Abstract
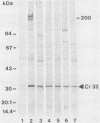
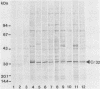
Western blotting (immunoblotting) of Cowdria ruminantium antigens with goat or mouse antiserum identified a periodate-resistant, proteinase K-sensitive immunodominant antigen of 32,000 daltons. This protein, designated Cr32, could be demonstrated in goat choroid plexus infected with one of two different Cowdria stocks. Antisera against nine different Cowdria stocks from Africa and the Caribbean region recognized Cr32, which indicates that this protein contains conserved antigenic determinants.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOVARNICK M. R., MILLER J. C., SNYDER J. C. The influence of certain salts, amino acids, sugars, and proteins on the stability of rickettsiae. J Bacteriol. 1950 Apr;59(4):509–522. doi: 10.1128/jb.59.4.509-522.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezuidenhout J. D. The present state of Cowdria ruminantium cultivation in cell lines. Onderstepoort J Vet Res. 1987 Sep;54(3):205–210. [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Du Plessis J. L. A method for determining the Cowdria ruminantium infection rate of Amblyomma hebraeum: effects in mice injected with tick homogenates. Onderstepoort J Vet Res. 1985 Jun;52(2):55–61. [PubMed] [Google Scholar]
- Du Plessis J. L., Camus E., Oberem P. T., Malan L. Heartwater serology: some problems with the interpretation of results. Onderstepoort J Vet Res. 1987 Sep;54(3):327–329. [PubMed] [Google Scholar]
- Fukushi H., Hirai K. Immunochemical diversity of the major outer membrane protein of avian and mammalian Chlamydia psittaci. J Clin Microbiol. 1988 Apr;26(4):675–680. doi: 10.1128/jcm.26.4.675-680.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongejan F., Morzaria S. P., Shariff O. A., Abdalla H. M. Isolation and transmission of Cowdria ruminantium (causal agent of heartwater disease) in Blue Nile Province, Sudan. Vet Res Commun. 1984 May;8(2):141–145. doi: 10.1007/BF02214705. [DOI] [PubMed] [Google Scholar]
- Jongejan F., Uilenberg G., Franssen F. F., Gueye A., Nieuwenhuijs J. Antigenic differences between stocks of Cowdria ruminantium. Res Vet Sci. 1988 Mar;44(2):186–189. [PubMed] [Google Scholar]
- Kocan K. M., Morzaria S. P., Voigt W. P., Kiarie J., Irvin A. D. Demonstration of colonies of Cowdria ruminantium in midgut epithelial cells of Amblyomma variegatum. Am J Vet Res. 1987 Mar;48(3):356–360. [PubMed] [Google Scholar]
- Palmer G. H., Barbet A. F., Musoke A. J., Katende J. M., Rurangirwa F., Shkap V., Pipano E., Davis W. C., McGuire T. C. Recognition of conserved surface protein epitopes on Anaplasma centrale and Anaplasma marginale isolates from Israel, Kenya and the United States. Int J Parasitol. 1988 Feb;18(1):33–38. doi: 10.1016/0020-7519(88)90033-1. [DOI] [PubMed] [Google Scholar]
- Palmer G. H., Oberle S. M., Barbet A. F., Goff W. L., Davis W. C., McGuire T. C. Immunization of cattle with a 36-kilodalton surface protein induces protection against homologous and heterologous Anaplasma marginale challenge. Infect Immun. 1988 Jun;56(6):1526–1531. doi: 10.1128/iai.56.6.1526-1531.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreau P., Morel P. C., Barre N., Durand P. Existence de la cowdriose (heartwater) à Cowdria ruminantium chez les ruminants des Antilles françaises (la Guadeloupe) et des Mascareignes (la Réunion et Ile Maurice). Rev Elev Med Vet Pays Trop. 1980;33(1):21–22. [PubMed] [Google Scholar]
- Provost A., Bezuidenhout J. D. The historical background and global importance of heartwater. Onderstepoort J Vet Res. 1987 Sep;54(3):165–169. [PubMed] [Google Scholar]
- Uilenberg G., Camus E., Barré N. Quelques observations sur une souche de Cowdria ruminantium isolée en Guadeloupe (Antilles françaises). Rev Elev Med Vet Pays Trop. 1985;38(1):34–42. [PubMed] [Google Scholar]
- Uilenberg G. Experimental transmission of Cowdria ruminantium by the Gulf coast tick Amblyomma maculatum: danger of introducing heartwater and benign African theileriasis onto the American mainland. Am J Vet Res. 1982 Jul;43(7):1279–1282. [PubMed] [Google Scholar]
- Uilenberg G. Heartwater (Cowdria ruminantium infection): current status. Adv Vet Sci Comp Med. 1983;27:427–480. [PubMed] [Google Scholar]
- du Plessis J. L., Kumm N. A. The passage of Cowdria ruminantium in mice. J S Afr Vet Med Assoc. 1971 Sep;42(3):217–221. [PubMed] [Google Scholar]