Abstract
Pregnant women who develop preeclampsia exhibit higher circulating levels of the soluble VEGF receptor-1 (sFlt-1). Recent findings suggest that soluble Flt-1 may contribute to the pathogenesis of preeclampsia by binding and neutralizing vascular endothelial growth factors (VEGF) and placental growth factor (PlGF). Existing literature identifies sFlt-1 as a 100 kDa glycoprotein, a product of an mRNA splice variant. We hypothesized that sFlt-1 expression may be more complex with multiple variants of sFlt-1 as well as multiple sources during normal pregnancy and preeclampsia. Using a combination of affinity purification of sFlt-1 by heparin-agarose and epitope specific antibodies, we performed Western blot analysis with epitope specific antibodies for sFlt-1. Plasma of preeclamptic women exhibits significantly higher amounts of a novel 145 kDa variant of sFlt-1, along with the 100 kDa isoform. We identified sFlt-1 variants in the conditioned medium from placental explant cultures that are hypoxia responsive with varying sizes, including 185, 145,100 and 60 kDa forms, as well as antigenicity. The 145 kDa was similar in antigenicity to the 100 kDa found in plasma whereas the 185 and 60 kDa sFlt-1 demonstrated different epitopes. Deglycosylation studies also confirm that there are multiple sFlt-1 polypeptides. Co-immunoprecipitation with VEGF suggests that these different sFlt isoforms can bind VEGF and therefore, may be of functional importance. Finally, comparison of sFlt-1 in the conditioned medium obtained from cultured cytotrophoblasts, peripheral blood mononuclear cells (PBMCs) and human uterine microvascular cells (HUtMVECs) exhibit mainly the100 kDa sFlt-1. Collectively these data suggest the presence of multiple isoforms of sFlt-1 in the circulation of women with preeclampsia as well as in uncomplicated pregnancies and the possibility of multiple sources. Placental hypoxia may contribute to sFlt-1 over expression but other regulatory mechanisms cannot be ruled out.
Keywords: Villous explant culture, Conditioned medium, Preeclampsia, Plasma, Heparin-agarose, New soluble Flt-1 variants, sFlt-1, Deglycosylation, VEGF binding
1. Introduction
Preeclampsia is a pregnancy specific disorder characterized by new onset hypertension and proteinuria after 20 weeks of gestation. This syndrome affects 5–7% of pregnancies and is associated with significant fetal/neonatal and maternal morbidity and mortality [1]. Excess circulating soluble vascular endothelial growth factor (VEGF) receptor-1, also known as sFlt-1, is thought to be a major player in the pathogenesis of preeclampsia [2]. Serum soluble Flt-1 concentrations are higher in preeclampsia and weeks prior to the onset of the disease [3]. Soluble Flt-1 is believed to exert its biological activity by binding and thereby neutralizing the vascular endothelial growth factors (VEGF) and placental growth factor (PlGF) [4,5]. In a pregnant rat model, adenovirus transfection with sFlt-1 results in features similar to those observed with preeclampsia [2]. Based on the dramatic rise in sFlt-1 over the course of gestation and the dramatic fall soon after delivery, it has been hypothesized that the placenta is the primary source of this circulating anti-angiogenic factor. Placental production of sFlt-1 was reported by Clark et al. [6]. Isolated placental cytotrophoblasts also produce sFlt-1 [7,8]. However, we have shown that maternal peripheral blood mononuclear cells (PBMCs) are also a potential source of sFlt-1 [9]. Outside of pregnancy, sFlt-1 has been observed in vascular endothelial cells [5], peripheral blood mononuclear cells [10,11] and vascular smooth muscle cells [12].
Soluble Flt-1 is a truncated, splice-variant form of the membrane-bound Flt-1 protein. Preeclamptic placentas are believed to be hypoxic as they show increased levels of HIF-α proteins and its downstream marker proteins including sFlt-1 [13,14]. The Flt-1 gene is hypoxia responsive with an authentic hypoxia response element (HRE) in the promoter region [15] and many in vitro studies have shown the up regulation of Flt-1 proteins and RNA using villous explants [9,16,17] and cytotrophoblast cells [7,8].
Several lines of evidence, however, lend biologic plausibility to our hypothesis that multiple molecular weight forms of sFlt-1 exist, with sources and function. Much of the existing literature of Flt focuses on 185 kDa form that is found on the cell surface and the 100 kDa soluble form. The Flt-1 gene codes for a transcript of 7.4 Kb and splice variants of 3.0 and 2.4 Kb size mRNA. The 7.4 Kb mRNA codes for a 150 KDa protein that when glycosylated results in a 185 kDa membrane bound protein on the cell surface [18]. This membrane bound protein has an extracellular domain that has 7 IgG like domains, transmembrane domain and intracellular domain. The splice variant mRNA codes for a soluble protein that is identical to the membrane bound Flt-1 up to the sixth IgG like domain. The splice variant lacks the transmembrane domain but acquires short stretch of sequence from the 5′ end of intron 13 that codes for 31 amino acids unique to the sFlt-1 protein. The soluble Flt-1 is a 100 kDa protein that also is variably glycosylated. Recent reports suggest that the 13th intron of the Flt-1 gene contains six potential poly adenylation and splice sites that could make the sFlt-1 transcripts vary in size any where from 3.0 to 7.5 Kb [19,20]. Whether these lengthy transcripts will actually code for protein or are mainly involved in regulation is currently not known. However, in a recent report by Slay and colleagues, a new sFlt protein was identified, referred to as sFlt-14 that is expressed in vascular smooth muscle cells by a unique transcript [12]. A 3.0 Kb long transcript has been identified that uses an unknown splice site in intron 14. Thus the mRNA for sFlt-14 contains part of n'terminal sequence of exon 14 followed by 480 nucleotide sequence of intron 14 differing from the membrane bound Flt-1 and also the sFlt-1. This mRNA is associated with a cellular and soluble form of sFlt with approximate molecular weights of 115 and 135, respectively. Additionally the authors report that sFlt-14 is found to be over expressed in preeclampsia also [12]. These findings support our hypothesis of the existence of other sFlt-1 variants.
Based on the existing literature it is clear that further research is needed to characterize sFlt-1 variants, and their sources and regulation as well as their functional relevance. This is particularly important in furthering our understanding of preeclampsia pathogenesis a serious condition that still remains completely misunderstood and is lacking a cure.
We aimed this study (1) to elucidate the existence of sFlt-1 variants in plasma of women with preeclampsia and (2) to compare the sFlt-1 production using the explants from normal pregnant and preeclamptic women and also to study the effect of in vitro hypoxia. We have used multiple cell types that include villous explants, cytotrophoblasts, maternal peripheral blood mononuclear cells (PBMCs) and human uterine micro vascular endothelial cells (HUtMVECs). Additionally deglycosylation of sFlt-1 proteins to study the polypeptide nature and VEGF co-immunoprecipitation study to show the binding of sFlt were also performed.
2. Methods
2.1. Study population and sample collection
This study is a part of an ongoing investigation of preeclampsia at Magee-Womens Hospital (Pittsburgh, PA). The study was approved by the University of Pittsburgh Institutional Review Board and informed consent was obtained from all subjects. Placentas (n = 6 in each group) and maternal venous blood (n = 14 in each group) were obtained from women with uncomplicated, normotensive pregnancies and pregnancies complicated by preeclampsia. Plasma samples were collected prior to delivery and placental samples immediately after delivery. Plasma samples were stored at −70°C until assayed. Clinical characteristics are presented in Table1 Exclusion criteria included prior preeclampsia, illicit drug use and preexisting medical conditions such as diabetes, chronic hypertension, and renal disease. Preeclampsia was diagnosed by the presence of gestational hypertension (an absolute blood pressure ≥ 140 mm Hg systolic and/or 90 mm Hg diastolic after 20 weeks of gestation), proteinuria (greater than 300 mg per 24-h urine collection, ≥2+ on a voided or or ≥1+ on a catheterized random urine sample, or a protein/creatinine ratio of >0.3), and hyperuricemia (≥1 standard deviation above reference values for the gestational age the sample was obtained (e.g. term, >5.5 mg/dL)) beginning after the 20th week of pregnancy with resolution of blood pressure and proteinuria postpartum [21]. We include hyperuricemia in our classification as it identifies a more homogeneous group of gestational hypertensive women with a greater frequency of adverse fetal outcomes [22]. The diagnosis of preeclampsia was determined retrospectively based on medical chart review by a jury of research and clinical investigators.
Table 1.
Characteristics of the entire study population.
| Normal pregnancy (n = 20) | Preeclampsiaa (n = 20) | |
|---|---|---|
| Maternal age (years) | 27.94 ± 1.6 | 27.05 ± 1.3 |
| Gestational weeks at delivery | 39.5 ± 0.2 | 36.2 ± 0.7* |
| Maternal race (n) | ||
| White | 17 | 18 |
| African-American | 2 | 2 |
| Others | 1 | 0 |
| Nulliparous (n) | 16 | 20 |
| Mode of delivery (n) | ||
| C-section | 12 | 9 |
| Birth weight (g) | 3496.3 ± 99.9** | 2600.5 ± 220.7 |
| Systolic BP (mm Hg) | 117.1 ± 2.6 | 159.4 ± 2.7** |
| Diastolic BP (mm Hg) | 71.6 ± 1.5 | 92. 4 ± 1.0** |
| Proteinuriab (n) | 0 | 20 |
| Uric acid (mg/dL) | ND | 6.73 ± 0.34 |
Mean±SEM; ND, not determined.
p = 0.003,
p = 0.001 compared to normal pregnancy.
Preeclampsia definition is based on the Working Group Report (2003) and hyperuricemia of 1SD above normal for gestational age.
1–3+dip, protein/creatinine ratio ≥ 0.3, 24 h protein > 300 mg.
2.2. Villous explant culture
Villous explants were prepared as described by us previously [23] with modifications. Several cotyledons from third trimester placentas were excised at random and rinsed extensively in sterile saline to remove blood. Decidua and large vessels were removed from the villous placenta by blunt dissection. Villous tissue was then finely dissected into 5–10 mg pieces while in an iced sterile saline bath. The pieces were extensively washed two or three more times before culture.
After removing excess buffer using sterile gauze, villous tissue was weighed and precisely 600 mg of tissue was collected. Fifty milligrams of villous tissue was placed into each well of a 24 well plate (Becton Dickinson, Franklin Lakes, NJ) containing 1.0 ml of Medium 199 (Mediatech, Cellgro, Herndon, VA) supplemented with 5% Fetal Bovine Serum (FBS, Summit Technology, Ft. Collins, CO) and antibiotics. Explants were incubated at 37°C for 24 h on an orbital shaker (60 rpm, Belly Dancer, Stovall Life Science Inc., Greensboro, NC) under standard tissue culture conditions of 5% CO2-balance room air (nonhypoxic condition, pO2 ∼ 147 mm Hg or 20.94% O2) in a cell culture incubator (Forma Scientific, Marietta, OH).
Reduced O2 (“hypoxia”, pO2 ∼ 15 mm Hg or 2% O2–5% CO2-balance nitrogen) exposures were carried out in a Hypoxic chamber/Glove box (Coy Laboratory Products, Grass Lake, MI) with an oxygen probe for continuous monitoring and an orbital shaker. At the end of the incubation period, explants were removed, excess medium removed with sterile cotton gauze and samples were flash frozen in liquid nitrogen and stored at −70°C. The medium (12 ml) was pooled and then stored at −70°C in aliquots.
2.3. Cytotrophoblast culture
Cytotrophoblast cells were isolated from term placenta according to published protocols [24]. Briefly, villous explants were prepared and thoroughly washed to remove any blood. These explants were digested with trypsin/DNase/Dispase solution made in M199 medium buffered with HEPES. Cells were separated based on their buoyant density in a continuous Percoll gradient (Sigma Chemical Co., St Louis, MO). Cells were identified using density beads (Sigma Chemical Co., St Louis, MO). Cells that band between 1.048 and 1.062 were collected and washed with culture medium by centrifugation. Cytotrophoblasts were grown for 24 h in RPMI medium (Cellgro, Mediatech Inc., Herndon, VA) containing 5% fetal bovine serum.
2.4. Peripheral blood mononuclear cells (PBMCs)
Peripheral blood was drawn from normal pregnant women at term and the PBMCs were purified using ficoll gradients and were cultured following published protocol [9].
2.5. Human uterine micro vascular endothelial cells (HUtMVECs)
Human uterine micro vascular endothelial cells (HUtMVECs) were purchased from Lonza (Lonza, Walkersville, MD, USA) and were grown in culture following manufacturer's protocol using Endothelial basal Medium-2 supplemented with hEGF, hydroxycortisone, GA-1000, fetal bovine serum, VEGF, hFGF-β, IGF-1 and ascorbic acid. Conditioned medium from these cultures were collected and stored in aliquots at −70 C.
2.6. Heparin-agarose enrichment of soluble Flt
Patient plasma samples and conditioned medium from explant cultures, cytotrophoblasts, HUtMVECs and peripheral blood mononuclear cells (PBMCs) were enriched for soluble Flt-1 by heparin-agarose affinity chromatography using published protocols [19,25]. Briefly, heparin-agarose beads (Sigma Chemical Company, St. Louis, MO) – 30 μl packed volume and either 0.5 ml of conditioned medium (diluted to 1 ml with PBS) or 100 μl of plasma sample (diluted 1:10 with PBS) – were incubated at 4°C for 1 h with continuous mixing. The heparin-agarose/sFlt conjugate was centrifuged and the pellet was washed three times with 1× PBS buffer. After the final wash, the beads were resuspended in 1× Laemmli's solution and processed for Western blot analysis.
2.7. Western analysis
Total proteins from villous explants were extracted using our published procedures [23]. A HIF-1α monoclonal antibody (Transduction Laboratories, Lexington, KY, cat # H72320) was diluted to 1.25 μg/ml in TBS buffer (Tris buffered saline) containing 0.05% Tween-20. Membranes containing 10 μg proteins, electrophoresed separately, were probed with a monoclonal anti-β-actin antibody (Clone AC-15, Sigma, St. Louis, MO; 2.2 μg/ml) to account for loading variations. The membrane was further stained with Coomassie blue to confirm equal loading. For the characterization of sFlt-1 proteins four different antibodies were used: (1) sc-386 from Santa Cruz Biotechnology that recognizes extracellular domain (immunogen is 17 amino acid peptide corresponding to the last 50 amino acids in the carboxyl terminus), not found in sFlt-1, (2) V4262 from Sigma Chemical Company that recognizes the amino acid terminus epitope present in both Flt-1 and sFlt-1 (immunogen is amino acids in the 1 to 251 region of the amino terminus), (3) AF321, R&D Systems, similar to Sigma antibody (immunogen is a recombinant sFlt protein containing amino acids 27 to 687) and (4) 36–1100 from Zymed that is specific for human sFlt-1 (immunogen is a polypeptide against human sFlt, amino acids 672–685, accession # AAC50060). A diagrammatic representation of the different antibodies and their potential binding sites is shown in Fig. 3A.
2.8. Immunoassay for sFlt-1
Soluble Flt-1 was measured in conditioned media from explants cultured under normoxic and hypoxic conditions for 24 h using the Quantakine kit from R&D Systems (Minneapolis, MN) following manufacturer's instructions. Sensitivity of the assay was 5.01 pg/ml, with an intra-assay coefficient of variation of 2.6–3.8% and an inter assay coefficient of variation of 7.0–8.1%.
2.9. Co-immunoprecipitation
The heparin-agarose enriched sFlt-1 from the conditioned medium (hypoxia exposed preeclamptic explants) were pooled and incubated with 1.0 μg recombinant VEGF (R&D Systems) for 4 h incubation at 4 C. Anti-VEGF antibody (cat # 554539, BD Pharmingen, San Diego, CA) was added to this fraction and the incubation was continued for another 4 h. The immune complexes were pulled down using Protein-A agarose incubation for 30 min.
The protein-A agarose and immune complexes were recovered by centrifugation and pellets were washed three times with 1 ml of Radioimmunoassay buffer (RIPA buffer, PBS with 1% NP-40, 1% sodium deoxycholate, 0.1% SDS and one μl per ml of Protease inhibitor cocktail, 1 mM phenylmethyl sulfonylflouride, and sodium vanadate). Finally the agarose–IP complexes were resuspended in IX Laemmli buffer (1X Laemmli buffer 50 mM Tris HCl, pH 6.8, 2% SDS, 10% Glycerol) containing 5 mM DTT, 0.5 mM phenyl methyl sulfonyl fluoride (PMSF), 1 mM sodium vanadate and one μl per ml of protease inhibitors cocktail (1000×, Protease Inhibitor Cocktail Set III, Calbiochem, San Diego, CA) and subjected to Western blot analysis for sFlt-1.
2.10. Deglycosylation
To purify and concentrate the 100 and 145 kDa proteins and the high molecular weight proteins, we initially separated the heparin-agarose fraction on an SDS containing-8% polyacrylamide gel. Using the colored molecular weight markers as guide, the region between 100 and 150 KDa and the region above that were excised from the gel. Electro elution of the proteins was performed using dialysis membranes filled with protein running buffer and the gel section. Electrophoresis was carried out overnight at 30 V. The eluted proteins were concentrated using Amicon spin columns (Microcon Ultracell YM-3, Millipore, Bedford, MA) and were then used in deglycosylation studies.
Enzymatic deglycosylation was carried out using a kit from Prozyme Glyko following the manufacturer's protocol (Prozyme Inc., San Leandro, CA). After denaturing the proteins by heating at 100 °C for 5 min in the presence of SDS and mercaptoethanol, the heparin-agarose fractions of either the conditioned medium or plasma containing the enriched sFlt-1 were subjected to deglycosylation with a cocktail of five deglycosylation enzymes (N-Glycanase, Sialidase A, O-Glycanase, β (1–4) Galactosidase and β-N-Acetylglucosaminidase). The deglycosylated proteins were subjected to Western blot analysis as described above.
2.11. Densitometry
The developed films from Western analysis were scanned using a Hewlett Packard laser scanner (Scanjet 5370C, Hewlett Packard, Palo Alta, CA) into a PICT or TIFF file in grey scale. Densitometry was carried out using an automated digitizing software UN-SCANIT™ Gel Version 4.3 (Silk Scientific Inc., Orem, Utah).
2.12. Data analysis
The data in all graphs and the table are presented as mean ±SEM. They were analyzed by one or two factor analysis of variance (ANOVA). When appropriate, post hoc comparisons between individual group means were made by Bonferroni/Spearman test. A p value of less than 0.05 was considered to be significant.
3. Results
3.1. Soluble Flt-1 in plasma
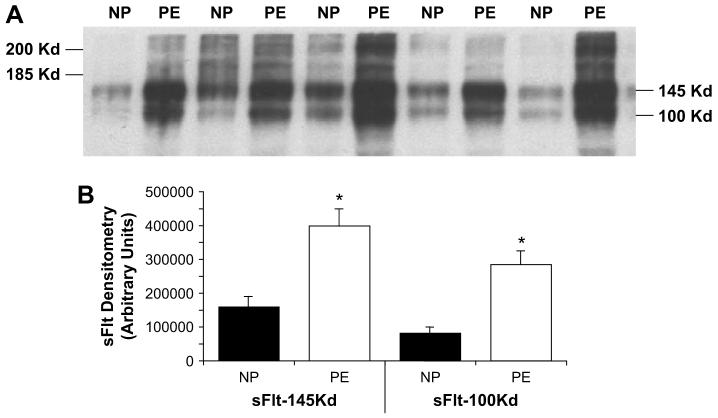
In order to demonstrate the existence of other sFlt-1 variants, we used heparin-agarose binding to enrich sFlt-1 in plasma samples from women with normal pregnancy and preeclampsia (n = 14 each) followed by Western blot analysis using an antibody from Sigma that recognizes both membrane bound Flt-1 and the soluble form sFlt-1. Since plasma samples were used in this experiment, only soluble forms will be detected by this approach. Two major bands at 100 and 145 kDa were observed in both groups of women (Fig. 1A). These two isoforms were also significantly higher in preeclamptic plasma with the 145 kDa form approximately 2.5-fold higher (398,348 ± 49,927 vs. 159,572 ± 30,763 arbitrary densitometric units) and the 100 KDa from approximately 3.5-fold higher (283,732 ± 41,018 with vs 80,828 ± 17,142) in women preeclampsia compared to normotensive women with uncomplicated pregnancies (p < 0.001 in both) (Fig. 1B). Other isoforms were present but it was difficult to quantify them in these gels. Using other antibodies, we were also able to identify variants of sFlt-1 with different epitopes of the sFlt-1 protein suggesting that other novel sFlt-1 variants exist. The recognition pattern of different antibodies for sFlt-1 is shown in Fig. 3 using plasma samples from normal and preeclamptic women. In addition to the 145 and 100 kDa proteins, the plasma samples also showed protein bands at 150 and 60 kDa when probed with antibodies obtained from Santa Cruz and Zymed Incorporations. These two antibodies recognize different epitopes than that of antibodies from Sigma and R&D companies.
Fig. 1.
Variants of soluble Flt in plasma from normal pregnant (NP) and preeclamptic women (PE). Western analysis for sFlt-1 was performed using antibody from Sigma, Inc. (St. Louis, MO) after heparin-agarose enrichment. Equal volume of plasma (100 μl) from each patient was used to enrich sFlt using heparin-agarose. (A) Representative Western blot profile demonstrating the 100 and 145 kDa major bands and (B) densitometric quantitation of these two sFlt-1 variants (n = 14 in each group; *p < 0.05).
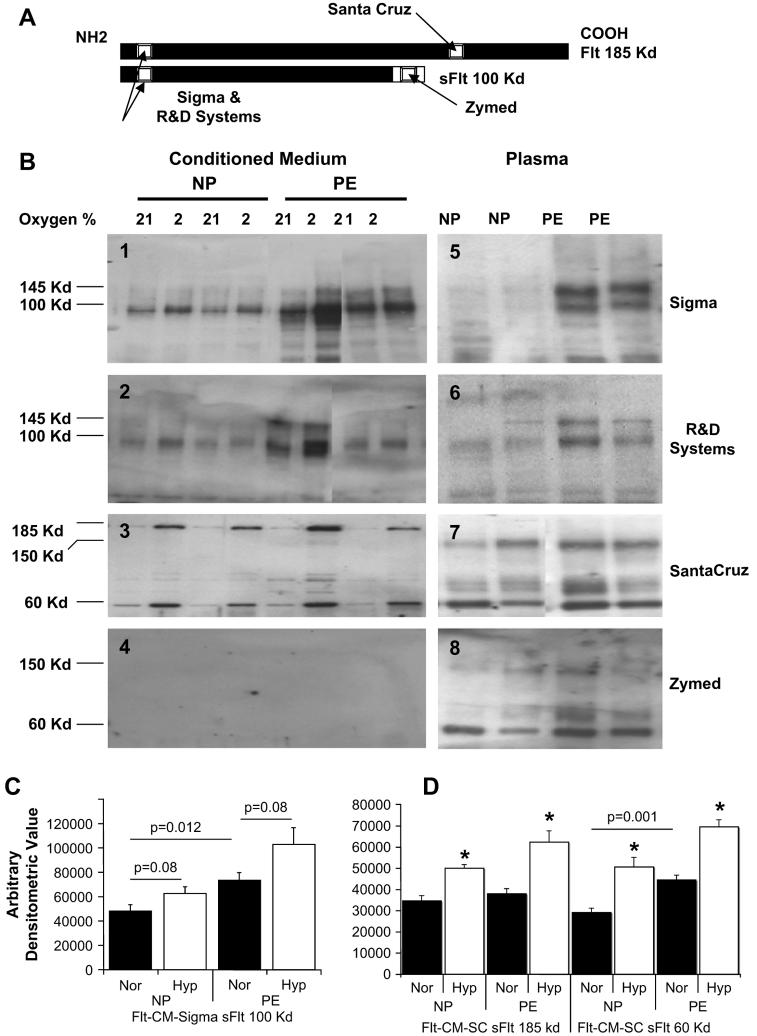
Fig. 3.
Characterization of sFlt-1 variants using different antibodies in both villous explant conditioned media and plasma from normal pregnant (NP) and preeclamptic women (PE). (A) Schematic representation of Flt (185 kDa) and sFlt (100 kDa) epitopes that are recognized by antibodies from Sigma Chemical Co., R&D Systems, Santa Cruz, and Zymed as reported by the respective manufacturers. (B) Panels 1–4 are representative Western blots using Sigma (1), R&D Systems (2), Santa Cruz (3) and Zymed (4) antibodies on villous explant conditioned media under 21% oxygen and 2% oxygen conditions. Panels 5–8 are representative Western blots using plasma samples from women with normal pregnancies and preeclampsia with the same order of antibodies. These are shown in adjacent panels to indicate the variability in sFlt-1 protein sources, molecular weight and antigenicity. (C) The 100 kDa sFlt-1 protein mass is significantly higher in preeclamptic villous explant conditioned media compared to normal pregnancy (p = 0.012) and hypoxia up regulated in response to (p = 0.08) using the Sigma antibody and densitometric quantitation. (B) Similarly, there is significant difference between normal pregnancy and preeclampsia for the 60 kDa sFlt-1 protein and significant hypoxic up regulation for both 185 and 60 kDa sFlt-1 proteins using the Santa Cruz antibody (p < 0.05 for all).
3.2. Characterization of sFlt-1 in conditioned medium from explant cultures of normal and preeclamptic women
To quantify the sFlt-1 in the conditioned medium we employed two different approaches. First one was a direct measurement of sFlt-1 in the conditioned medium using the ELISA kit from R&D systems. The second method involved heparin-agarose enrichment of the sFlt proteins and Western blot analysis using four different antibodies followed by densitometry.
3.3. Estimation of sFlt-1 in the condition medium by ELISA
Placental explants were incubated either under standard culture conditions (21% oxygen and 5% CO2) or under low oxygen conditions (2% oxygen/5% CO2) for 24 h. To validate the hypoxia response, total proteins were prepared from these explants and were subjected to Western blot analysis to show the HIF protein induction (Fig. 2A). The HIF-1α protein levels were significantly higher in preeclamptic explants compared to normal pregnant explants exposed to 21% oxygen (Fig. 2B, p < 0.05). Under 2% oxygen explants from both groups up regulated HIF-1α proteins significantly (above 2.0 fold, p < 0.05).
Fig. 2.

Soluble Flt-1 is increased under hypoxic conditions in villous explant conditioned media from both uncomplicated pregnant (NP) and preeclamptic women (PE). Villous explants were prepared from placentas of normal pregnant and women with preeclampsia (n = 6 in each group) and incubated at 21% and 2% oxygen for 24 h. Western analysis was performed to validate the hypoxia responsive HIF-1α protein increase (A). (B) Densitometric quantitation of HIF-1α protein mass demonstrated a two-fold increase in response to hypoxia in both groups (*p < 0.05). (C) Soluble Flt-1 concentrations by ELISA in conditioned media from these experiments. Compared to normal pregnancy the preeclamptic conditioned medium under 21% oxygen showed elevated sFlt-1 levels that was not statistically significant (p = 0.097). Compared to 21% oxygen, 2% oxygen exposure resulted in significantly higher sFlt-1 concentrations in normal pregnant villous explant conditioned media (p = 0.03); sFlt-1 concentrations were higher in preeclampsia under 2% oxygen; however, this was not statistically significant (p = 0.085).
The sFlt-1 concentration in the conditioned medium was estimated by ELISA (Fig. 2C). Under standard culture conditions (21% oxygen, 5% CO2), the conditioned medium from preeclamptic explants had 13170 pg/ml of sFlt compared to 8095 pg/ml of sFlt-1 by normal pregnant explants (p = 0.097). Under hypoxic conditions, increased levels of sFlt-1 were observed in both groups (1.5 fold, NP p = 0.0.03 and PE 0.085). Two of the PE explants expressed very high sFlt-1 even under normoxic conditions. The other four preeclamptic explants showed either marginal increase or equal amounts of sFlt-1 as seen in explants of normal pregnancy under normoxic conditions.
3.4. Heparin-agarose enrichment and characterization of sFlt-1 variants in the explant conditioned medium
The antigenic recognition pattern of the four different antibodies, their potential epitopes are shown in Fig. 3A.
Conditioned medium from the explant cultures were subjected to heparin-agarose affinity enrichment of sFlt-1 proteins and the sFlt-1 was analyzed by Western blots. Fig. 3B, panels 1–4, shows representative Western blot profiles of the heparin-agarose fractions of conditioned medium using four antibodies. Sigma antibody recognizes the 100 kDa sFlt-1 proteins (3B, panel 1) that have been described in the literature and additionally a band at 145 kDa. The R&D systems antibody shows a similar profile as that of Sigma antibody (panel 2). Panel 3 shows a profile of antibody from Santa Cruz Biotechnology indicating predominance of 185 and 60 kDa proteins. The fourth panel (3B, panel 4) using the sFlt-1 antibody from Zymed, indicates, in contrast to the results in the plasma samples, that this antibody does not recognize any protein in the conditioned medium from the explant culture. All the bands that were identified in the conditioned medium were up regulated under hypoxic exposure (Fig. 3C and D) although not significantly (p = 0.08). Under 21% conditions, however, the preeclamptic group showed significantly higher amounts of the 100 kDa sFlt-1 protein (both Sigma and R&D antibody, p = 0.012) compared to controls.
In order to compare the sFlt-1 variants in the plasma samples, representative profile of each antibody is shown in Fig. 3B, panels 5–8, adjacent to the profiles obtained for the conditioned medium. The Zymed antibody that did not recognize any protein in the conditioned medium, now recognizes a 150 and 60 kDa bands mainly and a few other minor bands (3B, panel 8). The pattern is identical to that of Santa Cruz antibody (3B, panel 7). Antibodies from Sigma (as shown in Figs. 1A and 3B, panel 5) and R&D systems (panel 6) recognize two major bands at 145 and 100 kDa in the plasma as seen in the conditioned medium. In addition the plasma samples exhibit few high molecular weight bands with a wide range of sizes. The 145 kDa is the new sFlt-1 isoform that has similar antigenic epitope as that of the 100 kDa sFlt-1 that has been previously reported. Additionally, the bands observed at 150 and 60 kDa using antibodies from Santa Cruz and Zymed companies are new forms of sFlt-1 that are novel in their size and molecular weight.
Fig. 5.
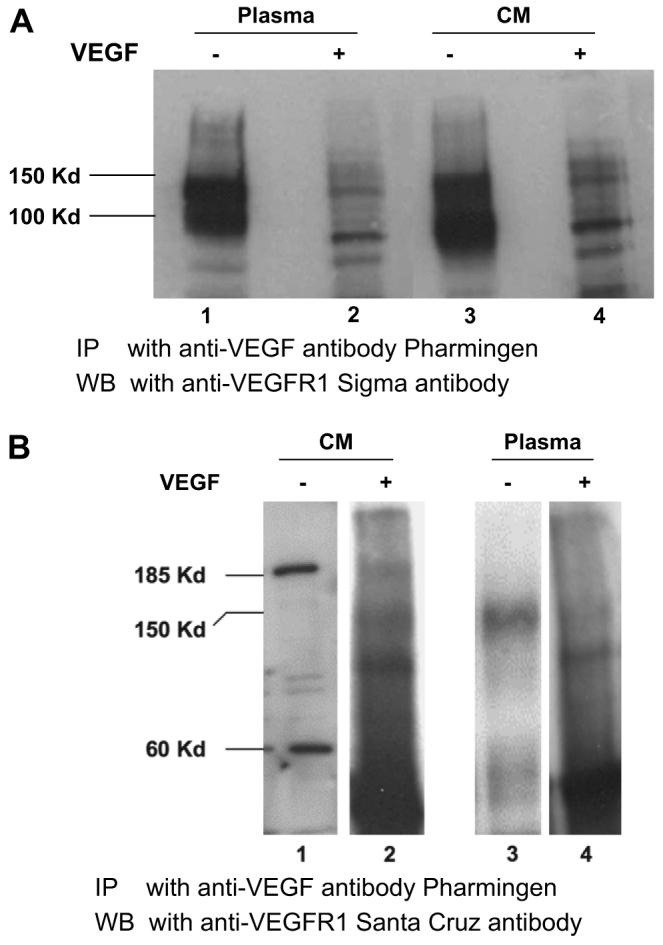
Soluble Flt-1 variants bind exogenous VEGF protein. In order to show that these variants are capable of binding VEGF, their functional target, VEGF (1 μg) was added to the heparin-agarose enriched sFlt-1 fraction, immunoprecipitated the sFlt-1/VEGF complex with anti-VEGF antibody (Pharmingen, San Diego, CA) and then probed for sFlt-1 with Sigma antibody (A). Lanes 1 and 3 show the input heparin-agarose sFlt-1 from plasma and conditioned medium, respectively. Lanes 2 and 4 show the sFlt-1 receptors that were immunoprecipitated using anti VEGF antibody. Immunoprecipitates from the same experiment were analyzed by Western blot using antibody from Santa Cruz (B) to demonstrate that the 150 and 185 kDa variants were also capable of binding VEGF protein.
3.5. Deglycosylation of heparin-bound proteins of plasma samples
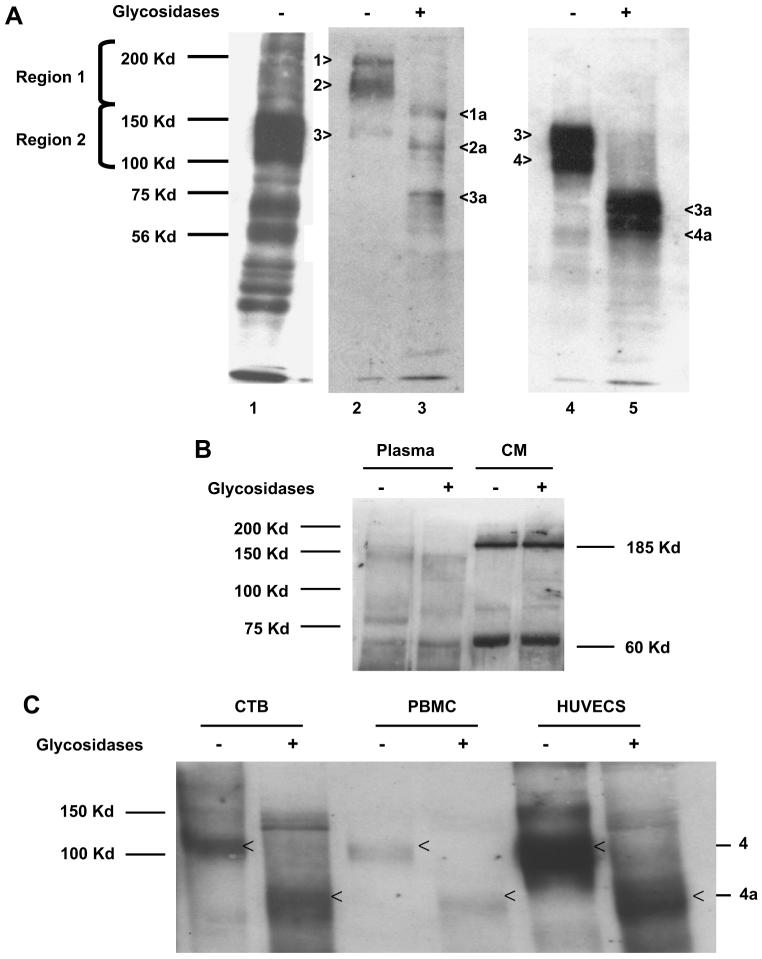
To determine whether the difference in sizes of these sFlt-1 variants is due to differential gycosylation of a single polypeptide, we performed deglycosylation studies using pooled samples from preeclamptic patients only. We electro eluted sFlt-1 in the heparin-agarose fractions from the gel in two regions. Region I contained proteins above 150 kDa and region II contained proteins between 100 and 150 kDa (Fig. 4A). We used a deglycosylation kit from Prozyme that involves five different glycosidases to remove simple and complex n-linked and o-linked glycosyl moieties completely. Lane 1 contains the heparin-agarose fraction from a preeclamptic plasma, intentionally overexposed to show different sized bands, probed with Sigma ab. Lane 2 contains the input from Region I with proteins of approximate molecular weights of 200, 185 and 145 kDa and Lane 4 contains input from Region II showing the 145 and 100 kDa proteins. The 145 sFlt-1 in lane 2 may be the same as in Lane 4, a carry over in the electro elution process. The respective deglycosylated forms are shown in lanes 3 and 5. Lane 3 shows deglycosylated proteins at 80,120 and 150 kDa and lane 5 shows two major bands at 70 and 80 kDa. The 200 kDa band is deglycosylated to a band at 150; the 185 is deglycosylated to 120 kDa; 145 is deglycosylated to 80 kDa and the 100 kDa is deglycosylated to 70 kDa. The bands are labeled 1–4 and the corresponding deglycosylated forms are 1a–4a, respectively. This indicated that the soluble VEGF receptors are not single polypeptides that are differentially glycosylated, instead they are polypeptides of different lengths.
Fig. 4.
Deglycosylation yields sFlt-1 polypeptide variants of differing molecular weights in both villous explant conditioned media and maternal plasma. (A) Western analysis for sFlt-1 was performed using antibody from Sigma, Inc. (St. Louis, MO) after heparin-agarose enrichment of pooled preeclamptic plasma (A, Lane 1); Lanes 2–4 show the proteins eluted from regions 1 and 2, and Lanes 3 and 5 are deglycosylated sFlt-1 variants, respectively. The bands are numbered 1–4 and the deglycosylated forms are denoted by 1a–4a, respectively. (B) Pooled plasma from preeclamptic woman and pooled preeclamptic villous explant hypoxic conditioned media were treated as noted above but with a different antibody from Santa Cruz Biotechnology. The representative Western blot demonstrates unglycosylated forms of sFlt-1, specifically the 150 kDa band in plasma and 185 and 60 kDa bands in conditioned medium. (C) Conditioned medium from cytotrophoblasts (CTB), peripheral blood mononuclear cells (PBMCs) and human uterine microvascular endothelial cells (HUtMVECs) treated with glycosidases also demonstrated that the 100 kDa sFlt-1 is the major form (Band 4 in Lane 4 of Fig. 4A) and when deglycosylated it gives rise to a polypeptide with a molecular weight of 60–70 kDa (Band 4a in lane 5 of 4A) using the Sigma antibody.
The sFlt-1 variants identified by the Santa Cruz antibody both in the plasma and conditioned medium appear to be non-glycosylated forms, since deglycosylation did not cause any change in their respective sizes (Fig. 4B).
3.6. Soluble Flt-1 in conditioned medium from cultures of cytotrophoblasts, HUtMVECs and PBMCs
We compared the heparin agarose fraction from conditioned medium obtained from cytotrophoblasts, HUtMVECs and PBMCs to determine the potential sources of these variants. Under standard culture conditions (21% oxygen) these three cell types produce mainly the 100–120 kDa sFlt-1 protein (Band 4 in 4A) and we can observe traces of the 145 kDa sFlt-1 band (Band 3 in 4A) also. When deglycosylated, sFlt-1 from these systems gives identical profile with a ∼70 kDa protein that was observed using plasma samples and explant conditioned medium (Fig. 4C) and Sigma antibody suggesting that these are similar in size, antigenicity and extent of glycosylation. Santa Cruz antibody failed to detect any sFlt-1 variants in these conditioned medium, either due to the fact there were none or not present in detectable quantities (data not shown).
3.7. Co-immunoprecipitation with VEGF and probe for VEGF receptors
In order to find out whether the different isoforms are capable of binding VEGF protein, we performed co-immunoprecipitation studies. We added 1 μg of VEGF recombinant protein to the heparin-agarose enriched fraction from conditioned medium and plasma, used a VEGF specific antibody to pull the protein complexes down and performed Western analysis for sFlt-1 using the Sigma ab. Fig. 5A shows that both 145 and 100 kDa proteins are capable of binding VEGF.
We also analyzed the 185 and 60 kDa proteins from conditioned medium and the 150 kDa protein from plasma as recognized by the Santa Cruz ab (Fig. 2B), in a similar way (Fig. 5B) and the results show that these high molecular variants also bind VEGF.
4. Discussion
The mechanism of sFlt-1 expression during pregnancy and particularly with preeclampsia is complex. Growing evidence suggests that there may be multiple RNA transcripts for sFlt-1, various tissue sources, and that the mechanisms other than hypoxia may be involved in the regulation of this protein.
The major findings of this report are: (1) identification of variants of sFlt-1 protein based on molecular weight and antigenicity both in plasma of pregnant women and in conditioned medium obtained from placental explant culture, (2) increased sFlt-1 production by explants from preeclamptic pregnancies compared to normal pregnant controls, (3) confirmation of soluble Flt-1 protein up regulation in villous explants exposed to hypoxic conditions, (4) identification of varying sizes of sFlt-1 polypeptides despite deglycosylation suggesting the presence of novel mRNA transcripts and (5) providing evidence for VEGF binding functional potential of these sFlt-1 variants.
Heparin-agarose affinity chromatography or batch purification has been successfully used in the purification of soluble Flt-1 [5,6,19]. Heparin is a negatively charged polymer of a regular disaccharide repeat sequence with a high degree of sulfation and expected to bind many proteins [26] and this binding is reversible depending on ionic strength. Park and Lee have shown that the fourth IgG-like domain of the sFlt-1 is the major heparin-binding site [27]. Therefore we chose to enrich the sFlt-1 proteins present in the conditioned medium and plasma obtained from patients and further characterized with specific assays.
Initial reports of sFlt-1 indicated an approximately 90 kDa protein that also exists in glycosylated forms with molecular weight ranging from 100 to 120 kDa [5,6]. The mRNA size that codes for this sFlt-1 protein was reported as either 2.4 or 3.0 Kb, the difference in length being the 3′ untranslated sequence. Other naturally occurring splice variants of Flt-1, other than the sFlt-1 referenced above, have been reported earlier. These variants contain 4–5 of the extracellular IgG like loops, and can bind to VEGF; however, they are unable to cause signal transduction because of the tyrosine kinase domain is missing [28]. The membrane-bound Flt-1 (185 kDa) is thought to be produced by mRNA that is approximately 7.0–8.0 Kb. However, recent studies indicate that the Flt-1 gene has multiple poly-adenylation sites and splice sites in intron 13 that could make the sFlt-1 transcripts vary in size by up to 4000 bases at the 3′ untranslated regions depending on which site is utilized [19,20]. Thus the 7.0–8.0 Kb mRNA that was previously reported by many investigators could be a mixed population of mRNA transcripts for Flt-1 as well as sFlt-1. Sequences in the 3′ region could potentially be involved in stabilization and differential expression of sFlt-1 mRNA. Although these two reports suggest multiple different mRNA lengths for sFlt-1, they do not necessarily show sFlt-1 proteins with varying sizes. In other words these mRNA variants with lengthy 3′ ends may code for a single sFlt-1 with differential glycosylation that changes their size. Our study focused on sFlt-1 protein of various sizes rather than mRNA. We also used deglycosylation to specifically address distinct polypeptides for sFlt-1.
In a recent report, a novel smooth muscle specific sFlt-14 was identified that codes for an intracellular form and a soluble form of 115 and 135 kDa sizes, respectively. Evidence is provided for the inclusion of intron 14 that results in larger size mRNA transcript and protein [12] and presence of amino acids that are not found in either the 185 kDa Flt-1 or the 100 kDa sFlt-1. The authors suggest that this may be one of those rare occurrences where the intronic sequences are translated into proteins as seen in sFlt-1 where the intronic sequences code for 31 amino acid stretch. This 135 kDa protein may be the 145 kDa protein that we have identified in plasma samples of preeclamptic women in our study. Gu et al identified a 150 kDa protein in the plasma of preeclamptic women [29] further supporting our current work that variants of sFlt are present and may vary by tissue type.
The use of four different antibodies that have different epitope recognition sites enabled us to identify distinct isoforms of sFlt-1 both in the conditioned medium from explant culture and in plasma of women with normal pregnancy and preeclampsia. Using an antibody from Sigma, we found sFlt-1 variants in plasma of pregnant women distinct from the previously demonstrated 100 kDa sFlt-1 form. These 145 and 100 kDa sFlt-1 proteins were significantly higher in women with preeclampsia. The 145 kDa is a novel variant that to our knowledge has not been reported in the literature. In these Western profiles we also observed other protein bands of varying molecular sizes but since the 100 and 145 kDa proteins were so highly expressed (almost 90%), quantitation of these other molecular weights sFlt-1 proteins was not feasible. In addition to the 145 kDa protein, the plasma samples demonstrated sFlt-1 proteins with molecular weights of 150 and 60 kDa. These two polypeptides were identified using antibodies from Santa Cruz and Zymed which do not share the same epitope recognition as that of the from Sigma and R&D systems antibodies. The explant conditioned medium demonstrated 100 and 145 kDa sFlt-1 proteins similar to the ones found in plasma. Expression of the 145 kDa sFlt-1 form was lower than the 100 kDa form in explant culture medium, media from cytotrophoblasts, PBMCs and endothelial cells than the 100 kDa form. This was in contrast to the plasma samples where the 145 kDa form was the predominant form. Interestingly the antibody from Zymed that failed to recognize any of the 185, 145, 100 and 60 kDa sFlt-1 forms in the explant medium whereas it recognized a 150 and 60 kDa sFlt-1 proteins in plasma. This suggests that the 150 and 60 kDa forms identified by this antibody in plasma may be from a source other than the placenta.
The 185 and 60 kDa proteins are recognized by the Santa Cruz antibody and not by the Sigma and R&D antibodies. This is an indication that these two are unique splice variants that may have skipped the first few amino acids at the 5′ amino-terminus that are the recognition sequence for the Sigma and R&D antibodies. Partial evidence to this statement comes from the work of Boocock et al. [28] that have identified splice variants that resemble our findings.
Soluble variants of proteins are usually produced by spliced transcripts or proteolytic cleavage of the membrane bound proteins by proteases. Our results of deglycosylation studies provide evidence for distinct sFlt-1 variants of different peptide length rather than differential glycosylation. There is a possibility that the 185 kDa sFlt-1 isoform may arise from the peptidyl cleavage by chymotrypsin-like proteases [30] or other membrane type-MMPs [31]. However, the deglycosylation step (Fig. 4B) did not further reduce the 185 kDa protein from conditioned medium. This raises the possibility of an unglycosylated variant of sFlt-1 that is remarkably different in polypeptide size (requires about 1638 amino acids and 4914 bp of coding sequence). The largest mRNA size known for the Flt-1 protein is 7680 bp (Gene bank accession # X51602.1) with a coding region of 4017 bp resulting in an unglycosylated Flt-1 protein with a theoretical size of 150 kDa (SWISSPORT # P17948). Previous studies indicated that the sFlt-1 transcript might be of different sizes mainly due to the variation in the 3′ UTR that is not translated but contributes to post-transcriptional regulation. Our findings along with Wang and Sley may provide the first evidence for the occurrence of sFlt-1 molecules that are distinct polypeptides based on size [12,29].
There have been many attempts to identify the source of sFlt-1 in preeclamptic pregnancies. Since circulating sFlt-1 concentrations drop dramatically after delivery of the placenta, it is widely accepted that placenta is the primary source of sFlt-1 in pregnancy and preeclampsia. However, other cell types have been shown to produce sFlt-1 in the nonpregnant state. We have previously shown that PBMCs are an additional source of sFlt-1 in preeclampsia [9]. The placental compartment is comprised of different cell types including cytotrophoblasts, macrophages, endothelial cells, smooth muscle cells, and cells from maternal blood. All these cells are capable of expressing sFlt-1 protein. Our finding of different variants of sFlt-1, raised the question about the origin or source of these varying isoforms. Using placental explant cultures to study the sFlt-1 protein profile we found the prominent sFlt-1 under standard culture conditions was the 100 kDa protein, although we detected the 145 kDa form to a lesser degree. Both these isoforms were hypoxia responsive. Using standard culture conditions with cytotrophoblasts, maternal PBMCs and the human uterine microvascular cells, we also demonstrated that the 100 kDa is the major form of sFlt-1 produced by these cell types. The 145 kDa protein was also produced under these conditions but to a lesser degree. Thus the increased production of the 145 kDa sFlt-1 protein seen during preeclampsia may be disease specific.
Consistent with published reports [7,9,16,17] we found increased sFlt-1 production by placental explants from women with preeclampsia with some variations. In our hands, the amount of sFlt-1 produced by preeclamptic explants was only marginally higher (p = 0.097) than sFlt-1 produced by explants from normal pregnancy, cultured under standard 21% oxygen condition and as estimated by ELISA. On the other hand densitometry performed on all the different sFlt-1 bands demonstrated significant differences between sFlt-1 in conditioned medium from normal and preeclamptic explants (p < 0.05). There are a number of possible explanations for the above finding. First, this could be due to the selective epitope recognition of the R&D antibody that may not account for all of the sFlt-1 species in the conditioned medium. Second, of the six patients with preeclampsia, only two had markedly higher amounts of sFlt-1 in placental explants while the other four had only slightly higher sFlt-1 than normal pregnancy under 21% oxygen condition. We did not see the robust difference in the circulating sFlt-1 levels of explant conditioned medium that was seen in the patients. This could be explained by the physical isolation of the explant tissue from cellular interaction and association with other circulating factors that may be required to result in the over production of sFlt-1. Exposure of these explants to hypoxic conditions for 24 h (a drastic measure) does show sFlt-1 regulation in agreement with published reports. However, in spite of 24 h incubation at 2% oxygen, the fold induction of sFlt-1 proteins is only about 1.4–1.5 in both groups and fails to mimic the in vivo differences found in the circulation of pregnant women. This observation suggests that placental hypoxia contributes to sFlt-1 production in preeclampsia, but also suggests in vitro vs. in vivo differences or other mechanisms including hypoxia independent (dys) regulatory pathways involved in sFlt-1 over expression in vivo.
There is evidence supporting both hypoxic and non-hypoxic regulation of sFlt-1 in other systems. Induction of uteroplacental ischemia alone resulted in significant elevation of both mRNA and protein for sFlt-1 in non-human primates [32]. These investigators suggest that placental and peripheral blood mononuclear cells are involved in the sFlt-1 production. Likewise there are other instances where sFlt-1 can be up regulated by non-hypoxic mechanisms. Hypertension produced by reduced uterine perfusion in pregnant rats has been shown to result in sFlt-1 up regulation [33]. Lockwood et al have shown that treating first trimester decidual cells with thrombin increases sFlt-1 [34]. Treatment with angiotensin II, autoantibodies from preeclamptic women targeted against angiotensin receptor and atorvastatin have also been reported to cause up regulation of sFlt-1 in pregnant mice, human placental villous explants and trophoblast cells [35,36].
While our study findings are novel and exciting, we acknowledge some limitations. The multiple isoforms reported here are based on heparin-agarose binding and immunoreactivity. We also used response to low oxygen tension. Although co-immunoprecipitation with VEGF study suggests the functional potential of these variants, additional functional and molecular studies are needed for confirmation. In addition purification of the various protein bands and sequencing by mass spectrophotometry would provide additional evidence that these are indeed related to sFlt-1. Peptide sequencing of the 185 and 60 kDa proteins from the conditioned medium is necessary to identify the precise nature of these two isoforms, i.e., whether they are native proteins or resultants of proteolytic cleavage of the membrane bound Flt-1 protein. Finally evaluation of the sequences of the splice variants of sFlt-1 at the mRNA level using cell-specific and transcript specific probes will provide better understanding regarding the biology of this receptor. These approaches are currently in progress.
Collectively our study provides evidence for multiple sources and multiple sFlt-1 transcripts as well as varying tissue sources of sFlt-1 protein during pregnancy and especially during preeclampsia. It appears that a global dysregulation is involved in the sFlt-1 expression during preeclampsia. When the responsible factors involved are identified it should shed new light in our understanding the etiology of the pathogenesis of preeclampsia.
Acknowledgements
We gratefully acknowledge the assistance of the PEPP staff (Perinatal Exposures and Preeclampsia Prevention) in procuring samples used in this study. The work was partially presented at the 53rd Society for Gynecological Investigations meeting, March 14–17, 2007, Reno, NV USA and at the 13th International Federation of Placental Association meeting, Kingston, ON Canada, August 17–21, 2007. Part of this work was supported by PO1-HD30367 and RO3-HD055219.
References
- 1.Ilekis JV, Reddy UM, Roberts JM. Preeclampsia–a pressing problem: an executive summary of a National Institute of Child Health and Human Development workshop. Reprod Sci. 2007;14:508–23. doi: 10.1177/1933719107306232. [DOI] [PubMed] [Google Scholar]
- 2.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–58. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–83. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed A, Dunk C, Ahmad S, Khaliq A. Regulation of placental vascular endothelial growth factor (VEGF) and placenta growth factor (PIGF) and soluble Flt-1 by oxygen–a review. Placenta. 2000;21(Suppl A):S16–24. doi: 10.1053/plac.1999.0524. [DOI] [PubMed] [Google Scholar]
- 5.Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci USA. 1993;90:10705–9. doi: 10.1073/pnas.90.22.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark DE, Smith SK, He Y, Day KA, Licence DR, Corps AN, et al. A vascular endothelial growth factor antagonist is produced by the human placenta and released into the maternal circulation. Biol Reprod. 1998;59:1540–8. doi: 10.1095/biolreprod59.6.1540. [DOI] [PubMed] [Google Scholar]
- 7.Li H, Gu B, Zhang Y, Lewis DF, Wang Y. Hypoxia-induced increase in soluble Flt-1 production correlates with enhanced oxidative stress in trophoblast cells from the human placenta. Placenta. 2005;26:210–7. doi: 10.1016/j.placenta.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Nagamatsu T, Fujii T, Kusumi M, Zou L, Yamashita T, Osuga Y, et al. Cytotrophoblasts up-regulate soluble fms-like tyrosine kinase-1 expression under reduced oxygen: an implication for the placental vascular development and the pathophysiology of preeclampsia. Endocrinology. 2004;145:4838–45. doi: 10.1210/en.2004-0533. [DOI] [PubMed] [Google Scholar]
- 9.Rajakumar A, Michael HM, Rajakumar PA, Shibata E, Hubel CA, Karumanchi SA, et al. Extra-placental expression of vascular endothelial growth factor receptor-1, (Flt-1) and soluble Flt-1 (sFlt-1), by peripheral blood mononuclear cells (PBMCs) in normotensive and preeclamptic pregnant women. Placenta. 2005;26:563–73. doi: 10.1016/j.placenta.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Barleon B, Reusch P, Totzke F, Herzog C, Keck C, Martiny-Baron G, et al. Soluble VEGFR-1 secreted by endothelial cells and monocytes is present in human serum and plasma from healthy donors. Angiogenesis. 2001;4:143–54. doi: 10.1023/a:1012245307884. [DOI] [PubMed] [Google Scholar]
- 11.Clauss M, Weich H, Breier G, Knies U, Rockl W, Waltenberger J, et al. The vascular endothelial growth factor receptor Flt-1 mediates biological activities. Implications for a functional role of placenta growth factor in monocyte activation and chemotaxis. J Biol Chem. 1996;271:17629–34. doi: 10.1074/jbc.271.30.17629. [DOI] [PubMed] [Google Scholar]
- 12.Sela S, Itin A, Natanson-Yaron S, Greenfield C, Goldman-Wohl D, Yagel S, et al. A novel human-specific soluble vascular endothelial growth factor receptor 1: cell-type-specific splicing and implications to vascular endothelial growth factor homeostasis and preeclampsia. Circ Res. 2008;102:1566–74. doi: 10.1161/CIRCRESAHA.108.171504. [DOI] [PubMed] [Google Scholar]
- 13.Rajakumar A, Brandon HM, Daftary A, Ness R, Conrad KP. Evidence for the functional activity of hypoxia-inducible transcription factors overexpressed in preeclamptic placentae. Placenta. 2004;25:763–9. doi: 10.1016/j.placenta.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 14.Soleymanlou N, Jurisica I, Nevo O, Ietta F, Zhang X, Zamudio S, et al. Molecular evidence of placental hypoxia in preeclampsia. J Clin Endocrinol Metab. 2005;90:4299–308. doi: 10.1210/jc.2005-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerber HP, Condorelli F, Park J, Ferrara N. Differential transcriptional regulation of the two vascular endothelial growth factor receptor genes. Flt-1, but not Flk-1/KDR, is up-regulated by hypoxia. J Biol Chem. 1997;272:23659–67. doi: 10.1074/jbc.272.38.23659. [DOI] [PubMed] [Google Scholar]
- 16.Ahmad S, Ahmed A. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ Res. 2004;95:884–91. doi: 10.1161/01.RES.0000147365.86159.f5. [DOI] [PubMed] [Google Scholar]
- 17.Nevo O, Soleymanlou N, Wu Y, Xu J, Kingdom J, Many A, et al. Increased expression of sFlt-1 in in vivo and in vitro models of human placental hypoxia is mediated by HIF-1. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1085–93. doi: 10.1152/ajpregu.00794.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shibuya M. Structure and function of VEGF/VEGF-receptor system involved in angiogenesis. Cell Struct Funct. 2001;26:25–35. doi: 10.1247/csf.26.25. [DOI] [PubMed] [Google Scholar]
- 19.Huckle WR, Roche RI. Post-transcriptional control of expression of sFlt-1, an endogenous inhibitor of vascular endothelial growth factor. J Cell Biochem. 2004;93:120–32. doi: 10.1002/jcb.20142. [DOI] [PubMed] [Google Scholar]
- 20.Thomas C, Andrews JI, Liu KZ. Intronic polyadenylation signal sequences and alternate splicing generate human soluble Flt1 variants and regulate the abundance of soluble Flt1 in the placenta. FASEB J. 2007;21:3885–95. doi: 10.1096/fj.07-8809com. [DOI] [PubMed] [Google Scholar]
- 21.Roberts JM, Pearson G, Cutler J, Lindheimer M. Summary of the NHLBI Working Group on Research on Hypertension During Pregnancy. Hypertension. 2003;41:437–45. doi: 10.1161/01.HYP.0000054981.03589.E9. [DOI] [PubMed] [Google Scholar]
- 22.Roberts JM, Bodnar LM, Lain KY, Hubel CA, Markovic N, Ness RB, et al. Uric acid is as important as proteinuria in identifying fetal risk in women with gestational hypertension. Hypertension. 2005;46:1263–9. doi: 10.1161/01.HYP.0000188703.27002.14. [DOI] [PubMed] [Google Scholar]
- 23.Rajakumar A, Doty K, Daftary A, Harger G, Conrad KP. Impaired oxygen-dependent reduction of HIF-1alpha and -2alpha proteins in pre-eclamptic placentae. Placenta. 2003;24:199–208. doi: 10.1053/plac.2002.0893. [DOI] [PubMed] [Google Scholar]
- 24.Nelson DM, Smith SD, Furesz TC, Sadovsky Y, Ganapathy V, Parvin CA, et al. Hypoxia reduces expression and function of system A amino acid transporters in cultured term human trophoblasts. Am J Physiol, Cell Physiol. 2003;284:C310–5. doi: 10.1152/ajpcell.00253.2002. [DOI] [PubMed] [Google Scholar]
- 25.Kendall RL, Wang G, Thomas KA. Identification of a natural soluble form of the vascular endothelial growth factor receptor, FLT-1, and its heterodimerization with KDR. Biochem Biophys Res Commun. 1996;226:324–8. doi: 10.1006/bbrc.1996.1355. [DOI] [PubMed] [Google Scholar]
- 26.Margalit H, Fischer N, Ben-Sasson SA. Comparative analysis of structurally defined heparin binding sequences reveals a distinct spatial distribution of basic residues. J Biol Chem. 1993;268:19228–31. [PubMed] [Google Scholar]
- 27.Park M, Lee ST. The fourth immunoglobulin-like loop in the extracellular domain of FLT-1, a VEGF receptor, includes a major heparin-binding site. Biochem Biophys Res Commun. 1999;264:730–4. doi: 10.1006/bbrc.1999.1580. [DOI] [PubMed] [Google Scholar]
- 28.Boocock CA, Charnock-Jones DS, Sharkey AM, McLaren J, Barker PJ, Wright KA, et al. Expression of vascular endothelial growth factor and its receptors flt and KDR in ovarian carcinoma. J Natl Cancer Inst. 1995;87:506–16. doi: 10.1093/jnci/87.7.506. [DOI] [PubMed] [Google Scholar]
- 29.Gu Y, Lewis DF, Wang Y. Placental productions and expressions of soluble endoglin, soluble fms-like tyrosine kinase receptor-1, and placental growth factor in normal and preeclamptic pregnancies. J Clin Endocrinol Metab. 2008;93:260–6. doi: 10.1210/jc.2007-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Gu Y, Zhang Y, Lewis DF, Alexander JS, Granger DN. Increased chymotrypsin-like protease (chymase) expression and activity in placentas from women with preeclampsia. Placenta. 2007;28:263–9. doi: 10.1016/j.placenta.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka SS, Togooka Y, Sato H, Seiki M, Tojo H, Tachi C. Expression and localization of membrane type matrix metalloproteinase-1 (MT1-MMP) in trophoblast cells of cultured mouse blastocysts and ectoplacental cones. Placenta. 1998;19:41–8. doi: 10.1016/s0143-4004(98)90097-2. [DOI] [PubMed] [Google Scholar]
- 32.Makris A, Thornton C, Thompson J, Thomson S, Martin R, Ogle R, et al. Uteroplacental ischemia results in proteinuric hypertension and elevated sFLT-1. Kidney Int. 2007;71:977–84. doi: 10.1038/sj.ki.5002175. [DOI] [PubMed] [Google Scholar]
- 33.Gilbert JS, Babcock SA, Granger JP. Hypertension produced by reduced uterine perfusion in pregnant rats is associated with increased soluble fms-like tyrosine kinase-1 expression. Hypertension. 2007;50:1142–7. doi: 10.1161/HYPERTENSIONAHA.107.096594. [DOI] [PubMed] [Google Scholar]
- 34.Lockwood CJ, Toti P, Arcuri F, Norwitz E, Funai EF, Huang ST, et al. Thrombin regulates soluble fms-like tyrosine kinase-1 (sFlt-1) expression in first trimester decidua: implications for preeclampsia. Am J Pathol. 2007;170:1398–405. doi: 10.2353/ajpath.2007.060465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou CC, Ahmad S, Mi T, Abbasi S, Xia L, Day MC, et al. Autoantibody from women with preeclampsia induces soluble Fms-like tyrosine kinase-1 production via angiotensin type 1 receptor and calcineurin/nuclear factor of activated T-cells signaling. Hypertension. 2008;51:1010–9. doi: 10.1161/HYPERTENSIONAHA.107.097790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou CC, Ahmad S, Mi T, Xia L, Abbasi S, Hewett PW, et al. Angiotensin II induces soluble fms-Like tyrosine kinase-1 release via calcineurin signaling pathway in pregnancy. Circ Res. 2007;100:88–95. doi: 10.1161/01.RES.0000254703.11154.18. [DOI] [PMC free article] [PubMed] [Google Scholar]