Abstract
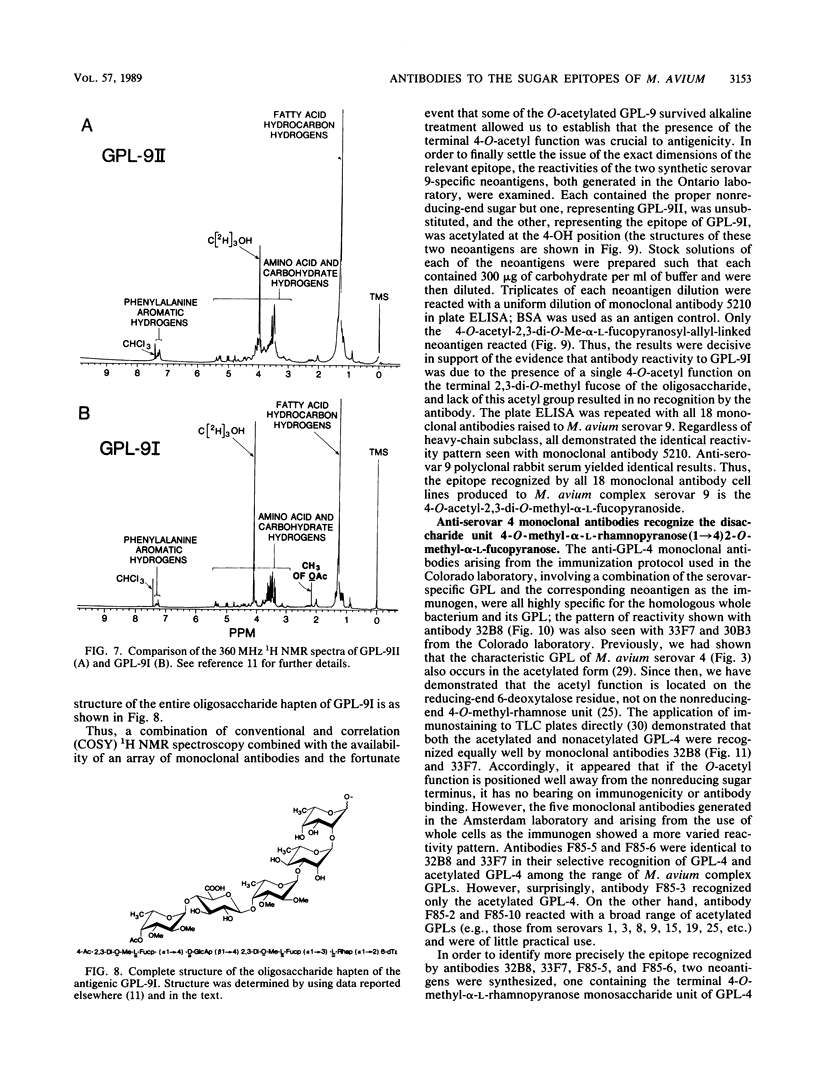
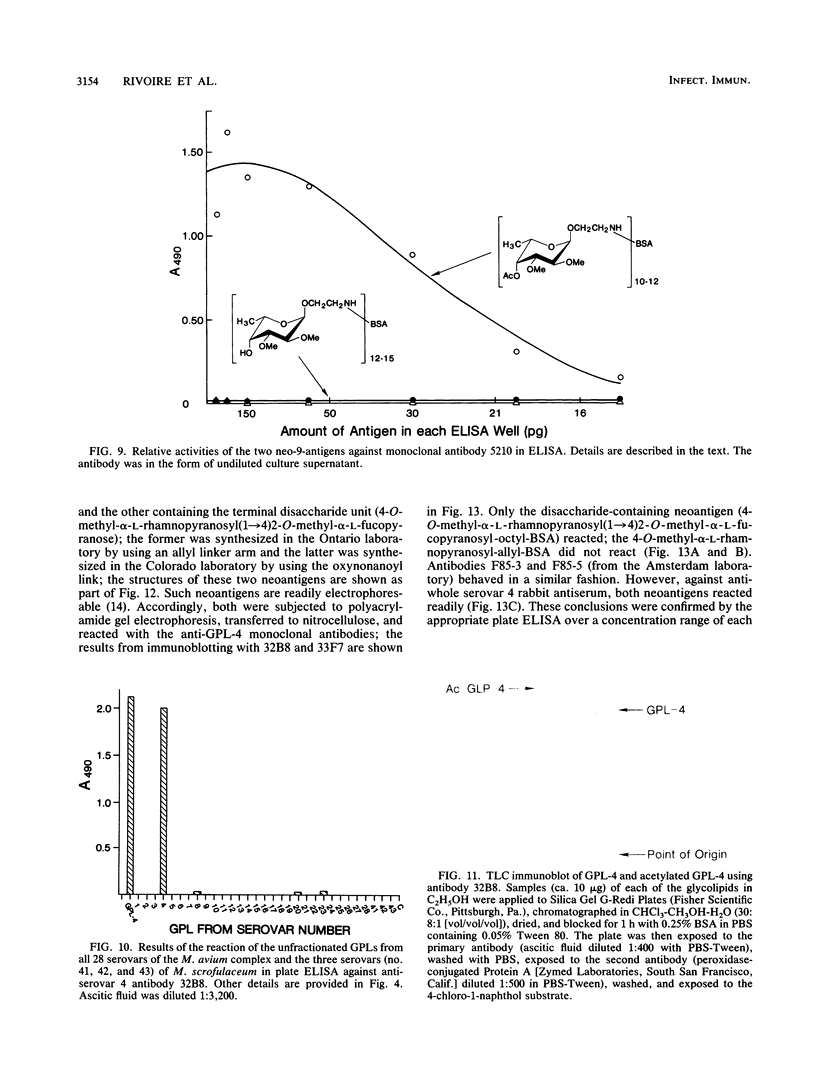
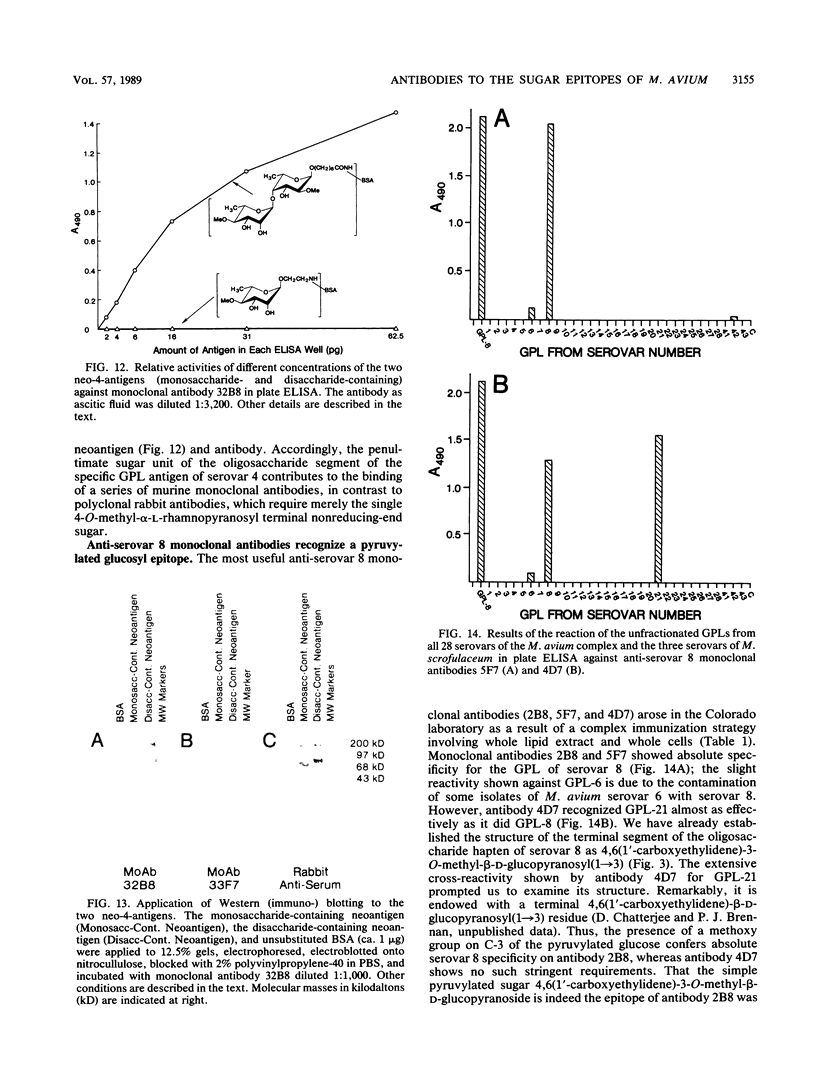
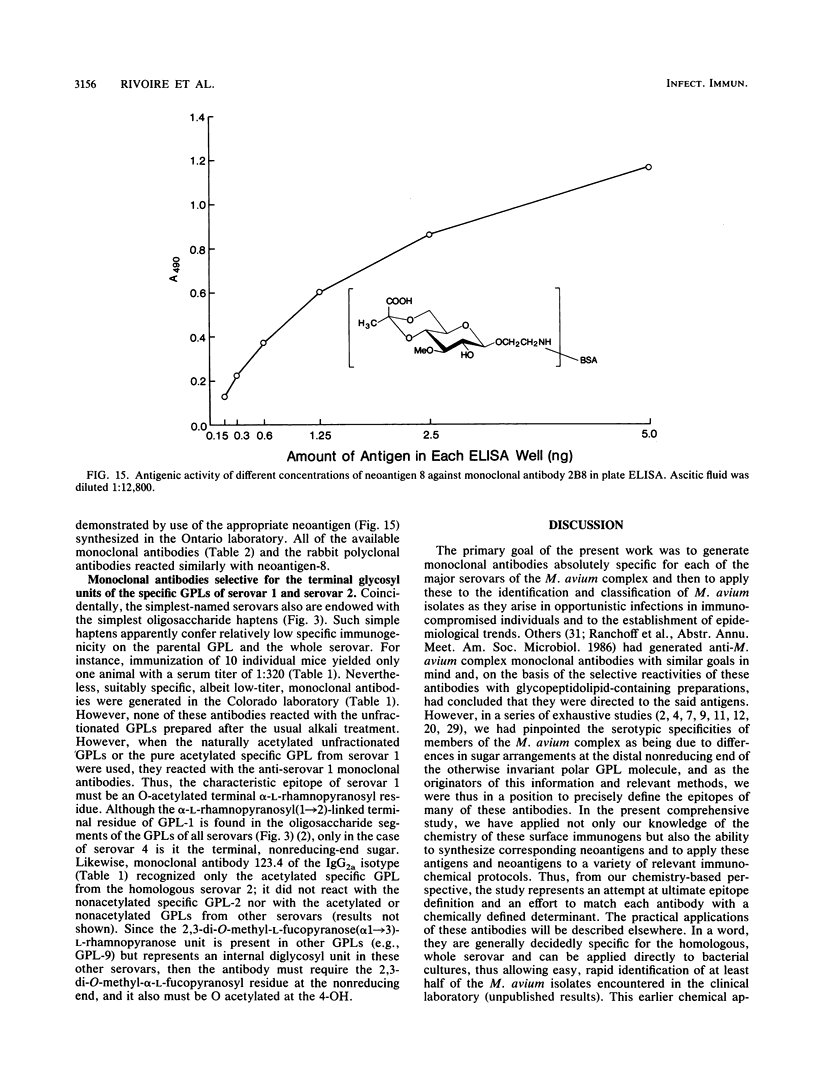
Monoclonal antibodies have been generated to the unique distal sugar epitopes on the oligosaccharide haptens of the glycopeptidolipid antigens of clinically prominent members of the Mycobacterium avium serocomplex. Thus, antibodies are described that recognize the distal O-acetyl-alpha-L-rhamnopyranosyl residue of the specific glycopeptidolipid of M. avium serovar 1, the 4-O-acetyl-2,3-di-O-methyl-alpha-L-fucopyranose of serovar 2, the 4-O-methyl-alpha-L-rhamnopyranosyl-(1----4)-2-O-methyl-alpha-L- fucopyranosyl unit of serovar 4, the 4,6-(1'-carboxyethylidene)-3-O-methyl-beta-D-glucopyranosyl unit of serovar 8 [and the 4,6-(1'-carboxyethylidene)-beta-D-glucopyranosyl residue of serovar 21], and the 4-O-acetyl-2,3-di-O-methyl-alpha-L-fucopyranosyl-(1----4)-beta-D- glucuronopyranosyl unit of serovar 9. Epitope definition was arrived at through use of the pure, chemically defined glycopeptidolipid antigens and neoglycoproteins containing the chemically synthesized distal sugars of some select serovars. These monoclonal antibodies combined with the already published information on the structure of the antigen determinants and the tools used to arrive at these structures provide powerful means for fundamental studies on the role of these antigens in immunopathogenesis and for the precise mapping of the epidemiology of opportunistic infections caused by M. avium.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bozic C. M., McNeil M., Chatterjee D., Jardine I., Brennan P. J. Further novel amido sugars within the glycopeptidolipid antigens of Mycobacterium avium. J Biol Chem. 1988 Oct 15;263(29):14984–14991. [PubMed] [Google Scholar]
- Brennan P. J., Aspinall G. O., Shin J. E. Structure of the specific oligosaccharides from the glycopeptidolipid antigens of serovars in the Mycobacterium avium-Mycobacterium intracellulare-Mycobacterium scrofulaceum complex. J Biol Chem. 1981 Jul 10;256(13):6817–6822. [PubMed] [Google Scholar]
- Brennan P. J., Goren M. B. Structural studies on the type-specific antigens and lipids of the mycobacterium avium. Mycobacterium intracellulare. Mycobacterium scrofulaceum serocomplex. Mycobacterium intracellulare serotype 9. J Biol Chem. 1979 May 25;254(10):4205–4211. [PubMed] [Google Scholar]
- Brennan P. J., Heifets M., Ullom B. P. Thin-layer chromatography of lipid antigens as a means of identifying nontuberculous mycobacteria. J Clin Microbiol. 1982 Mar;15(3):447–455. doi: 10.1128/jcm.15.3.447-455.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan P. J., Mayer H., Aspinall G. O., Nam Shin J. E. Structures of the glycopeptidolipid antigens from serovars in the Mycobacterium avium/Mycobacterium intracellulare/Mycobacterium scrofulaceum serocomplex. Eur J Biochem. 1981 Mar 16;115(1):7–15. doi: 10.1111/j.1432-1033.1981.tb06190.x. [DOI] [PubMed] [Google Scholar]
- Brockhaus M., Magnani J. L., Blaszczyk M., Steplewski Z., Koprowski H., Karlsson K. A., Larson G., Ginsburg V. Monoclonal antibodies directed against the human Leb blood group antigen. J Biol Chem. 1981 Dec 25;256(24):13223–13225. [PubMed] [Google Scholar]
- Camphausen R. T., Jones R. L., Brennan P. J. Structure and relevance of the oligosaccharide hapten of Mycobacterium avium serotype 2. J Bacteriol. 1986 Nov;168(2):660–667. doi: 10.1128/jb.168.2.660-667.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee D., Aspinall G. O., Brennan P. J. The presence of novel glucuronic acid-containing, type-specific glycolipid antigens within Mycobacterium spp. Revision of earlier structures. J Biol Chem. 1987 Mar 15;262(8):3528–3533. [PubMed] [Google Scholar]
- Chatterjee D., Bozic C., Aspinall G. O., Brennan P. J. Glucuronic acid- and branched sugar-containing glycolipid antigens of Mycobacterium avium. J Biol Chem. 1988 Mar 25;263(9):4092–4097. [PubMed] [Google Scholar]
- Chatterjee D., Cho S. N., Stewart C., Douglas J. T., Fujiwara T., Brennan P. J. Synthesis and immunoreactivity of neoglycoproteins containing the trisaccharide unit of phenolic glycolipid I of Mycobacterium leprae. Carbohydr Res. 1988 Dec 1;183(2):241–260. doi: 10.1016/0008-6215(88)84078-3. [DOI] [PubMed] [Google Scholar]
- Cho S. N., Hunter S. W., Gelber R. H., Rea T. H., Brennan P. J. Quantitation of the phenolic glycolipid of Mycobacterium leprae and relevance to glycolipid antigenemia in leprosy. J Infect Dis. 1986 Mar;153(3):560–569. doi: 10.1093/infdis/153.3.560. [DOI] [PubMed] [Google Scholar]
- Cohn M. L., Waggoner R. F., McClatchy J. K. The 7H11 medium for the cultivation of mycobacteria. Am Rev Respir Dis. 1968 Aug;98(2):295–296. doi: 10.1164/arrd.1968.98.2.295. [DOI] [PubMed] [Google Scholar]
- Dhariwal K. R., Liav A., Vatter A. E., Dhariwal G., Goren M. B. Haptenic oligosaccharides in antigenic variants of mycobacterial C-mycosides antagonize lipid receptor activity for mycobacteriophage D4 by masking a methylated rhamnose. J Bacteriol. 1986 Oct;168(1):283–293. doi: 10.1128/jb.168.1.283-293.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaylord H., Brennan P. J. Leprosy and the leprosy bacillus: recent developments in characterization of antigens and immunology of the disease. Annu Rev Microbiol. 1987;41:645–675. doi: 10.1146/annurev.mi.41.100187.003241. [DOI] [PubMed] [Google Scholar]
- Gaylord H., Brennan P. J., Young D. B., Buchanan T. M. Most Mycobacterium leprae carbohydrate-reactive monoclonal antibodies are directed to lipoarabinomannan. Infect Immun. 1987 Nov;55(11):2860–2863. doi: 10.1128/iai.55.11.2860-2863.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good R. C. Opportunistic pathogens in the genus Mycobacterium. Annu Rev Microbiol. 1985;39:347–369. doi: 10.1146/annurev.mi.39.100185.002023. [DOI] [PubMed] [Google Scholar]
- Jardine I., Scanlan G., McNeil M., Brennan P. J. Plasma desorption mass spectrometric analysis of mycobacterial glycolipids. Anal Chem. 1989 Mar 1;61(5):416–422. doi: 10.1021/ac00180a008. [DOI] [PubMed] [Google Scholar]
- Kolk A. H., Ho M. L., Klatser P. R., Eggelte T. A., Kuijper S., de Jonge S., van Leeuwen J. Production and characterization of monoclonal antibodies to Mycobacterium tuberculosis, M. bovis (BCG) and M. leprae. Clin Exp Immunol. 1984 Dec;58(3):511–521. [PMC free article] [PubMed] [Google Scholar]
- Lindberg A. A., Hellerqvist C. G. Bacteriophage attachment sites, serological specificity, and chemical composition of the lipopolysaccharides of semirough and rough mutants of Salmonella typhimurium. J Bacteriol. 1971 Jan;105(1):57–64. doi: 10.1128/jb.105.1.57-64.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil M., Gaylord H., Brennan P. J. N-formylkansosaminyl-(1----3)-2-O-methyl-D-rhamnopyranose: the type-specific determinant of serovariant 14 of the Mycobacterium avium complex. Carbohydr Res. 1988 Jun 15;177:185–198. doi: 10.1016/0008-6215(88)85052-3. [DOI] [PubMed] [Google Scholar]
- McNeil M., Tsang A. Y., Brennan P. J. Structure and antigenicity of the specific oligosaccharide hapten from the glycopeptidolipid antigen of Mycobacterium avium serotype 4, the dominant Mycobacterium isolated from patients with acquired immune deficiency syndrome. J Biol Chem. 1987 Feb 25;262(6):2630–2635. [PubMed] [Google Scholar]
- Nishimori K., Yugi H., Naiki M., Sugimura T., Tanaka Y., Nonomura I., Yokomizo Y., Kubo S. Production and characterization of serovar-specific monoclonal antibodies to serovars 4, 8, and 9 of Mycobacterium intracellulare. Infect Immun. 1987 Mar;55(3):711–715. doi: 10.1128/iai.55.3.711-715.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudelman E., Hakomori S., Kannagi R., Levery S., Yeh M. Y., Hellström K. E., Hellström I. Characterization of a human melanoma-associated ganglioside antigen defined by a monoclonal antibody, 4.2. J Biol Chem. 1982 Nov 10;257(21):12752–12756. [PubMed] [Google Scholar]
- Nudelman E., Kannagi R., Hakomori S., Parsons M., Lipinski M., Wiels J., Fellous M., Tursz T. A glycolipid antigen associated with Burkitt lymphoma defined by a monoclonal antibody. Science. 1983 Apr 29;220(4596):509–511. doi: 10.1126/science.6836295. [DOI] [PubMed] [Google Scholar]
- Rao A. S., Liao J., Kabat E. A., Osserman E. F., Harboe M., Nimmich W. Immunochemical studies on human monoclonal macroglobulins with specificities for 3,4-pyruvylated D-galactose and 4,6-pyruvylated D-glucose. J Biol Chem. 1984 Jan 25;259(2):1018–1026. [PubMed] [Google Scholar]
- Takeo K., Aspinall G. O., Brennan P. J., Chatterjee D. Synthesis of tetrasaccharides related to the antigenic determinants from the glycopeptidolipid antigens of serovars 9 and 25 in the Mycobacterium avium-M. intracellulare-M. scrofulaceum serocomplex. Carbohydr Res. 1986 Aug 1;150:133–150. doi: 10.1016/0008-6215(86)80011-8. [DOI] [PubMed] [Google Scholar]
- Wolinsky E. Nontuberculous mycobacteria and associated diseases. Am Rev Respir Dis. 1979 Jan;119(1):107–159. doi: 10.1164/arrd.1979.119.1.107. [DOI] [PubMed] [Google Scholar]
- Yanagihara D. L., Barr V. L., Knisley C. V., Tsang A. Y., McClatchy J. K., Brennan P. J. Enzyme-linked immunosorbent assay of glycolipid antigens for identification of mycobacteria. J Clin Microbiol. 1985 Apr;21(4):569–574. doi: 10.1128/jcm.21.4.569-574.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L. S. Mycobacterium avium complex infection. J Infect Dis. 1988 May;157(5):863–867. doi: 10.1093/infdis/157.5.863. [DOI] [PubMed] [Google Scholar]