Abstract
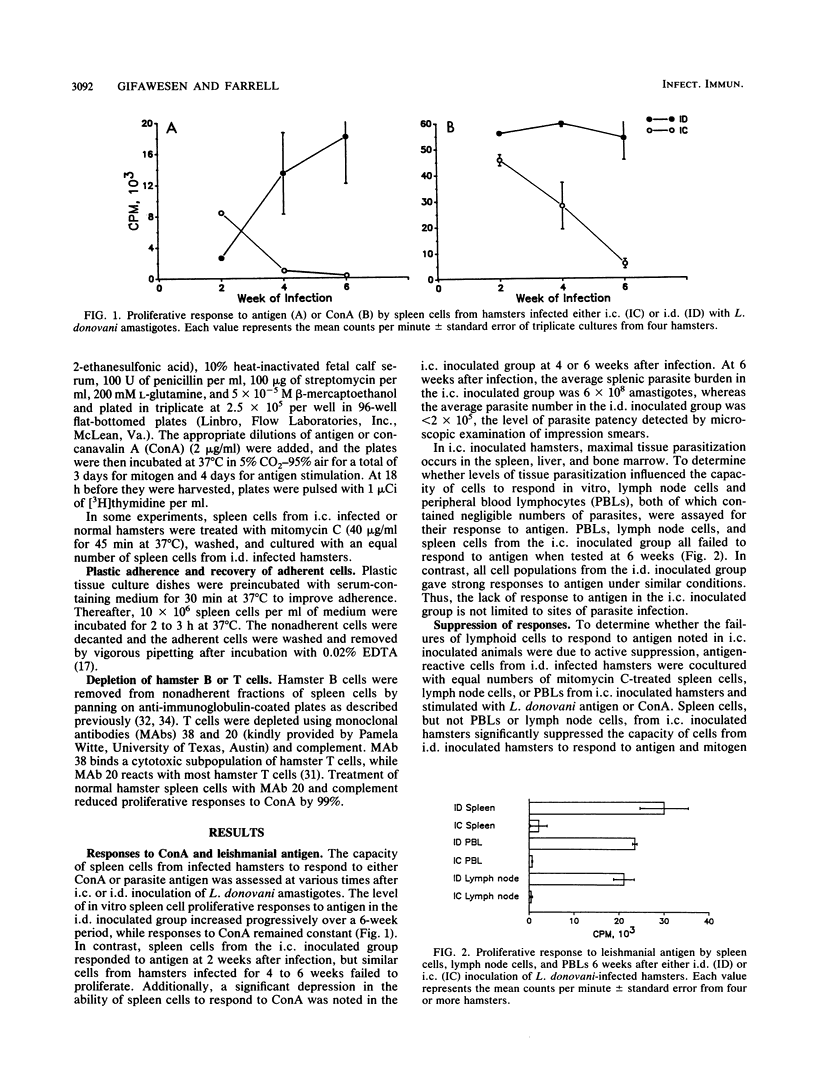
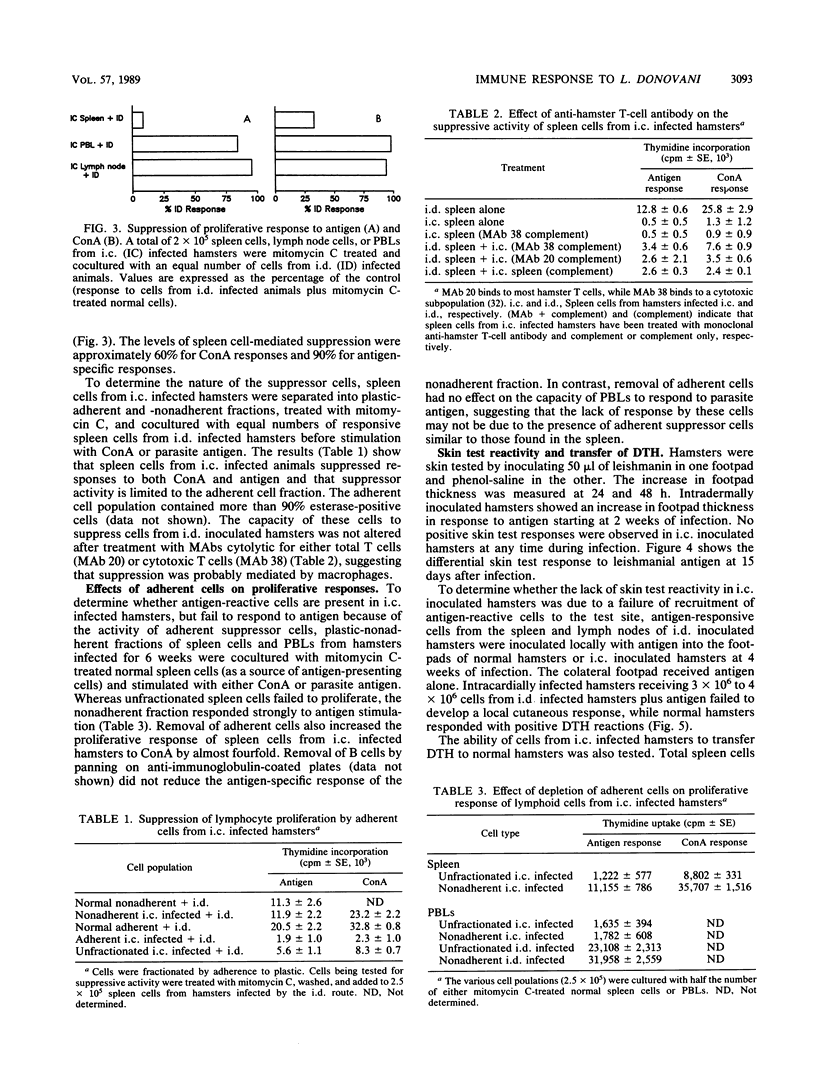
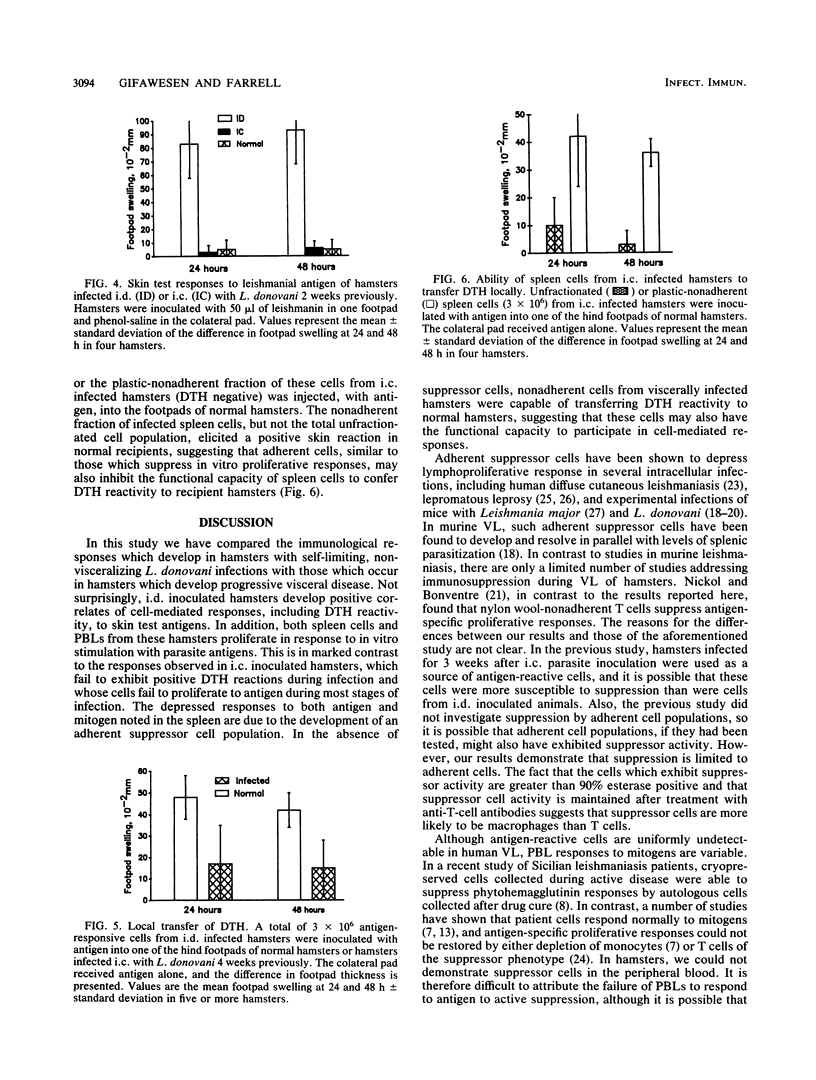
Leishmania donovani infection in golden hamsters was studied as a model for human kala-azar. After intradermal inoculation of L. donovani amastigotes, hamsters developed positive skin reactions (delayed-type hypersensitivity [DTH]) to parasite antigens and lymphoid cells from these hamsters proliferated to parasite antigens in vitro and transferred DTH reactivity to normal recipients. In contrast, hamsters infected by the intracardial route developed progressive visceral infections and failed to respond to skin test antigens. Spleen cells, lymph node cells, and peripheral blood lymphocytes (PBLs) from these hamsters were unresponsive to parasite antigens in vitro, and spleen cells failed to transfer DTH to normal recipients. Spleen cells, but not PBLs, displayed depressed responses to T-cell mitogens and also suppressed the proliferative response of cells from hamsters inoculated intradermally. Removal of adherent cells restored the capacity of spleen cells, but not PBLs, to respond to parasite antigens. The nonadherent population of these spleen cells also transferred DTH to normal recipients. The adherent suppressor cells, which have the characteristics of macrophages, appear to be localized to the spleen and are apparently not responsible for the failure of peripheral lymphoid cells to respond to antigen. These studies suggest that hamsters with visceral infections develop a population of antigen-reactive cells and that in the absence of suppression these cells may express functional activities, including the capacity to elicit DTH responses.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agu W. E., Farrell J. P., Soulsby E. J. Proliferative glomerulonephritis in experimental Leishmania donovani infection of the golden hamster. Comp Immunol Microbiol Infect Dis. 1981;4(3-4):353–368. doi: 10.1016/0147-9571(81)90021-7. [DOI] [PubMed] [Google Scholar]
- Badaro R., Jones T. C., Carvalho E. M., Sampaio D., Reed S. G., Barral A., Teixeira R., Johnson W. D., Jr New perspectives on a subclinical form of visceral leishmaniasis. J Infect Dis. 1986 Dec;154(6):1003–1011. doi: 10.1093/infdis/154.6.1003. [DOI] [PubMed] [Google Scholar]
- Barral A., Carvalho E. M., Badaró R., Barral-Netto M. Suppression of lymphocyte proliferative responses by sera from patients with American visceral leishmaniasis. Am J Trop Med Hyg. 1986 Jul;35(4):735–742. doi: 10.4269/ajtmh.1986.35.735. [DOI] [PubMed] [Google Scholar]
- Bunn-Moreno M. M., Madeira E. D., Miller K., Menezes J. A., Campos-Neto A. Hypergammaglobulinaemia in Leishmania donovani infected hamsters: possible association with a polyclonal activator of B cells and with suppression of T cell function. Clin Exp Immunol. 1985 Feb;59(2):427–434. [PMC free article] [PubMed] [Google Scholar]
- Carvalho E. M., Badaró R., Reed S. G., Jones T. C., Johnson W. D., Jr Absence of gamma interferon and interleukin 2 production during active visceral leishmaniasis. J Clin Invest. 1985 Dec;76(6):2066–2069. doi: 10.1172/JCI112209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho E. M., Teixeira R. S., Johnson W. D., Jr Cell-mediated immunity in American visceral leishmaniasis: reversible immunosuppression during acute infection. Infect Immun. 1981 Aug;33(2):498–500. doi: 10.1128/iai.33.2.498-500.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cillari E., Liew F. Y., Lo Campo P., Milano S., Mansueto S., Salerno A. Suppression of IL-2 production by cryopreserved peripheral blood mononuclear cells from patients with active visceral leishmaniasis in Sicily. J Immunol. 1988 Apr 15;140(8):2721–2726. [PubMed] [Google Scholar]
- Farrell J. P. Leishmania donovani: acquired resistance to visceral leishmaniasis in the golden hamster. Exp Parasitol. 1976 Aug;40(1):89–94. doi: 10.1016/0014-4894(76)90069-2. [DOI] [PubMed] [Google Scholar]
- Garnham P. C., Humphrey J. H. Problems in leishmaniasis related to immunology. Curr Top Microbiol Immunol. 1969;48:29–42. doi: 10.1007/978-3-642-46163-7_2. [DOI] [PubMed] [Google Scholar]
- Ghose A. C., Haldar J. P., Pal S. C., Mishra B. P., Mishra K. K. Phytohaemagglutinin-induced lymphocyte transformation test in Indian kala-azar. Trans R Soc Trop Med Hyg. 1979;73(6):725–726. doi: 10.1016/0035-9203(79)90032-4. [DOI] [PubMed] [Google Scholar]
- Hahn H., Kaufmann S. H., Falkenberg F., Chahinin M., Horn W. Peritoneal exudate T lymphocytes with specificity to sheep red blood cells. II. Inflammatory helper T cells and effector T cells in mice with delayed-type hypersensitivity and in suppressed mice. Immunology. 1979 Sep;38(1):51–55. [PMC free article] [PubMed] [Google Scholar]
- Haldar J. P., Ghose S., Saha K. C., Ghose A. C. Cell-mediated immune response in Indian kala-azar and post-kala-azar dermal leishmaniasis. Infect Immun. 1983 Nov;42(2):702–707. doi: 10.1128/iai.42.2.702-707.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho M., Koech D. K., Iha D. W., Bryceson A. D. Immunosuppression in Kenyan visceral leishmaniasis. Clin Exp Immunol. 1983 Feb;51(2):207–214. [PMC free article] [PubMed] [Google Scholar]
- MANSON-BAHR P. E. Immunity in kala-azar. Trans R Soc Trop Med Hyg. 1961 Nov;55:550–555. doi: 10.1016/0035-9203(61)90078-5. [DOI] [PubMed] [Google Scholar]
- Milon G., Marchal G., Seman M., Truffa-Bachi P., Zilberfarb V. Is the delayed-type hypersensitivity observed after a low dose of antigen mediated by helper T cells? J Immunol. 1983 Mar;130(3):1103–1107. [PubMed] [Google Scholar]
- Murray H. W., Carriero S. M., Donelly D. M. Presence of a macrophage-mediated suppressor cell mechanism during cell-mediated immune response in experimental visceral leishmaniasis. Infect Immun. 1986 Nov;54(2):487–493. doi: 10.1128/iai.54.2.487-493.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Masur H., Keithly J. S. Cell-mediated immune response in experimental visceral leishmaniasis. I. Correlation between resistance to Leishmania donovani and lymphokine-generating capacity. J Immunol. 1982 Jul;129(1):344–350. [PubMed] [Google Scholar]
- Nickol A. D., Bonventre P. F. Immunosuppression associated with visceral leishmaniasis of hamsters. Parasite Immunol. 1985 Jul;7(4):439–449. doi: 10.1111/j.1365-3024.1985.tb00089.x. [DOI] [PubMed] [Google Scholar]
- Nickol A. D., Bonventre P. F. Visceral leishmaniasis in congenic mice of susceptible and resistant phenotypes: immunosuppression by adherent spleen cells. Infect Immun. 1985 Oct;50(1):160–168. doi: 10.1128/iai.50.1.160-168.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Daly J. A., Cabrera Z. Serum protein from Leishmania brasiliensis infected hamster that suppress lymphocyte response of normal hamster lymphocytes. Z Parasitenkd. 1986;72(3):293–298. doi: 10.1007/BF00928738. [DOI] [PubMed] [Google Scholar]
- Sacks D. L., Lal S. L., Shrivastava S. N., Blackwell J., Neva F. A. An analysis of T cell responsiveness in Indian kala-azar. J Immunol. 1987 Feb 1;138(3):908–913. [PubMed] [Google Scholar]
- Salgame P. R., Mahadevan P. R., Antia N. H. Mechanism of immunosuppression in leprosy: presence of suppressor factor(s) from macrophages of lepromatous patients. Infect Immun. 1983 Jun;40(3):1119–1126. doi: 10.1128/iai.40.3.1119-1126.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathish M., Bhutani L. K., Sharma A. K., Nath I. Monocyte-derived soluble suppressor factor(s) in patients with lepromatous leprosy. Infect Immun. 1983 Dec;42(3):890–899. doi: 10.1128/iai.42.3.890-899.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott P. A., Farrell J. P. Experimental cutaneous leishmaniasis. I. Nonspecific immunodepression in BALB/c mice infected with Leishmania tropica. J Immunol. 1981 Dec;127(6):2395–2400. [PubMed] [Google Scholar]
- Sprent J., Miller J. F., Mitchell G. F. Antigen-induced selective recruitment of circulating lymphocytes. Cell Immunol. 1971 Apr;2(2):171–181. doi: 10.1016/0008-8749(71)90036-0. [DOI] [PubMed] [Google Scholar]
- Stauber L. A. Characterization of strains of Leishmania donovani. Exp Parasitol. 1966 Feb;18(1):1–11. doi: 10.1016/0014-4894(66)90002-6. [DOI] [PubMed] [Google Scholar]
- Witte P. L., Stein-Streilein J., Streilein J. W. Description of phenotypically distinct T-lymphocyte subsets which mediate helper/DTH and cytotoxic functions in the Syrian hamster. J Immunol. 1985 May;134(5):2908–2915. [PubMed] [Google Scholar]
- Witte P. L., Streilein J. W. Thy-1 antigen is present on B and T lymphocytes of the Syrian hamster. J Immunol. 1983 Dec;131(6):2903–2907. [PubMed] [Google Scholar]
- Wyler D. J. Circulating factor from a kala-azar patient suppresses in vitro antileishmanial T cell proliferation. Trans R Soc Trop Med Hyg. 1982;76(3):304–306. doi: 10.1016/0035-9203(82)90174-2. [DOI] [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]


