.html
- Steadman D.W, Rolett B. A chronostratigraphic analysis of landbird extinction on Tahuata, Marquesas Islands. J. Archaeol. Sci. 1996;23:81–94. doi:10.1006/jasc.1996.0007 [Google Scholar]
- Su B, et al. Polynesian origins: insights from the Y-chromosome. Proc. Acad. Nat. Sci. Phila. 2000;97:8225–8228. doi: 10.1073/pnas.97.15.8225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyke E.C. Rhyncogonus of the Mangarevan expedition. Occas. Pap. Bernice P. Bishop Mus. 1937;13:89–129. [Google Scholar]
- Wagner W.L, Funk V.A, editors. Hawaiian biogeography. Smithsonian Institution Press; Washington, DC: 1995. [Google Scholar]
- Whitehead P.J.P. Zoological specimens from Captain Cook's voyages. J. Soc. Bibliogr. Nat. Hist. 1969;5:161–201. [Google Scholar]
- Wright S.D, Yong C.G, Dawson J.W, Whittaker D.J, Gardner R.C. Riding the ice age El Nino? Pacific biogeography and evolution of Metrosideros subg. Metrosideros (Myrtaceae) inferred from nuclear ribosomal DNA. Proc. Natl Acad. Sci. USA. 2000;97:4118–4123. doi: 10.1073/pnas.050351197. doi:10.1073/pnas.050351197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S.D, Yong C.G, Wichman S.R, Dawson J.W, Gardner R.C. Stepping stones to Hawaii: a trans-equatorial dispersal pathway for Metrosideros (Myrtaceae) inferred from nrDNA (ITS+ETS) J. Biogeogr. 2001;28:769–774. doi:10.1046/j.1365-2699.2001.00605.x [Google Scholar]
- Zimmerman E.C. Introduction. vol. 1. University of Hawaii Press; Honolulu, HI: 1948. Insects of Hawaii. [Google Scholar]