Abstract
The Streptococcus mutans GS-5 gene, scrB, coding for sucrose 6-phosphate hydrolase activity has been cloned into Escherichia coli utilizing the bacteriophage replacement vector lambda L47.1. DNA sequences containing the gene were initially subcloned into the moderate-copy-number plasmid vector pLG339 to yield active subclones. However, due to the instability of the resultant chimeric plasmids, the gene was subsequently subcloned into the low-copy-number vector pOU61 to yield the stable hybrid plasmid pMH613. Both plasmids contain a 6.6-kilobase EcoRI fragment from strain GS-5 and express both hydrolase and sucrase activities. The relative position of the gene in the insert has been determined after Tn5 mutagenesis and deletion analysis. The cloned enzyme was purified to near homogeneity after gel filtration and anion-exchange chromatography, chromatofocusing, and preparative polyacrylamide gel electrophoresis. The purified enzyme displayed a molecular mass of 58 kilodaltons, which is significantly higher than the 48-kilodalton enzyme previously purified from S. mutans GS-5. These results suggest that processing of the hydrolase occurs in S. mutans.
Full text
PDF

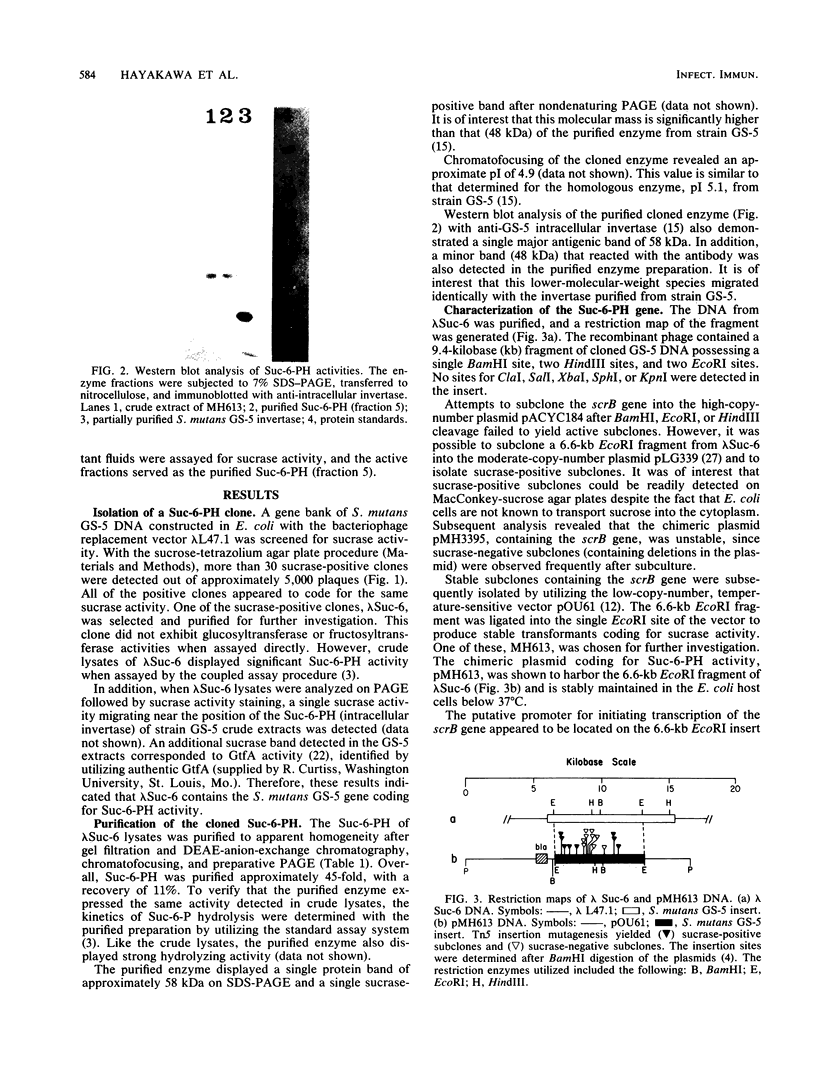


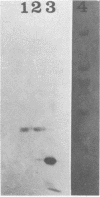
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chassy B. M., Porter E. V. Initial characterization of sucrose-6-phosphate hydrolase from Streptococcus mutans and its apparent identity with intracellular invertase. Biochem Biophys Res Commun. 1979 Jul 12;89(1):307–314. doi: 10.1016/0006-291x(79)90979-3. [DOI] [PubMed] [Google Scholar]
- Emr S. D., Hall M. N., Silhavy T. J. A mechanism of protein localization: the signal hypothesis and bacteria. J Cell Biol. 1980 Sep;86(3):701–711. doi: 10.1083/jcb.86.3.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. Dental caries. Annu Rev Med. 1975;26:121–136. doi: 10.1146/annurev.me.26.020175.001005. [DOI] [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980 Jun;44(2):331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagusztyn-Krynicka E. K., Smorawinska M., Curtiss R., 3rd Expression of Streptococcus mutans aspartate-semialdehyde dehydrogenase gene cloned into plasmid pBR322. J Gen Microbiol. 1982 May;128(5):1135–1145. doi: 10.1099/00221287-128-5-1135. [DOI] [PubMed] [Google Scholar]
- Kuramitsu H. K. Characterization of invertase activity from cariogenic Streptococcus mutans. J Bacteriol. 1973 Sep;115(3):1003–1010. doi: 10.1128/jb.115.3.1003-1010.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen J. E., Gerdes K., Light J., Molin S. Low-copy-number plasmid-cloning vectors amplifiable by derepression of an inserted foreign promoter. Gene. 1984 Apr;28(1):45–54. doi: 10.1016/0378-1119(84)90086-6. [DOI] [PubMed] [Google Scholar]
- Maynard M. T., Kuramitsu H. K. Purification and antigenic properties of intracellular invertase from Streptococcus mutans. Infect Immun. 1979 Mar;23(3):873–883. doi: 10.1128/iai.23.3.873-883.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe M. M., Smith E. E., Cowman R. A. Invertase activity in Streptococcus mutans and Streptococcus sanguis. Arch Oral Biol. 1973 Apr;18(4):525–531. doi: 10.1016/0003-9969(73)90073-3. [DOI] [PubMed] [Google Scholar]
- Minah G. E., Loesche W. J. Sucrose metabolism by prominent members of the flora isolated from cariogenic and non-cariogenic dental plaques. Infect Immun. 1977 Jul;17(1):55–61. doi: 10.1128/iai.17.1.55-61.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry D., Nilsen L. J., Kuramitsu H. K. Mapping of a cloned glucosyltransferase gene in Streptococcus mutans. Infect Immun. 1985 Oct;50(1):130–135. doi: 10.1128/iai.50.1.130-135.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry D., Wondrack L. M., Kuramitsu H. K. Genetic transformation of putative cariogenic properties in Streptococcus mutans. Infect Immun. 1983 Aug;41(2):722–727. doi: 10.1128/iai.41.2.722-727.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves R. E., Sols A. Regulation of Escherichia coli phosphofructokinase in situ. Biochem Biophys Res Commun. 1973 Jan 23;50(2):459–466. doi: 10.1016/0006-291x(73)90862-0. [DOI] [PubMed] [Google Scholar]
- Robeson J. P., Barletta R. G., Curtiss R., 3rd Expression of a Streptococcus mutans glucosyltransferase gene in Escherichia coli. J Bacteriol. 1983 Jan;153(1):211–221. doi: 10.1128/jb.153.1.211-221.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Chepelinsky A. B., McKenney K. Studying promoters and terminators by gene fusion. Science. 1983 Nov 18;222(4625):734–739. doi: 10.1126/science.6356355. [DOI] [PubMed] [Google Scholar]
- Slee A. M., Tanzer J. M. Sucrose transport by Streptococcus mutans. Evidence for multiple transport systems. Biochim Biophys Acta. 1982 Nov 22;692(3):415–424. doi: 10.1016/0005-2736(82)90392-3. [DOI] [PubMed] [Google Scholar]
- St Martin E. J., Wittenberger C. L. Characterization of a phosphoenolpyruvate-dependent sucrose phosphotransferase system in Streptococcus mutans. Infect Immun. 1979 Jun;24(3):865–868. doi: 10.1128/iai.24.3.865-868.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker N. G., Fairweather N. F., Spratt B. G. Versatile low-copy-number plasmid vectors for cloning in Escherichia coli. Gene. 1982 Jun;18(3):335–341. doi: 10.1016/0378-1119(82)90172-x. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Tanzer J. M., Brown A. T., McInerney M. F. Identification, preliminary characterization, and evidence for regulation of invertase in Streptococcus mutans. J Bacteriol. 1973 Oct;116(1):192–202. doi: 10.1128/jb.116.1.192-202.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer J. M., Chassy B. M., Krichevsky M. I. Sucrose metabolism by Streptococcus mutans, SL-I. Biochim Biophys Acta. 1971 Feb 28;261(2):379–387. doi: 10.1016/0304-4165(72)90062-1. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruijn F. J., Lupski J. R. The use of transposon Tn5 mutagenesis in the rapid generation of correlated physical and genetic maps of DNA segments cloned into multicopy plasmids--a review. Gene. 1984 Feb;27(2):131–149. doi: 10.1016/0378-1119(84)90135-5. [DOI] [PubMed] [Google Scholar]