Abstract
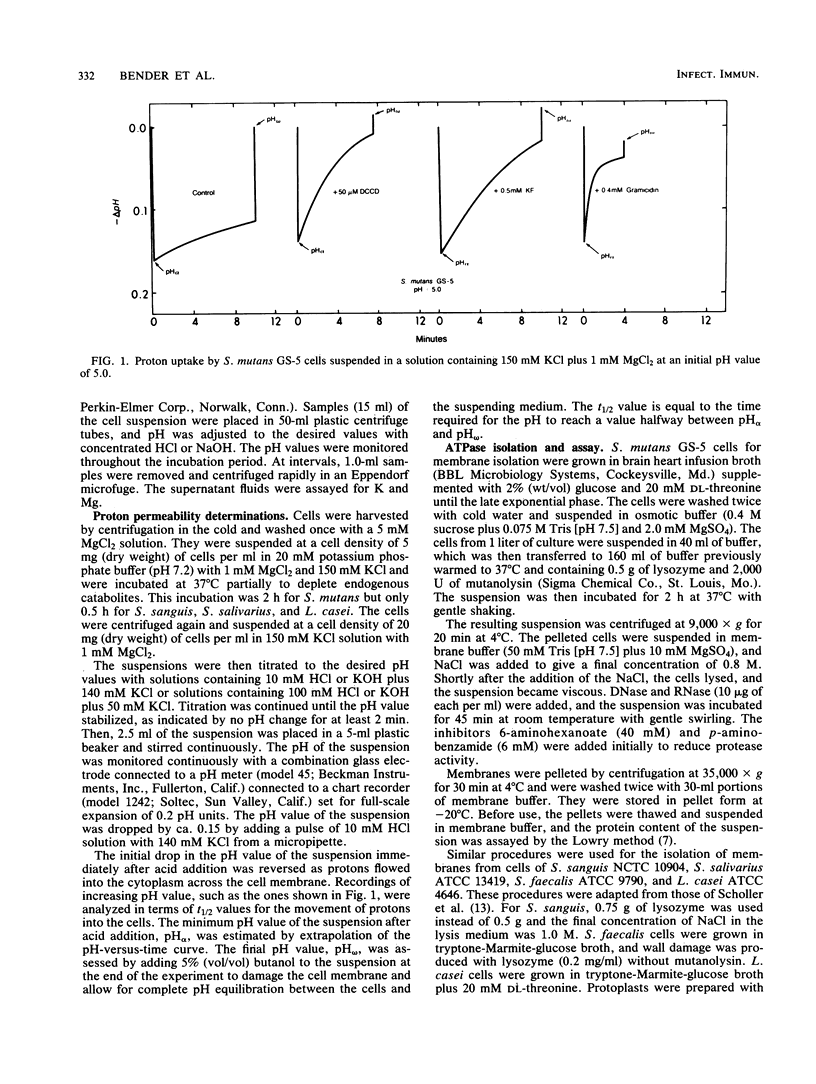
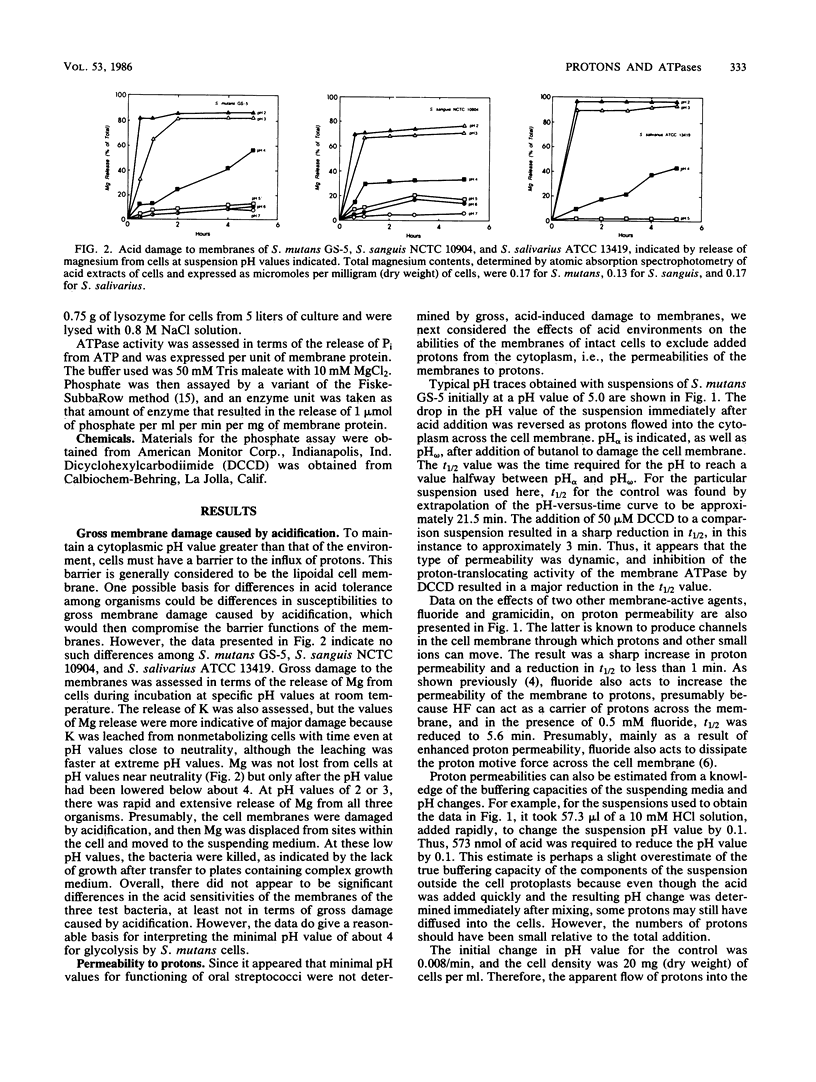
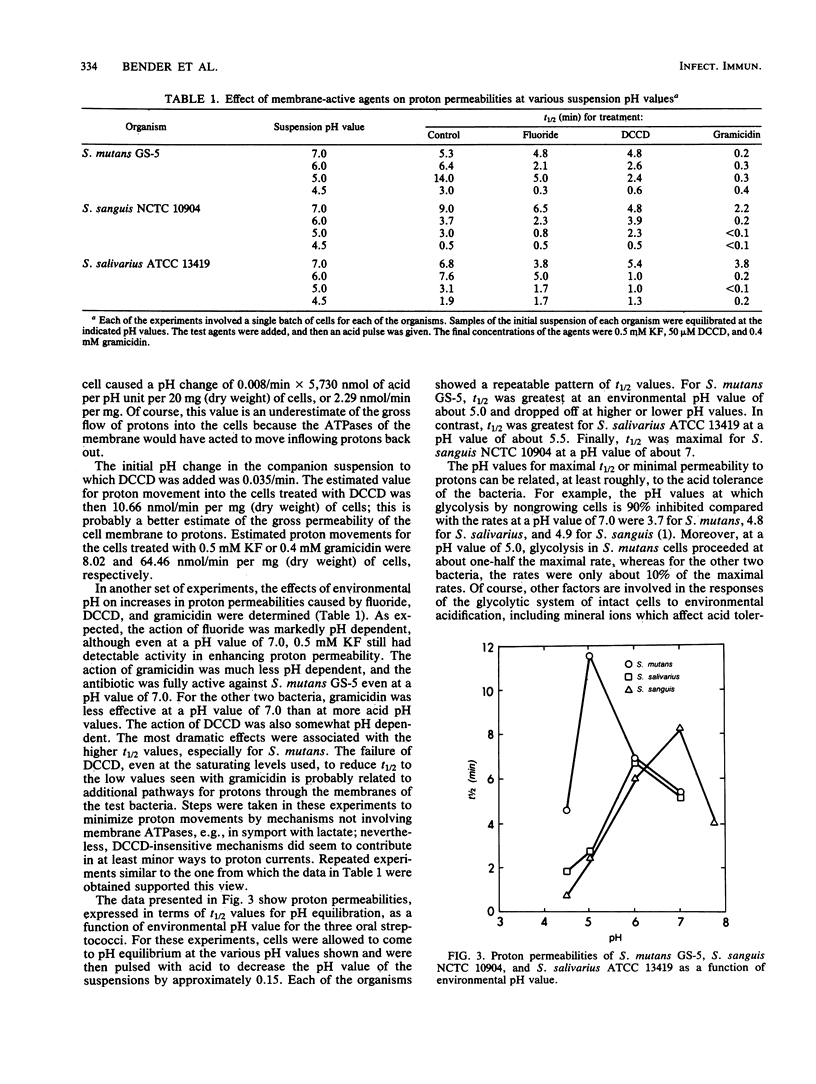
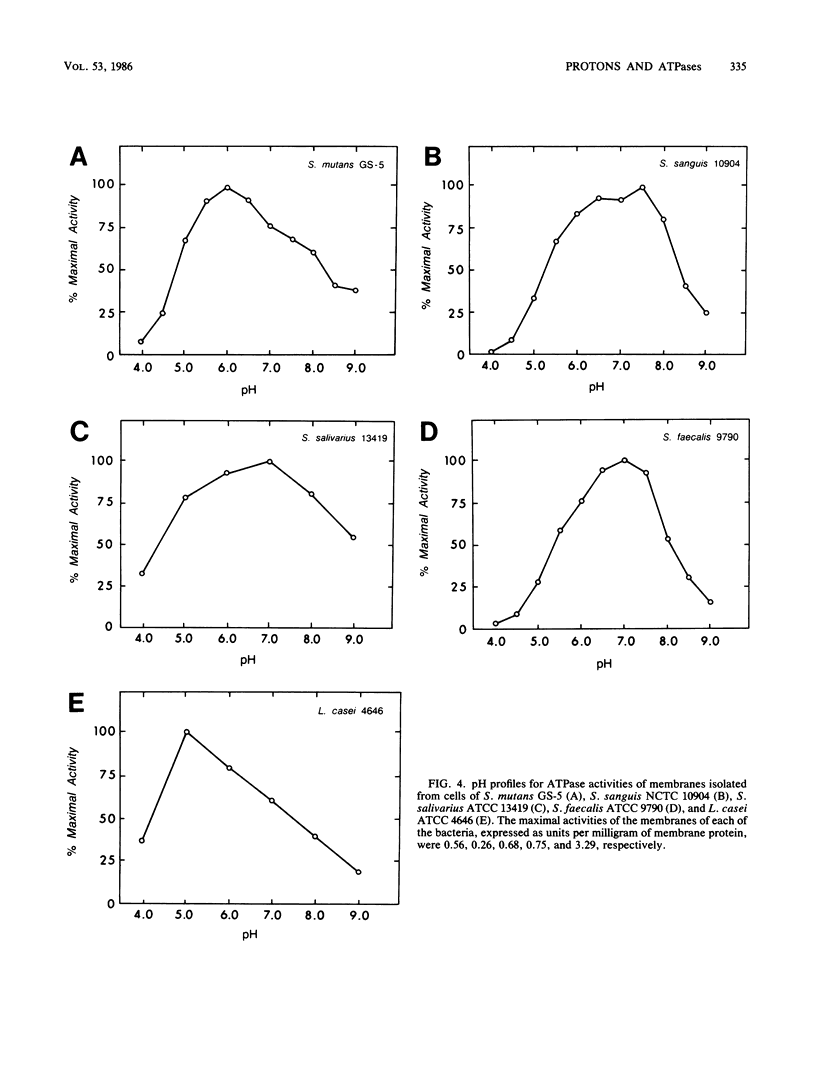
Differences in acid tolerance among representative oral streptococci were found to be related more closely to the dynamic permeabilities of the bacteria to protons than to differences in the sensitivities of cell membranes to gross damage caused by environmental acidification. For Streptococcus mutans GS-5, Streptococcus sanguis NCTC 10904, and Streptococcus salivarius ATCC 13419, gross membrane damage, indicated by the release of magnesium from whole cells, occurred at pH values below about 4 and was rapid and extensive at pH values of about 3 or less. A more aciduric, lactic acid bacterium, Lactobacillus casei ATCC 4646, was more resistant to environmental acidification, and gross membrane damage was evident only at pH values below 3. Assessments of the movements of protons into S. mutans cells after an acid pulse at various pH values indicated that permeability to protons was minimal at a pH value of about 5, at which the average half time for pH equilibration across the cell membrane was about 12 min. The corresponding values for the less aciduric organism S. sanguis were pH 7 and 8.2 min, and the values for the intermediate organism S. salivarius were pH 6 and 6.6 min. The ATPase inhibitor dicyclohexylcarbodiimide acted to increase markedly the permeability of each organism to protons, and this action indicated that permeability involved not only the passive inflow of protons but also active outflow through the proton-translocating membrane ATPase. Membranes were isolated from each of the bacteria, and pH profiles for ATPase activities indicated pH optima of about 7.5, 7.0, 6.0, and 5.0 for S. sanguis, S. salivarius, S. mutans, and L. casei, respectively. Thus, the pH profiles for the enzymes reflected the acid tolerances of the bacteria and the permeabilities of whole cells to protons.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bender G. R., Thibodeau E. A., Marquis R. E. Reduction of acidurance of streptococcal growth and glycolysis by fluoride and gramicidin. J Dent Res. 1985 Feb;64(2):90–95. doi: 10.1177/00220345850640021701. [DOI] [PubMed] [Google Scholar]
- Booth I. R. Regulation of cytoplasmic pH in bacteria. Microbiol Rev. 1985 Dec;49(4):359–378. doi: 10.1128/mr.49.4.359-378.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunick F. J., Kashket S. Enolases from fluoride-sensitive and fluoride-resistant streptococci. Infect Immun. 1981 Dec;34(3):856–863. doi: 10.1128/iai.34.3.856-863.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg A. D., Marquis R. E. Enhanced transmembrane proton conductance in Streptococcus mutans GS-5 due to ionophores and fluoride. Antimicrob Agents Chemother. 1981 May;19(5):807–812. doi: 10.1128/aac.19.5.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greger J. E., Eisenberg A. D. Adenosine 5'-triphosphate content of Streptococcus mutans GS-5 during starvation in a buffered salt medium. Caries Res. 1985;19(4):314–319. doi: 10.1159/000260861. [DOI] [PubMed] [Google Scholar]
- Kashket S., Kashket E. R. Dissipation of the proton motive force in oral streptococci by fluoride. Infect Immun. 1985 Apr;48(1):19–22. doi: 10.1128/iai.48.1.19-22.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maloney P. C. Membrane H+ conductance of Streptococcus lactis. J Bacteriol. 1979 Oct;140(1):197–205. doi: 10.1128/jb.140.1.197-205.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis R. E., Porterfield N., Matsumura P. Acid-base titration of streptococci and the physical states of intracellular ions. J Bacteriol. 1973 May;114(2):491–498. doi: 10.1128/jb.114.2.491-498.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh P. D., Keevil C. W., Ellwood D. C. Relationship of bioenergetic processes to the pathogenic properties of oral bacteria. J Dent Res. 1984 Mar;63(3):401–406. doi: 10.1177/00220345840630030901. [DOI] [PubMed] [Google Scholar]
- Schachtele C. F., Jensen M. E. Comparison of methods for monitoring changes in the pH of human dental plaque. J Dent Res. 1982 Oct;61(10):1117–1125. doi: 10.1177/00220345820610100201. [DOI] [PubMed] [Google Scholar]
- Schöller M., Klein J. P., Sommer P., Frank R. Protoplast and cytoplasmic membrane preparations from Streptococcus sanguis and Streptococcus mutans. J Gen Microbiol. 1983 Oct;129(10):3271–3279. doi: 10.1099/00221287-129-10-3271. [DOI] [PubMed] [Google Scholar]
- Vadeboncoeur C., Proulx M., Trahan L. Effect of gramicidin D on the acidogenic properties of oral streptococci and human dental plaque. J Dent Res. 1982 May;61(5):632–635. doi: 10.1177/00220345820610050201. [DOI] [PubMed] [Google Scholar]


