Abstract
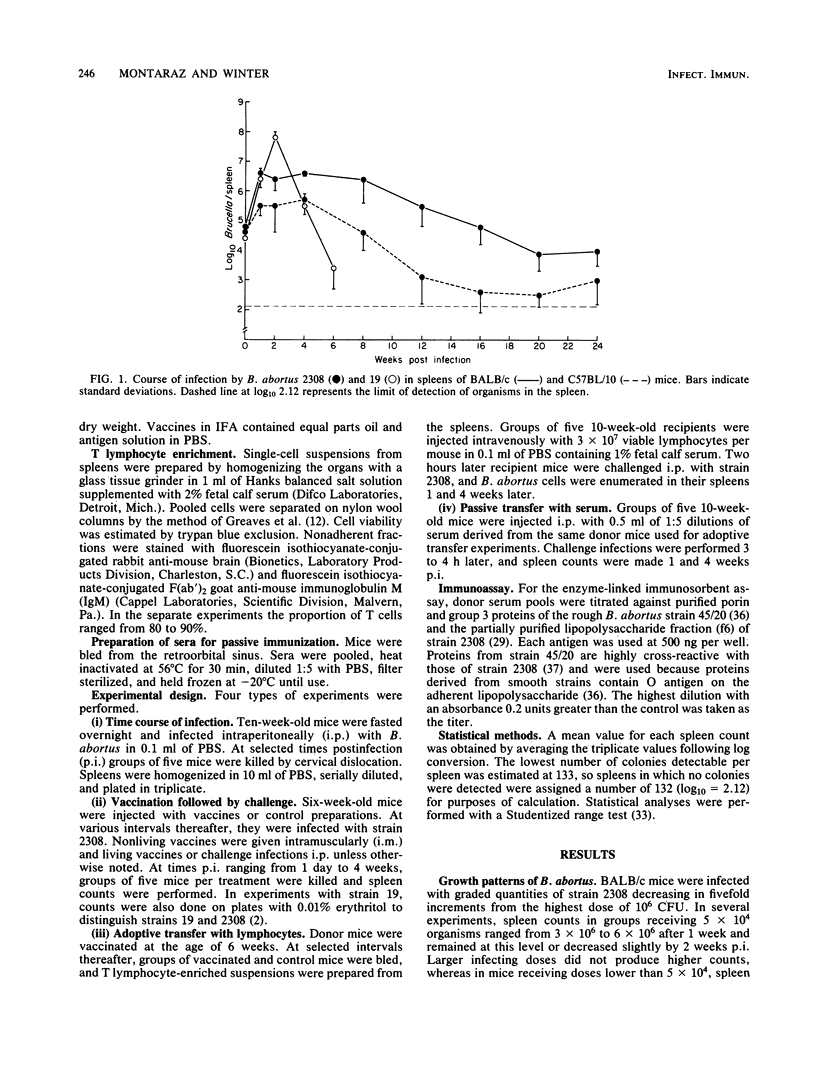
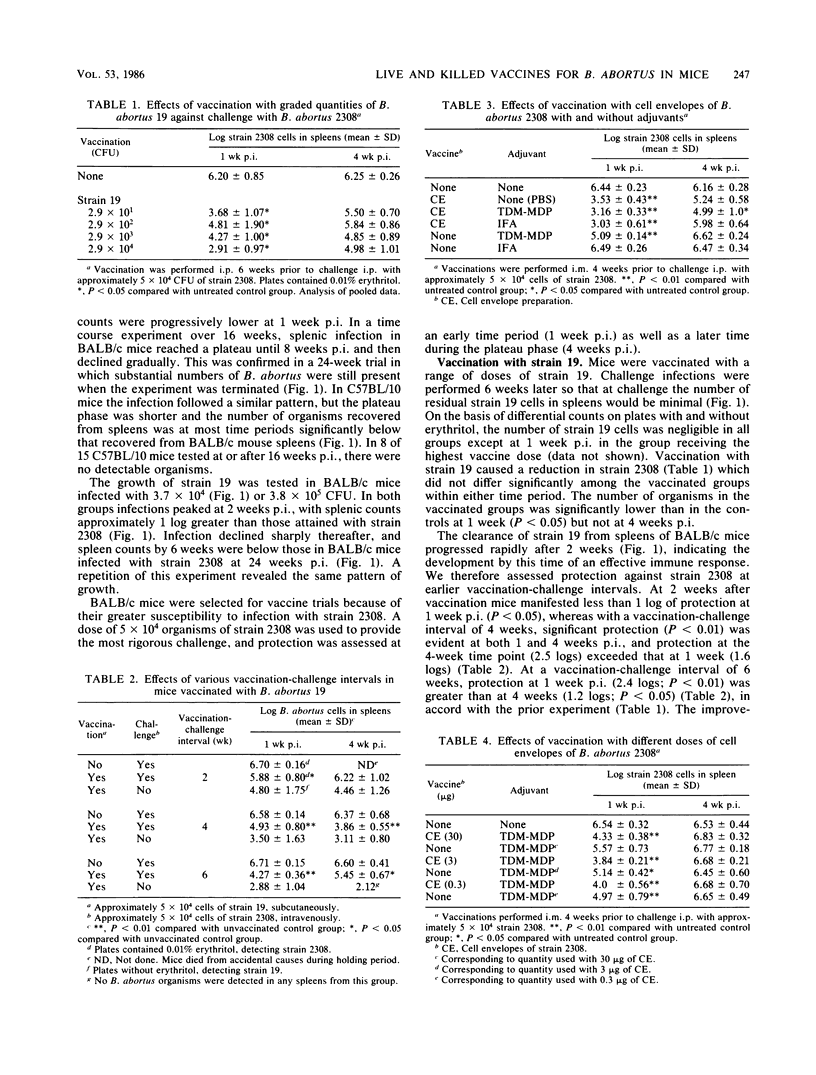
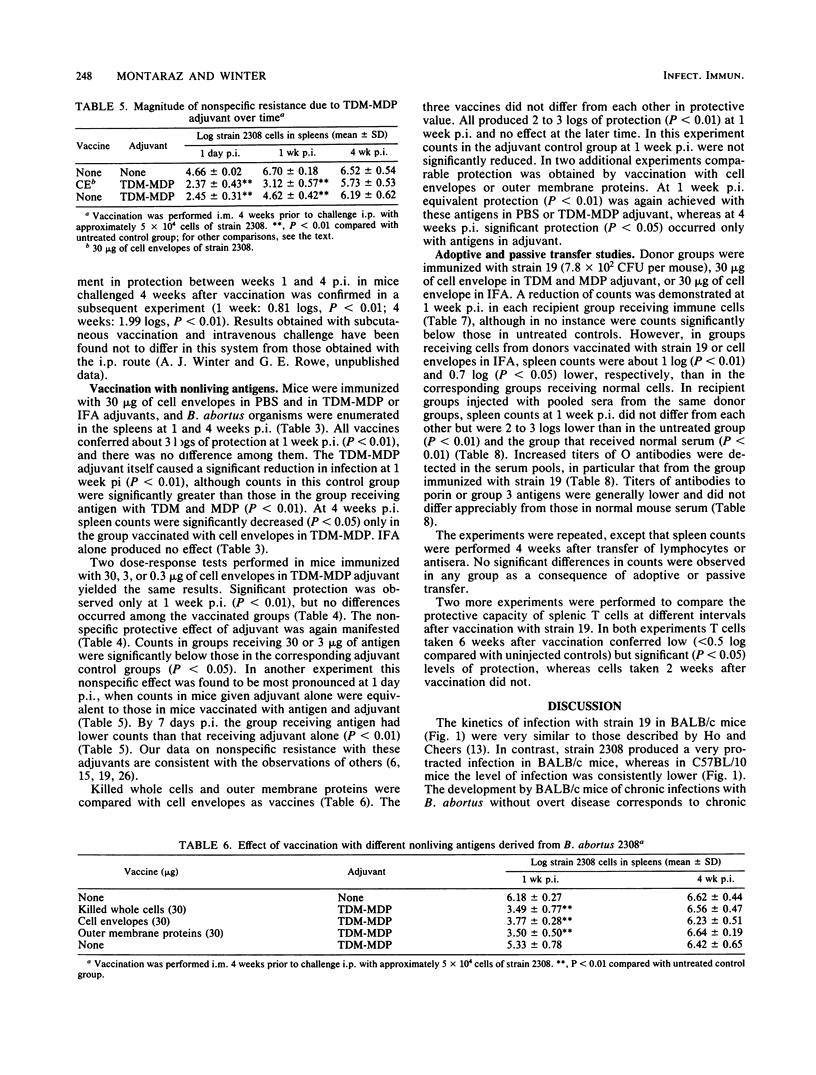
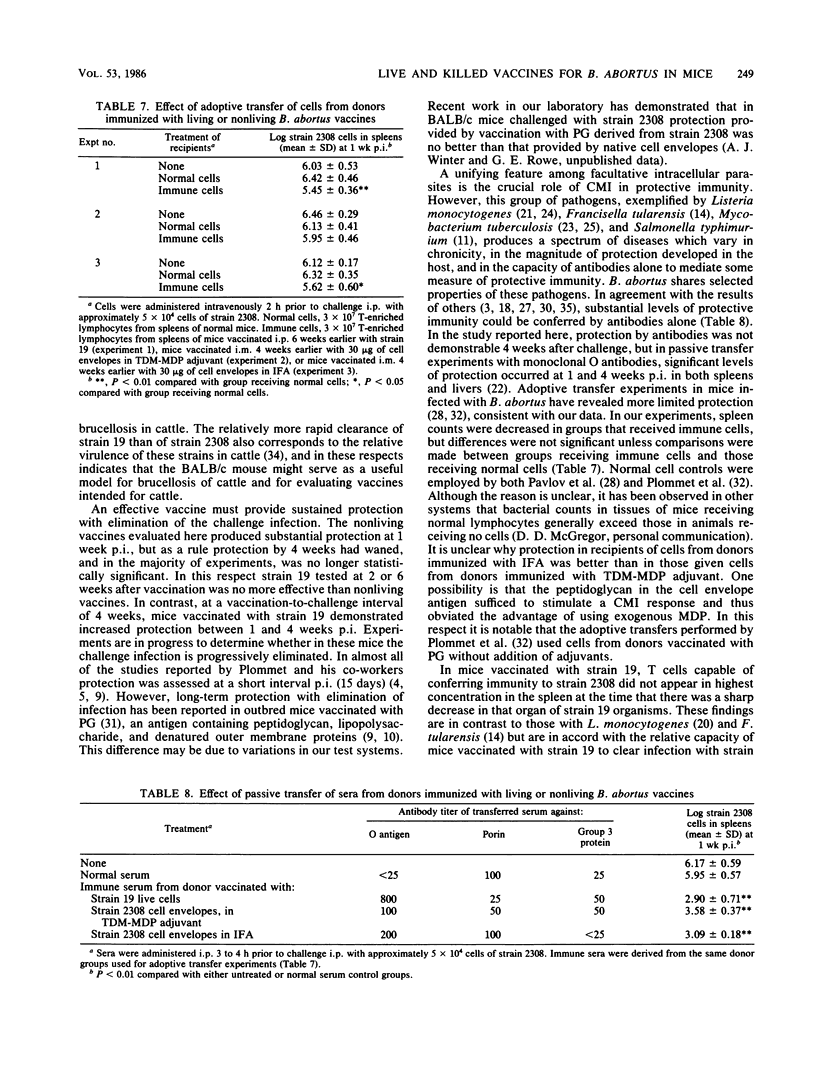
The BALB/c mouse was selected as a model for infection with Brucella abortus on the basis of protracted nonclinical infection produced by strain 2308, virulent for cattle, and relatively rapid clearance of strain 19, an attenuated strain used to vaccinate cattle. Protection in mice vaccinated with strain 19 was compared with that obtained with nonliving vaccines at early (1 week) and later (4 weeks) intervals after challenge with strain 2308 and assessed by enumeration of B. abortus organisms in the spleen. Mice challenged 4 weeks after vaccination with strain 19 exhibited significant protection at 1 and 4 weeks postinfection (p.i.), with an increased magnitude of protection at the later time. When challenged 6 weeks after vaccination with strain 19, the level of protection diminished between 1 and 4 weeks p.i. and at the later time was not always significantly different from controls. Mice immunized 4 weeks earlier with nonliving vaccines in mineral oil with t trehalose dimycolate (TDM) and muramyl dipeptide (MDP) demonstrated patterns of protection similar to those obtained following the 6 week vaccination-challenge interval with strain 19. Vaccination with cell envelopes derived from strain 2308 produced equivalent protection at 1 week p.i. whether administered in phosphate-buffered saline, incomplete Freund adjuvant, or the TDM and MDP adjuvant. Equivalent protection also followed vaccination with strain 2308 killed whole cells, cell envelopes, or outer membrane proteins in phosphate-buffered saline or in the TDM and MDP adjuvant. The TDM and MDP adjuvant alone induced nonspecific resistance, which peaked at 1 day p.i. and was still present at 1 week p.i., although by this time its magnitude was significantly less than the protection induced by antigen combined with the adjuvant. These data, together with the results of antibody assays and passive and adoptive transfer studies, suggested that protection at 1 week p.i. could be accounted for largely by an effect of O antibodies, with T cell-mediated immune responses having a subsidiary role.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam A., Petit J. F., Lefrancier P., Lederer E. Muramyl peptides. Chemical structure, biological activity and mechanism of action. Mol Cell Biochem. 1981 Dec 4;41:27–47. doi: 10.1007/BF00225295. [DOI] [PubMed] [Google Scholar]
- Bascoul S., Cannat A., Huguet M. F., Serre A. Studies on the immune protection to murine experimental brucellosis conferred by Brucella fractions. I. Positive role of immune serum. Immunology. 1978 Aug;35(2):213–221. [PMC free article] [PubMed] [Google Scholar]
- Bosseray N. Immunity to Brucella in mice vaccinated with a fraction (F8) or a killed vaccine (H38) with or without adjuvant. Level and duration of immunity in relation to dose of vaccine, recall injection and age of mice. Br J Exp Pathol. 1978 Aug;59(4):354–365. [PMC free article] [PubMed] [Google Scholar]
- Bosseray N., Plommet A. M., Plommet M. Theoretical, practical and statistical basis for a general control method of activity for anti-Brucella vaccines. Dev Biol Stand. 1984;56:257–270. [PubMed] [Google Scholar]
- Chedid L., Parant M., Parant F., Lefrancher P., Choay J., Lederer E. Enhancement of nonspecific immunity to Klebsiella pneumoniae infection by a synthetic immunoadjuvant (N-acetylmuramyl-L-alanyl-D-isoglutamine) and several analogs. Proc Natl Acad Sci U S A. 1977 May;74(5):2089–2093. doi: 10.1073/pnas.74.5.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheers C., Pagram F. Macrophage activation during experimental murine brucellosis: a basis for chronic infection. Infect Immun. 1979 Feb;23(2):197–205. doi: 10.1128/iai.23.2.197-205.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheers C. Pathogenesis and cellular immunity in experimental murine brucellosis. Dev Biol Stand. 1984;56:237–246. [PubMed] [Google Scholar]
- Dubray G., Bézard G. Isolation of three Brucella abortus cell-wall antigens protective in murine experimental brucellosis. Ann Rech Vet. 1980;11(4):367–373. [PubMed] [Google Scholar]
- Dubray G., Charriaut C. Evidence of three major polypeptide species and two major polysaccharide species in the Brucella outer membrane. Ann Rech Vet. 1983;14(3):311–318. [PubMed] [Google Scholar]
- Eisenstein T. K., Killar L. M., Sultzer B. M. Immunity to infection with Salmonella typhimurium: mouse-strain differences in vaccine- and serum-mediated protection. J Infect Dis. 1984 Sep;150(3):425–435. doi: 10.1093/infdis/150.3.425. [DOI] [PubMed] [Google Scholar]
- Ho M., Cheers C. Resistance and susceptibility of mice to bacterial infection. IV. Genetic and cellular basis of resistance to chronic infection with Brucella abortus. J Infect Dis. 1982 Sep;146(3):381–387. doi: 10.1093/infdis/146.3.381. [DOI] [PubMed] [Google Scholar]
- Kostiala A. A., McGregor D. D., Logie P. S. Tularaemia in the rat. I. The cellular basis on host resistance to infection. Immunology. 1975 May;28(5):855–869. [PMC free article] [PubMed] [Google Scholar]
- Leclerc C. D., Audibert F. M., Chedid L. A., Deriaud E. J., Masihi N. K., Lederer E. Comparison of immunomodulatory activities in mice and guinea pigs of a synthetic desmuramyl peptidolipid triglymyc. Infect Immun. 1984 Mar;43(3):870–875. doi: 10.1128/iai.43.3.870-875.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederer E. Synthetic immunostimulants derived from the bacterial cell wall. J Med Chem. 1980 Aug;23(8):819–825. doi: 10.1021/jm00182a001. [DOI] [PubMed] [Google Scholar]
- MACKANESS G. B. THE IMMUNOLOGICAL BASIS OF ACQUIRED CELLULAR RESISTANCE. J Exp Med. 1964 Jul 1;120:105–120. doi: 10.1084/jem.120.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIKI K., MACKANESS G. B. THE PASSIVE TRANSFER OF ACQUIRED RESISTANCE TO LISTERIA MONOCYTOGENES. J Exp Med. 1964 Jul 1;120:93–103. doi: 10.1084/jem.120.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madraso E. D., Cheers C. Polyadenylic acid-polyuridylic acid (poly A : U) and experimental murine brucellosis. II. Macrophages as target cells of poly A : U in experimental brucellosis. Immunology. 1978 Jul;35(1):77–84. [PMC free article] [PubMed] [Google Scholar]
- Masihi K. N., Brehmer W., Azuma I., Lange W., Müller S. Stimulation of chemiluminescence and resistance against aerogenic influenza virus infection by synthetic muramyl dipeptide combined with trehalose dimycolate. Infect Immun. 1984 Jan;43(1):233–237. doi: 10.1128/iai.43.1.233-237.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor D. D., Kostiala A. A. Role of Lymphocytes in Cellular resistance in infection. Contemp Top Immunobiol. 1976;5:237–266. [PubMed] [Google Scholar]
- Montaraz J. A., Winter A. J., Hunter D. M., Sowa B. A., Wu A. M., Adams L. G. Protection against Brucella abortus in mice with O-polysaccharide-specific monoclonal antibodies. Infect Immun. 1986 Mar;51(3):961–963. doi: 10.1128/iai.51.3.961-963.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. Nature of "memory" in T-cell-mediated antibacterial immunity: anamnestic production of mediator T cells. Infect Immun. 1975 Oct;12(4):754–760. doi: 10.1128/iai.12.4.754-760.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme I. M., Collins F. M. Protection against Mycobacterium tuberculosis infection by adoptive immunotherapy. Requirement for T cell-deficient recipients. J Exp Med. 1983 Jul 1;158(1):74–83. doi: 10.1084/jem.158.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parant M., Parant F., Chedid L., Drapier J. C., Petit J. F., Wietzerbin J., Lederer Enhancement of nonspecific immunity to bacterial infection by cord factor (6,6'-trehalose dimycolate). J Infect Dis. 1977 May;135(5):771–777. doi: 10.1093/infdis/135.5.771. [DOI] [PubMed] [Google Scholar]
- Pardon P. Resistance against a subcutaneous Brucella challenge of mice immunized with living or dead Brucella or by transfer of immune serum. Ann Immunol (Paris) 1977 Nov-Dec;128(6):1025–1037. [PubMed] [Google Scholar]
- Pavlov H., Hogarth M., McKenzie I. F., Cheers C. In vivo and in vitro effects of monoclonal antibody to Ly antigens on immunity to infection. Cell Immunol. 1982 Jul 15;71(1):127–138. doi: 10.1016/0008-8749(82)90502-0. [DOI] [PubMed] [Google Scholar]
- Plommet M., Plommet A. M. Effet des sérums immuns sur l'évolution de la brucellose expérimentale de la souris. Dev Biol Stand. 1984;56:271–281. [PubMed] [Google Scholar]
- Plommet M., Plommet A. M. Immune serum-mediated effects on brucellosis evolution in mice. Infect Immun. 1983 Jul;41(1):97–105. doi: 10.1128/iai.41.1.97-105.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulitzeanu D. Mechanism of immunity against brucella. Nature. 1965 Mar 13;205(976):1086–1088. doi: 10.1038/2051086a0. [DOI] [PubMed] [Google Scholar]
- Verstreate D. R., Creasy M. T., Caveney N. T., Baldwin C. L., Blab M. W., Winter A. J. Outer membrane proteins of Brucella abortus: isolation and characterization. Infect Immun. 1982 Mar;35(3):979–989. doi: 10.1128/iai.35.3.979-989.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstreate D. R., Winter A. J. Comparison of sodium dodecyl sulfate-polyacrylamide gel electrophoresis profiles and antigenic relatedness among outer membrane proteins of 49 Brucella abortus strains. Infect Immun. 1984 Oct;46(1):182–187. doi: 10.1128/iai.46.1.182-187.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter A. J., Verstreate D. R., Hall C. E., Jacobson R. H., Castleman W. L., Meredith M. P., McLaughlin C. A. Immune response to porin in cattle immunized with whole cell, outer membrane, and outer membrane protein antigens of Brucella abortus combined with trehalose dimycolate and muramyl dipeptide adjuvants. Infect Immun. 1983 Dec;42(3):1159–1167. doi: 10.1128/iai.42.3.1159-1167.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]


