Abstract
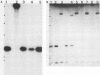
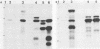
Type III group B streptococci (GBS) are the most common cause of neonatal sepsis and meningitis in the United States. The important role of the type III polysaccharide capsule and of the terminal sialic acid moiety of the capsule in the virulence of GBS has been demonstrated by using Tn916 mutagenesis. Several of the transposon insertion sites that resulted in defective type III capsule synthesis were located in a 30-kilobase (kb) region of the chromosome. Hybridization analysis of two other type III strains that differed in their relative virulence and of GBS serotypes Ia, Ib, Ic, and II showed that this region of the chromosome was highly conserved. A repetitive 1.4-kb sequence was found only in the 30-kb region of the more virulent type III strain, COH 1. The Escherichia coli maxicell in vivo expression system and an in vitro coupled transcription-translation system successfully identified the proteins expressed from the 30-kb region. Comparison of the proteins expressed from the same DNA fragments in these two assays indicated that some of these proteins may contain leader sequences that would ultimately result in their secretion to the cell surface. Identification and further characterization of the genes and their products will provide the foundation for understanding the genetic and biochemical events in GBS capsular polysaccharide production.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asada K., Nakatani S., Takanami M. Cloning of the contiguous 165-kilobase-pair region around the terminus of Escherichia coli K-12 DNA replication. J Bacteriol. 1985 Jul;163(1):398–400. doi: 10.1128/jb.163.1.398-400.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. D., Morrison D. A. Cloning of Streptococcus pneumoniae DNA fragments in Escherichia coli requires vectors protected by strong transcriptional terminators. Gene. 1987;55(2-3):179–187. doi: 10.1016/0378-1119(87)90278-2. [DOI] [PubMed] [Google Scholar]
- Echarti C., Hirschel B., Boulnois G. J., Varley J. M., Waldvogel F., Timmis K. N. Cloning and analysis of the K1 capsule biosynthesis genes of Escherichia coli: lack of homology with Neisseria meningitidis group B DNA sequences. Infect Immun. 1983 Jul;41(1):54–60. doi: 10.1128/iai.41.1.54-60.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawron-Burke C., Clewell D. B. Regeneration of insertionally inactivated streptococcal DNA fragments after excision of transposon Tn916 in Escherichia coli: strategy for targeting and cloning of genes from gram-positive bacteria. J Bacteriol. 1984 Jul;159(1):214–221. doi: 10.1128/jb.159.1.214-221.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn B., Collins J. A small cosmid for efficient cloning of large DNA fragments. Gene. 1980 Nov;11(3-4):291–298. doi: 10.1016/0378-1119(80)90069-4. [DOI] [PubMed] [Google Scholar]
- Hull R. A., Gill R. E., Hsu P., Minshew B. H., Falkow S. Construction and expression of recombinant plasmids encoding type 1 or D-mannose-resistant pili from a urinary tract infection Escherichia coli isolate. Infect Immun. 1981 Sep;33(3):933–938. doi: 10.1128/iai.33.3.933-938.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin T. R., Rubens C. E., Wilson C. B. Lung antibacterial defense mechanisms in infant and adult rats: implications for the pathogenesis of group B streptococcal infections in the neonatal lung. J Infect Dis. 1988 Jan;157(1):91–100. doi: 10.1093/infdis/157.1.91. [DOI] [PubMed] [Google Scholar]
- Roberts I., Mountford R., High N., Bitter-Suermann D., Jann K., Timmis K., Boulnois G. Molecular cloning and analysis of genes for production of K5, K7, K12, and K92 capsular polysaccharides in Escherichia coli. J Bacteriol. 1986 Dec;168(3):1228–1233. doi: 10.1128/jb.168.3.1228-1233.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubens C. E., Wessels M. R., Heggen L. M., Kasper D. L. Transposon mutagenesis of type III group B Streptococcus: correlation of capsule expression with virulence. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7208–7212. doi: 10.1073/pnas.84.20.7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeoka A. O., Rote N. S., Santos J. I., Hill H. R. Assessment of the virulence factors of group B streptococci: correlation with sialic acid content. J Infect Dis. 1983 May;147(5):857–863. doi: 10.1093/infdis/147.5.857. [DOI] [PubMed] [Google Scholar]
- Silver R. P., Finn C. W., Vann W. F., Aaronson W., Schneerson R., Kretschmer P. J., Garon C. F. Molecular cloning of the K1 capsular polysaccharide genes of E. coli. Nature. 1981 Feb 19;289(5799):696–698. doi: 10.1038/289696b0. [DOI] [PubMed] [Google Scholar]
- Silver R. P., Vann W. F., Aaronson W. Genetic and molecular analyses of Escherichia coli K1 antigen genes. J Bacteriol. 1984 Feb;157(2):568–575. doi: 10.1128/jb.157.2.568-575.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels M. R., Pozsgay V., Kasper D. L., Jennings H. J. Structure and immunochemistry of an oligosaccharide repeating unit of the capsular polysaccharide of type III group B Streptococcus. A revised structure for the type III group B streptococcal polysaccharide antigen. J Biol Chem. 1987 Jun 15;262(17):8262–8267. [PubMed] [Google Scholar]