Abstract
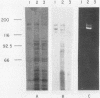
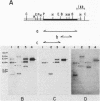
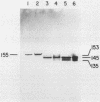
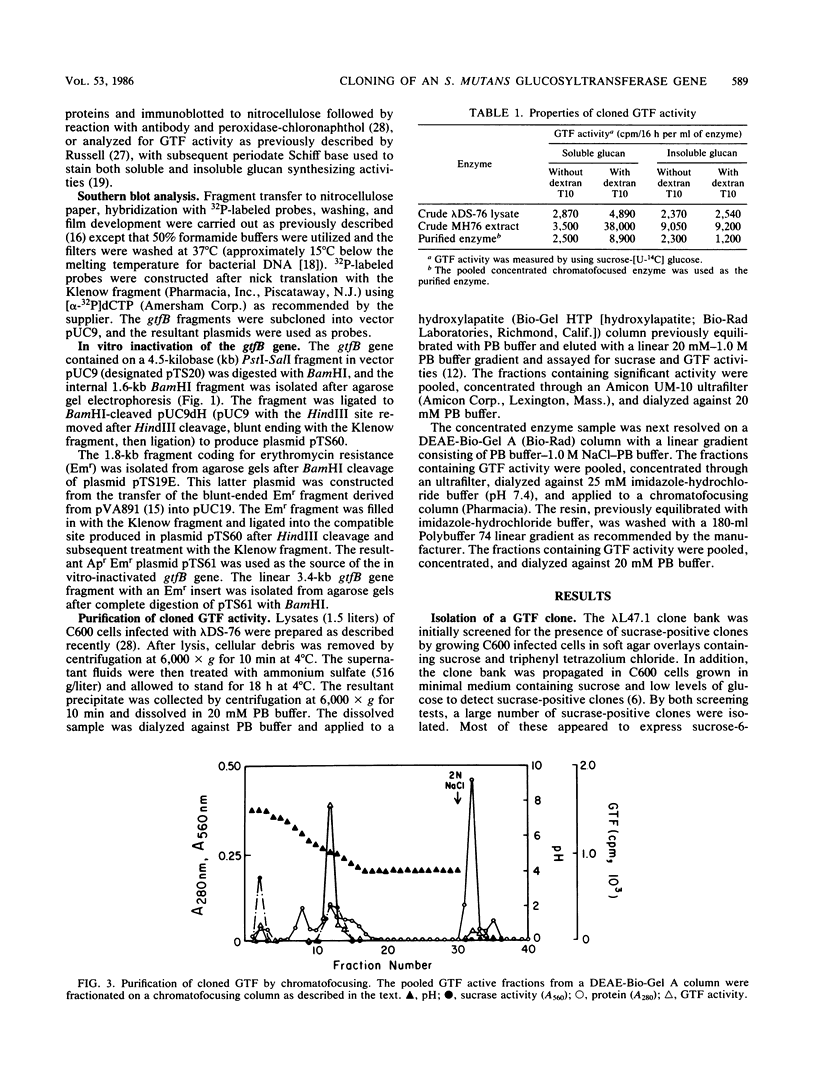
The gtfB gene coding for a glucosyltransferase (GTF) activity of Streptococcus mutans GS-5 was isolated on a 15.4-kilobase DNA fragment by using a lambda L47.1 gene library. The activity was catalyzed by gene products of 150 and 145 kilodaltons which reacted with antibodies directed against both soluble and insoluble glucan-synthesizing GTFs. The enzyme present in crude Escherichia coli extracts synthesized both soluble and insoluble glucans. The enzyme was partially purified from lysates of the lambda DS-76 clone and synthesized both types of glucans in a primer-independent fashion. In addition, the purified enzyme exhibited a pI of approximately 5.0. Southern blot analysis indicated that the cloned GTF gene represented a contiguous nucleotide sequence on the strain GS-5 chromosome. Furthermore, evidence for the existence of a distinct gene sharing partial homology with gtfB was also obtained. The gtfB gene was subcloned into plasmid pACYC184 into E. coli and exhibited GTF activity when carried on GS-5 inserts as small as 5 kilobases. The approximate location of the GTF promoter and the direction of gene transcription were also determined. The cloned enzyme was not secreted through the cytoplasmic membrane of E. coli, since most of the activity was found in the cytoplasm and, in lesser amounts, associated with the cytoplasmic membrane. The gtfB gene was insertionally inactivated by introducing a gene fragment coding for erythromycin resistance into the GTF coding region. After transformation of strain GS-5 with the altered gene, transformants defective in insoluble glucan synthesis were identified. These results indicate that the gtfB gene codes for a GTF involved in insoluble glucan synthesis in strain GS-5.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bratthall D. Demonstration of five serological groups of streptococcal strains resembling Streptococcus mutans. Odontol Revy. 1970;21(2):143–152. [PubMed] [Google Scholar]
- Emr S. D., Hall M. N., Silhavy T. J. A mechanism of protein localization: the signal hypothesis and bacteria. J Cell Biol. 1980 Sep;86(3):701–711. doi: 10.1083/jcb.86.3.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima K., Motoda R., Takada K., Ikeda T. Resolution of Streptococcus mutans glycosyltransferases into two components essential to water-insoluble glucan synthesis. FEBS Lett. 1981 Jun 15;128(2):213–216. doi: 10.1016/0014-5793(81)80083-x. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. Dental caries. Annu Rev Med. 1975;26:121–136. doi: 10.1146/annurev.me.26.020175.001005. [DOI] [PubMed] [Google Scholar]
- Gilpin M. L., Russell R. R., Morrissey P. Cloning and expression of two Streptococcus mutans glucosyltransferases in Escherichia coli K-12. Infect Immun. 1985 Aug;49(2):414–416. doi: 10.1128/iai.49.2.414-416.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazelbauer G. L., Harayama S. Mutants in transmission of chemotactic signals from two independent receptors of E. coli. Cell. 1979 Mar;16(3):617–625. doi: 10.1016/0092-8674(79)90035-7. [DOI] [PubMed] [Google Scholar]
- Jagusztyn-Krynicka E. K., Smorawinska M., Curtiss R., 3rd Expression of Streptococcus mutans aspartate-semialdehyde dehydrogenase gene cloned into plasmid pBR322. J Gen Microbiol. 1982 May;128(5):1135–1145. doi: 10.1099/00221287-128-5-1135. [DOI] [PubMed] [Google Scholar]
- Kuramitsu H. K. Characterization of extracellular glucosyltransferase activity of Steptococcus mutans. Infect Immun. 1975 Oct;12(4):738–749. doi: 10.1128/iai.12.4.738-749.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu H. K. Properties of a mutant of Streptococcus mutans altered in glucosyltransferase activity. Infect Immun. 1976 Feb;13(2):345–353. doi: 10.1128/iai.13.2.345-353.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu H. K., Wondrack L. Insoluble glucan synthesis by Streptococcus mutans serotype c strains. Infect Immun. 1983 Nov;42(2):763–770. doi: 10.1128/iai.42.2.763-770.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu H., Ingersoll L. Immunological relationships between glucosyltransferases from Streptococcus mutans serotypes. Infect Immun. 1976 Sep;14(3):636–644. doi: 10.1128/iai.14.3.636-644.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Evans R. P., Tobian J. A., Hartley D. L., Clewell D. B., Jones K. R. Novel shuttle plasmid vehicles for Escherichia-Streptococcus transgeneric cloning. Gene. 1983 Nov;25(1):145–150. doi: 10.1016/0378-1119(83)90176-2. [DOI] [PubMed] [Google Scholar]
- Maynard M. T., Kuramitsu H. K. Purification and antigenic properties of intracellular invertase from Streptococcus mutans. Infect Immun. 1979 Mar;23(3):873–883. doi: 10.1128/iai.23.3.873-883.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConaughy B. L., Laird C. D., McCarthy B. J. Nucleic acid reassociation in formamide. Biochemistry. 1969 Aug;8(8):3289–3295. doi: 10.1021/bi00836a024. [DOI] [PubMed] [Google Scholar]
- Mukasa H., Shimamura A., Tsumori H. Direct activity stains for glycosidase and glucosyltransferase after isoelectric focusing in horizontal polyacrylamide gel layers. Anal Biochem. 1982 Jul 1;123(2):276–284. doi: 10.1016/0003-2697(82)90446-8. [DOI] [PubMed] [Google Scholar]
- Mukasa H., Tsumori H., Shimamura A. Isolation and characterization of an extracellular glucosyltransferase synthesizing insoluble glucan from Streptococcus mutans serotype c. Infect Immun. 1985 Sep;49(3):790–796. doi: 10.1128/iai.49.3.790-796.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman B. M., White P., Mohan S. B., Cole J. A. Effect of dextran and ammonium sulphate on the reaction catalysed by a glucosyltransferase complex from Streptococcus mutans. J Gen Microbiol. 1980 Jun;118(2):353–366. doi: 10.1099/00221287-118-2-353. [DOI] [PubMed] [Google Scholar]
- Perry D., Kuramitsu H. K. Genetic transformation of Streptococcus mutans. Infect Immun. 1981 Jun;32(3):1295–1297. doi: 10.1128/iai.32.3.1295-1297.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry D., Nilsen L. J., Kuramitsu H. K. Mapping of a cloned glucosyltransferase gene in Streptococcus mutans. Infect Immun. 1985 Oct;50(1):130–135. doi: 10.1128/iai.50.1.130-135.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry D., Wondrack L. M., Kuramitsu H. K. Genetic transformation of putative cariogenic properties in Streptococcus mutans. Infect Immun. 1983 Aug;41(2):722–727. doi: 10.1128/iai.41.2.722-727.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucci M. J., Macrina F. L. Cloned gtfA gene of Streptococcus mutans LM7 alters glucan synthesis in Streptococcus sanguis. Infect Immun. 1985 Jun;48(3):704–712. doi: 10.1128/iai.48.3.704-712.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robeson J. P., Barletta R. G., Curtiss R., 3rd Expression of a Streptococcus mutans glucosyltransferase gene in Escherichia coli. J Bacteriol. 1983 Jan;153(1):211–221. doi: 10.1128/jb.153.1.211-221.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R. R., Coleman D., Dougan G. Expression of a gene for glucan-binding protein from Streptococcus mutans in Escherichia coli. J Gen Microbiol. 1985 Feb;131(2):295–299. doi: 10.1099/00221287-131-2-295. [DOI] [PubMed] [Google Scholar]
- Russell R. R. Glycosyltransferases of Streptococcus mutans strain Ingbritt. Microbios. 1978;23(93-94):136–146. [PubMed] [Google Scholar]
- Sato S., Kuramitsu H. K. Isolation and characterization of a fructosyltransferase gene from Streptococcus mutans GS-5. Infect Immun. 1986 Apr;52(1):166–170. doi: 10.1128/iai.52.1.166-170.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura A., Tsumori H., Mukasa H. Three kinds of extracellular glucosyltransferases from Streptococcus mutans 6715 (serotype g). FEBS Lett. 1983 Jun 27;157(1):79–84. doi: 10.1016/0014-5793(83)81120-x. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]