Abstract
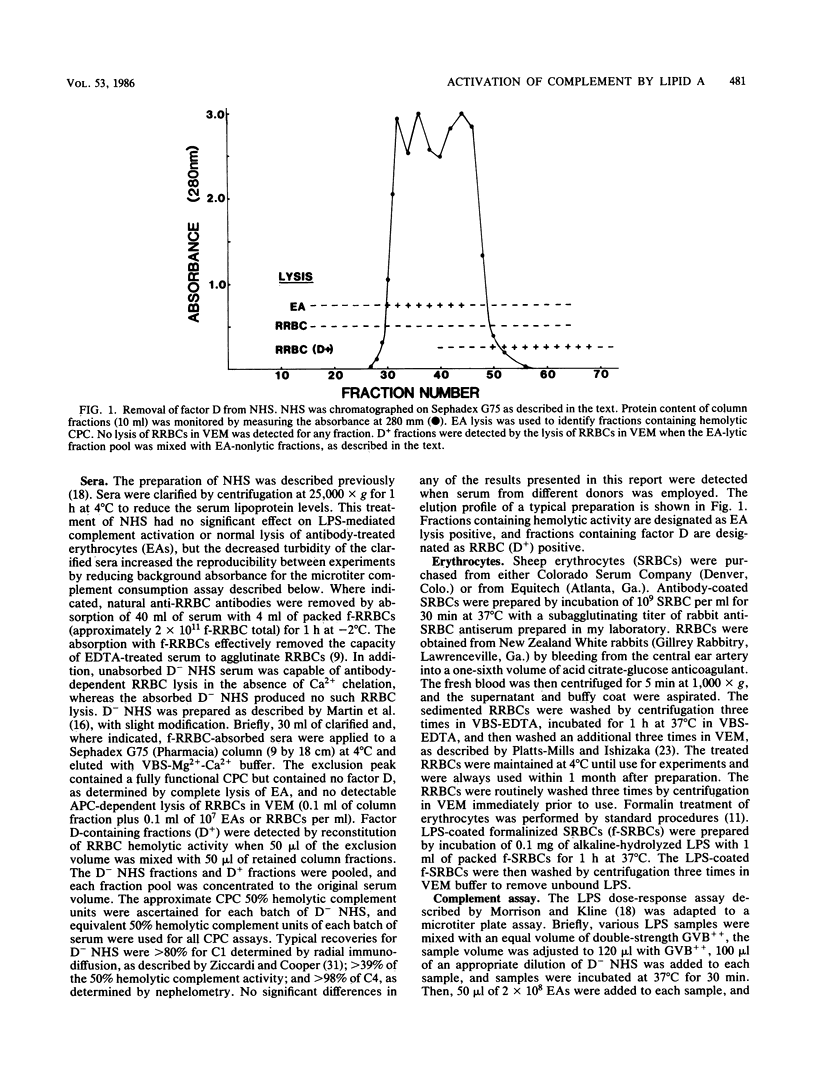
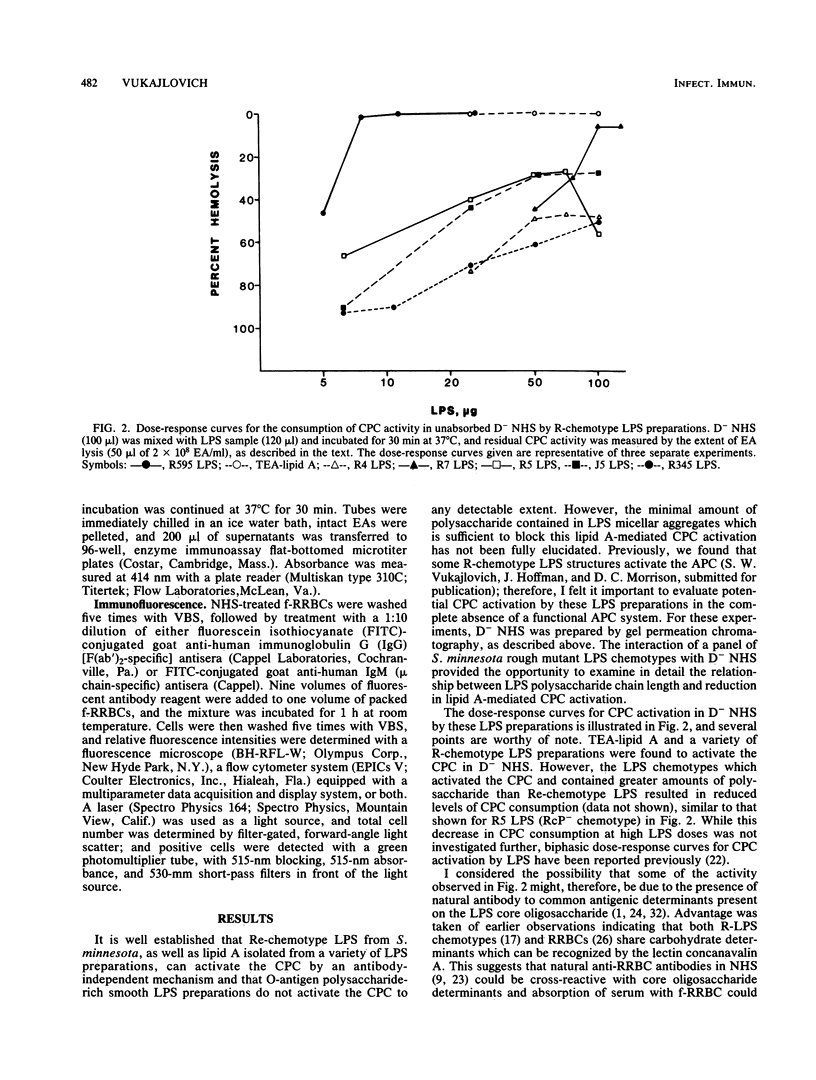
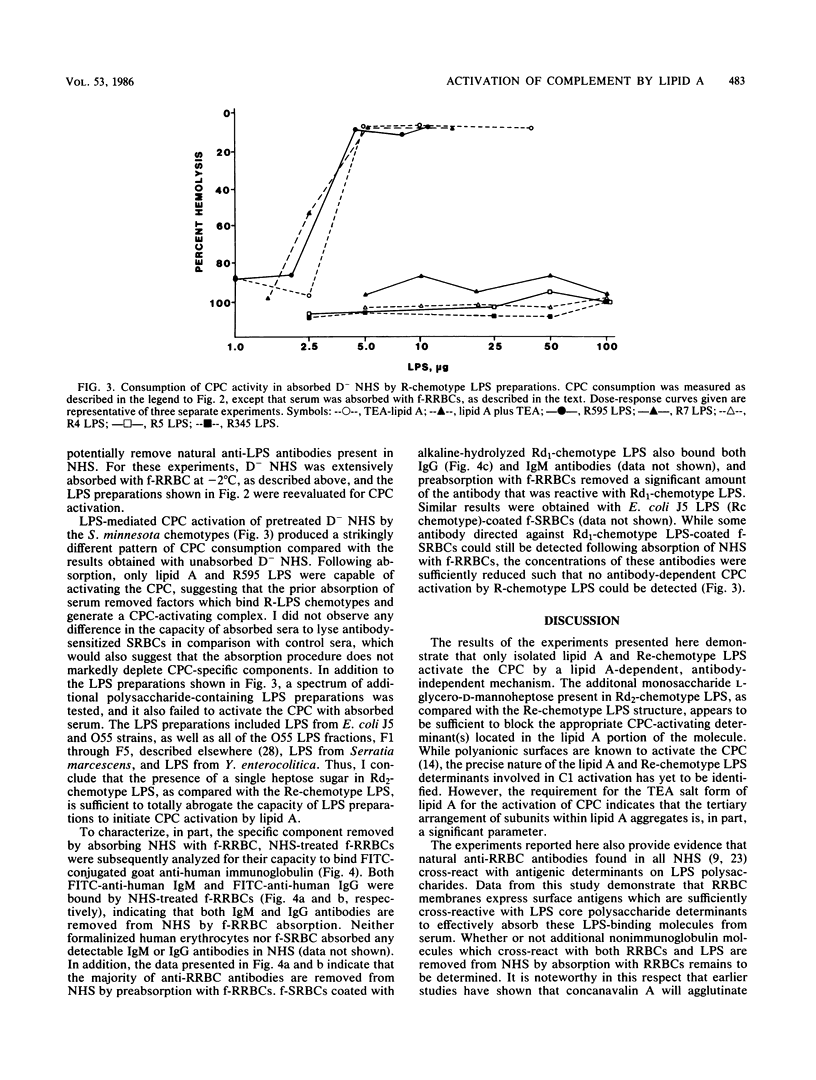
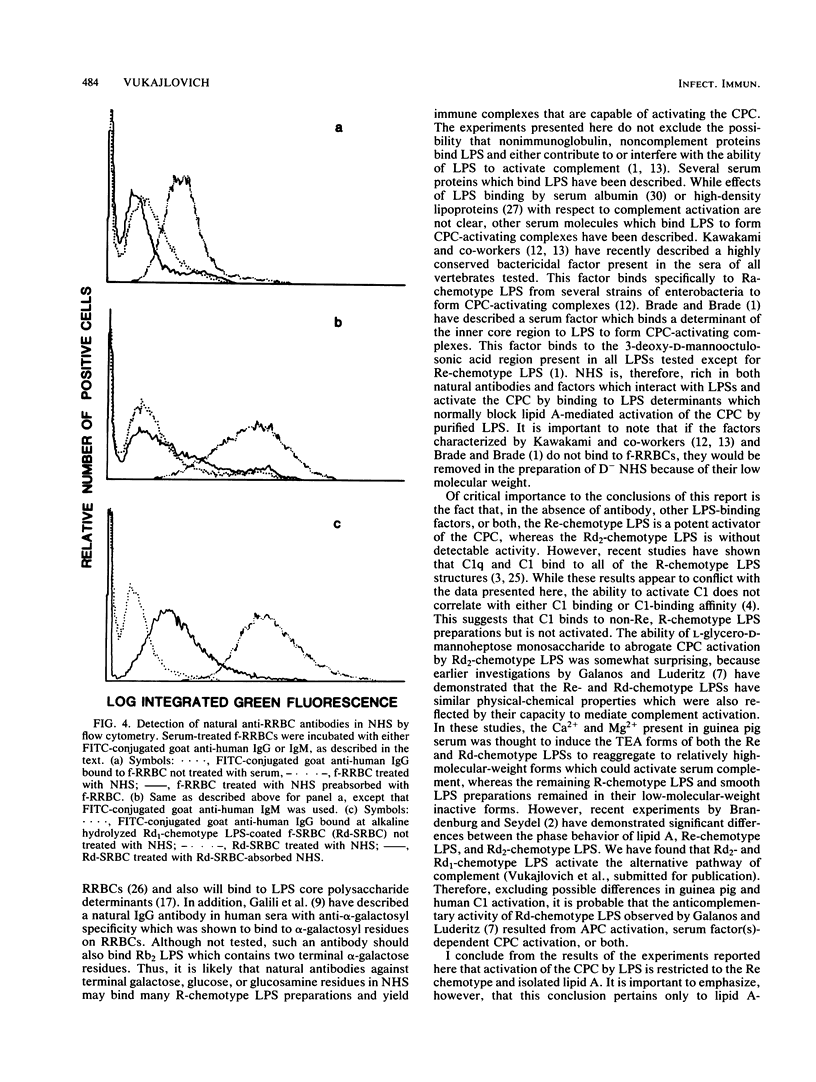
Incubation of most bacterial lipopolysaccharides (LPS) with normal human sera at 37 degrees C activates the serum complement system, resulting in decreased levels of hemolytic complement. A panel of R-chemotype LPS preparations isolated from Salmonella minnesota rough mutant strains, as well as smooth wild-type LPS from S. minnesota, Escherichia coli O55-B5, Serratia marcescens, and Yersinia enterolitica, were used to examine the effect of LPS polysaccharide chain length on LPS lipid (lipid A)-dependent activation of the classical pathway of complement (CPC). To examine specific lipid A-dependent activation of the CPC, sera deficient in alternative pathway of complement activity were prepared by the removal of factor D. Absorption of normal human sera with formalinized rabbit erythrocytes was found to remove natural antibodies, factors capable of forming LPS complexes which activate the CPC, or both. By using such factor D-depleted formalinized rabbit erythrocyte-absorbed normal human sera, only isolated lipid A and Re-chemotype LPS (R595 LPS) were found to activate the CPC. Thus, the presence of the additional monosaccharide L-glycero-D-mannoheptose in the Rd2 LPS oligosaccharide chain compared with the L-glycero-D-mannoheptose-deficient Re-chemotype LPS structure is sufficient to block lipid A-dependent activation of the CPC by LPS.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brade L., Brade H. A 28,000-dalton protein of normal mouse serum binds specifically to the inner core region of bacterial lipopolysaccharide. Infect Immun. 1985 Dec;50(3):687–694. doi: 10.1128/iai.50.3.687-694.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clas F., Schmidt G., Loos M. The role of the classical pathway for the bactericidal effect of normal sera against gram-negative bacteria. Curr Top Microbiol Immunol. 1985;121:19–72. doi: 10.1007/978-3-642-45604-6_3. [DOI] [PubMed] [Google Scholar]
- Cooper N. R. Activation and regulation of the first complement component. Fed Proc. 1983 Jan;42(1):134–138. [PubMed] [Google Scholar]
- Cooper N. R., Morrison D. C. Binding and activation of the first component of human complement by the lipid A region of lipopolysaccharides. J Immunol. 1978 Jun;120(6):1862–1868. [PubMed] [Google Scholar]
- Gaffin S. L., Badsha N., Vorster B. J. Properties of human anti-lipopolysaccharide gamma globulin: specificity and protective effects. Vox Sang. 1985;48(5):276–283. doi: 10.1111/j.1423-0410.1985.tb00183.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O. The role of the physical state of lipopolysaccharides in the interaction with complement. High molecular weight as prerequisite for the expression of anti-complementary activity. Eur J Biochem. 1976 Jun 1;65(2):403–408. doi: 10.1111/j.1432-1033.1976.tb10354.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Galili U., Rachmilewitz E. A., Peleg A., Flechner I. A unique natural human IgG antibody with anti-alpha-galactosyl specificity. J Exp Med. 1984 Nov 1;160(5):1519–1531. doi: 10.1084/jem.160.5.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewurz H., Shin H. S., Mergenhagen S. E. Interactions of the complement system with endotoxic lipopolysaccharide: consumption of each of the six terminal complement components. J Exp Med. 1968 Nov 1;128(5):1049–1057. doi: 10.1084/jem.128.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara I., Harada Y., Ihara S., Kawakami M. A new complement-dependent bactericidal factor found in nonimmune mouse sera: specific binding to polysaccharide of Ra chemotype Salmonella. J Immunol. 1982 Mar;128(3):1256–1260. [PubMed] [Google Scholar]
- Kawakami M., Ihara I., Ihara S., Suzuki A., Fukui K. A group of bactericidal factors conserved by vertebrates for more than 300 million years. J Immunol. 1984 May;132(5):2578–2581. [PubMed] [Google Scholar]
- Loos M., Bitter-Suermann D., Dierich M. Interaction of the first (C1), the second (C2) and the fourth (C4) component of complement with different preparations of bacterial lipopolysaccharides and with lipid A. J Immunol. 1974 Mar;112(3):935–940. [PubMed] [Google Scholar]
- Loos M. The classical complement pathway: mechanism of activation of the first component by antigen-antibody complexes. Prog Allergy. 1982;30:135–192. [PubMed] [Google Scholar]
- Martin A., Lachmann P. J., Halbwachs L., Hobart M. J. Haemolytic diffusion plate assays for factors B and D of the alternative pathway of complement activation. Immunochemistry. 1976 Apr;13(4):317–324. doi: 10.1016/0019-2791(76)90341-4. [DOI] [PubMed] [Google Scholar]
- Mayer H., Schlecht S., Gromska W. Reactivity of lipopolysaccharides from various salmonella SR and R chemotypes Ra-Re mutants with concanavalin A. Zentralbl Bakteriol Orig A. 1975 Nov;233(3):327–334. [PubMed] [Google Scholar]
- Morrison D. C., Kline L. F. Activation of the classical and properdin pathways of complement by bacterial lipopolysaccharides (LPS). J Immunol. 1977 Jan;118(1):362–368. [PubMed] [Google Scholar]
- Morrison D. C., Leive L. Fractions of lipopolysaccharide from Escherichia coli O111:B4 prepared by two extraction procedures. J Biol Chem. 1975 Apr 25;250(8):2911–2919. [PubMed] [Google Scholar]
- Morrison D. C., Verroust P., Weigle W. O. Anticomplementary activity of lipid A isolated from lipopolysaccharides. Proc Soc Exp Biol Med. 1973 Sep;143(4):1025–1030. doi: 10.3181/00379727-143-37462. [DOI] [PubMed] [Google Scholar]
- PILLEMER L., SCHOENBERG M. D., BLUM L., WURZ L. Properdin system and immunity. II. Interaction of the properdin system with polysaccharides. Science. 1955 Sep 23;122(3169):545–549. doi: 10.1126/science.122.3169.545. [DOI] [PubMed] [Google Scholar]
- Pangburn M. K., Morrison D. C., Schreiber R. D., Müller-Eberhard H. J. Activation of the alternative complement pathway: recognition of surface structures on activators by bound C3b. J Immunol. 1980 Feb;124(2):977–982. [PubMed] [Google Scholar]
- Platts-Mills T. A., Ishizaka K. Activation of the alternate pathway of human complements by rabbit cells. J Immunol. 1974 Jul;113(1):348–358. [PubMed] [Google Scholar]
- Pollack M., Huang A. I., Prescott R. K., Young L. S., Hunter K. W., Cruess D. F., Tsai C. M. Enhanced survival in Pseudomonas aeruginosa septicemia associated with high levels of circulating antibody to Escherichia coli endotoxin core. J Clin Invest. 1983 Dec;72(6):1874–1881. doi: 10.1172/JCI111150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemmer F., Loos M. Evidence for direct binding of the first component of complement, C1, to outer membrane proteins from Salmonella minnesota. Curr Top Microbiol Immunol. 1985;121:73–84. doi: 10.1007/978-3-642-45604-6_4. [DOI] [PubMed] [Google Scholar]
- Sumner J. B., Howell S. F. Identification of Hemagglutinin of Jack Bean with Concanavalin A. J Bacteriol. 1936 Aug;32(2):227–237. doi: 10.1128/jb.32.2.227-237.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulevitch R. J., Johnston A. R., Weinstein D. B. New function for high density lipoproteins. Their participation in intravascular reactions of bacterial lipopolysaccharides. J Clin Invest. 1979 Nov;64(5):1516–1524. doi: 10.1172/JCI109610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukajlovich S. W., Morrison D. C. Conversion of lipopolysaccharides to molecular aggregates with reduced subunit heterogeneity: demonstration of LPS-responsiveness in "endotoxin-unresponsive" C3H/HeJ splenocytes. J Immunol. 1983 Jun;130(6):2804–2808. [PubMed] [Google Scholar]
- Wollenweber H. W., Morrison D. C. Synthesis and biochemical characterization of a photoactivatable, iodinatable, cleavable bacterial lipopolysaccharide derivative. J Biol Chem. 1985 Dec 5;260(28):15068–15074. [PubMed] [Google Scholar]
- Ziccardi R. J., Cooper N. R. Direct demonstration and quantitation of the first complement component in human serum. Science. 1978 Mar 10;199(4333):1080–1082. doi: 10.1126/science.75568. [DOI] [PubMed] [Google Scholar]
- Ziegler E. J., McCutchan J. A., Fierer J., Glauser M. P., Sadoff J. C., Douglas H., Braude A. I. Treatment of gram-negative bacteremia and shock with human antiserum to a mutant Escherichia coli. N Engl J Med. 1982 Nov 11;307(20):1225–1230. doi: 10.1056/NEJM198211113072001. [DOI] [PubMed] [Google Scholar]


