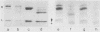Abstract
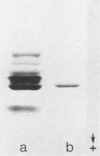
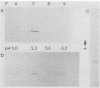
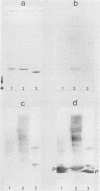
The opportunistic yeastlike fungi of the genus Candida comprise three species which are proteolytic in vitro. Among them, C. albicans and C. tropicalis are of foremost medical importance. However, a strict correlation between extracellular proteolytic activity and virulence is opposed by the low virulence of the third proteolytic species, C. parapsilosis. We purified the secretory acid proteinase of C. parapsilosis (clinical isolate 265). The enzyme is a carboxyl proteinase (EC 3.4.23) like all other secretory Candida proteinases handled so far. Proteinase 265 is distinguished by a lower molecular weight (approximately 33,000); it has increased hydrophobicity, which accounts for inhibition of the enzyme by hemin, and required the presence of nonionic detergent in the initial steps of purification. The enzyme already undergoes alkaline denaturation at neutrality. Its activity is thus confined to the acid microenvironment of the fungal cell wall. Within this range, the enzyme may degrade immunoglobulins like immunoglobulin A1 (IgA1), IgA2, and secretory IgA. No indication was found for glycosylation of proteinase 265 and the related enzyme of C. albicans CBS 2730. However, the comparable proteinase of C. tropicalis 293 was identified as a manno protein. Antiserum against proteinase 265 cross-reacted strongly with corresponding enzymes from other Candida species. Antisera against proteinases of C. albicans and C. tropicalis reacted only weakly with proteinase 265. Thus, secretory Candida proteinases are likely to possess common and species-specific antigenic sites. In contrast to C. albicans, infection of phagocytes by C. parapsilosis 265 was not accompanied by secretion of fungal proteinase. This lack of induction of the enzyme under conditions of infection may account for the low virulence of most isolates of C. parapsilosis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahearn D. G. Medically important yeasts. Annu Rev Microbiol. 1978;32:59–68. doi: 10.1146/annurev.mi.32.100178.000423. [DOI] [PubMed] [Google Scholar]
- Bistoni F., Vecchiarelli A., Cenci E., Sbaraglia G., Perito S., Cassone A. A comparison of experimental pathogenicity of Candida species in cyclophosphamide-immunodepressed mice. Sabouraudia. 1984;22(5):409–418. doi: 10.1080/00362178485380661. [DOI] [PubMed] [Google Scholar]
- Borg M., Kirk D., Baumgarten H., Rüchel R. A colorimetric assay for the assessment of cytotoxicity of yeasts. Sabouraudia. 1984;22(5):357–367. doi: 10.1080/00362178485380611. [DOI] [PubMed] [Google Scholar]
- Cutting J. A., Roth T. F. Staining of phospho-proteins on acrylamide gel electropherograms. Anal Biochem. 1973 Aug;54(2):386–394. doi: 10.1016/0003-2697(73)90367-9. [DOI] [PubMed] [Google Scholar]
- Dyess D. L., Garrison R. N., Fry D. E. Candida sepsis. Implications of polymicrobial blood-borne infection. Arch Surg. 1985 Mar;120(3):345–348. doi: 10.1001/archsurg.1985.01390270083014. [DOI] [PubMed] [Google Scholar]
- Elder J. H., Alexander S. endo-beta-N-acetylglucosaminidase F: endoglycosidase from Flavobacterium meningosepticum that cleaves both high-mannose and complex glycoproteins. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4540–4544. doi: 10.1073/pnas.79.15.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furth A. J., Bolton H., Potter J., Priddle J. D. Separating detergent from proteins. Methods Enzymol. 1984;104:318–328. doi: 10.1016/s0076-6879(84)04098-2. [DOI] [PubMed] [Google Scholar]
- Glass W. F., 2nd, Briggs R. C., Hnilica L. S. Use of lectins for detection of electrophoretically separated glycoproteins transferred onto nitrocellulose sheets. Anal Biochem. 1981 Jul 15;115(1):219–224. doi: 10.1016/0003-2697(81)90549-2. [DOI] [PubMed] [Google Scholar]
- Gripon J. C., Hofmann T. Inactivation of aspartyl proteinases by butane-2,3-dione. Modification of tryptophan and tyrosine residues and evidence against reaction of arginine residues. Biochem J. 1981 Jan 1;193(1):55–65. doi: 10.1042/bj1930055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975 Mar 25;415(1):29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Herzog V., Fahimi H. D. A new sensitive colorimetric assay for peroxidase using 3,3'-diaminobenzidine as hydrogen donor. Anal Biochem. 1973 Oct;55(2):554–562. doi: 10.1016/0003-2697(73)90144-9. [DOI] [PubMed] [Google Scholar]
- Johnson G. D., Davidson R. S., McNamee K. C., Russell G., Goodwin D., Holborow E. J. Fading of immunofluorescence during microscopy: a study of the phenomenon and its remedy. J Immunol Methods. 1982 Dec 17;55(2):231–242. doi: 10.1016/0022-1759(82)90035-7. [DOI] [PubMed] [Google Scholar]
- Kaehn K., Morr M., Kula M. R. Inhibition of the acid proteinase from Neurospora crassa by diazoacetyl-DL-norleucine methyl ester, 1,2-epoxy-3-(4-nitrophenoxy)propane and pepstatin. Hoppe Seylers Z Physiol Chem. 1979 Jun;360(6):791–794. [PubMed] [Google Scholar]
- Kwon-Chung K. J., Lehman D., Good C., Magee P. T. Genetic evidence for role of extracellular proteinase in virulence of Candida albicans. Infect Immun. 1985 Sep;49(3):571–575. doi: 10.1128/iai.49.3.571-575.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanoë J., Dunnigan J. Improvements of the Anson assay for measuring proteolytic activities in acidic pH range. Anal Biochem. 1978 Sep;89(2):461–471. doi: 10.1016/0003-2697(78)90375-5. [DOI] [PubMed] [Google Scholar]
- Macdonald F., Odds F. C. Inducible proteinase of Candida albicans in diagnostic serology and in the pathogenesis of systemic candidosis. J Med Microbiol. 1980 Aug;13(3):423–435. doi: 10.1099/00222615-13-3-423. [DOI] [PubMed] [Google Scholar]
- Macdonald F., Odds F. C. Virulence for mice of a proteinase-secreting strain of Candida albicans and a proteinase-deficient mutant. J Gen Microbiol. 1983 Feb;129(2):431–438. doi: 10.1099/00221287-129-2-431. [DOI] [PubMed] [Google Scholar]
- Macdonald F. Secretion of inducible proteinase by pathogenic Candida species. Sabouraudia. 1984;22(1):79–82. [PubMed] [Google Scholar]
- Marrie T. J., Cooper J. H., Costerton J. W. Ultrastructure of Candida parapsilosis endocarditis. Infect Immun. 1984 Aug;45(2):390–398. doi: 10.1128/iai.45.2.390-398.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odds F. C. Candida albicans proteinase as a virulence factor in the pathogenesis of Candida infections. Zentralbl Bakteriol Mikrobiol Hyg A. 1985 Dec;260(4):539–542. doi: 10.1016/s0176-6724(85)80069-9. [DOI] [PubMed] [Google Scholar]
- Osborn M., Weber K. The detertent-resistant cytoskeleton of tissue culture cells includes the nucleus and the microfilament bundles. Exp Cell Res. 1977 May;106(2):339–349. doi: 10.1016/0014-4827(77)90179-3. [DOI] [PubMed] [Google Scholar]
- Pistole T. G. Interaction of bacteria and fungi with lectins and lectin-like substances. Annu Rev Microbiol. 1981;35:85–112. doi: 10.1146/annurev.mi.35.100181.000505. [DOI] [PubMed] [Google Scholar]
- Plaut A. G. The IgA1 proteases of pathogenic bacteria. Annu Rev Microbiol. 1983;37:603–622. doi: 10.1146/annurev.mi.37.100183.003131. [DOI] [PubMed] [Google Scholar]
- Pontremoli S., Salamino F., Sparatore B., Melloni E., Morelli A., Benatti U., De Flora A. Isolation and partial characterization of three acidic proteinases in erythrocyte membranes. Biochem J. 1979 Sep 1;181(3):559–568. doi: 10.1042/bj1810559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radola B. J. High-resolution preparative isoelectric focusing. Methods Enzymol. 1984;104:256–275. doi: 10.1016/s0076-6879(84)04094-5. [DOI] [PubMed] [Google Scholar]
- Renart J., Sandoval I. V. Western blots. Methods Enzymol. 1984;104:455–460. doi: 10.1016/s0076-6879(84)04114-8. [DOI] [PubMed] [Google Scholar]
- Rubinstein E., Noriega E. R., Simberkoff M. S., Holzman R., Rahal J. J., Jr Fungal endocarditis: analysis of 24 cases and review of the literature. Medicine (Baltimore) 1975 Jul;54(4):331–334. [PubMed] [Google Scholar]
- Rüchel R. A variety of Candida proteinases and their possible targets of proteolytic attack in the host. Zentralbl Bakteriol Mikrobiol Hyg A. 1984 Jul;257(2):266–274. [PubMed] [Google Scholar]
- Rüchel R., Böning B. Detection of Candida proteinase by enzyme immunoassay and interaction of the enzyme with alpha-2-macroglobulin. J Immunol Methods. 1983 Jun 24;61(1):107–116. doi: 10.1016/0022-1759(83)90014-5. [DOI] [PubMed] [Google Scholar]
- Rüchel R., Böning B., Jahn E. Identification and partial characterization of two proteinases from the cell envelope of Candida albicans blastospores. Zentralbl Bakteriol Mikrobiol Hyg A. 1985 Dec;260(4):523–538. doi: 10.1016/s0176-6724(85)80068-7. [DOI] [PubMed] [Google Scholar]
- Rüchel R., Gross J. Preparations of continuous gradient gel slabs: a simple technique. Anal Biochem. 1979 Jan 1;92(1):91–98. doi: 10.1016/0003-2697(79)90629-8. [DOI] [PubMed] [Google Scholar]
- Rüchel R. On the renin-like activity of Candida proteinases and activation of blood coagulation in vitro. Zentralbl Bakteriol Mikrobiol Hyg A. 1983 Sep;255(2-3):368–379. [PubMed] [Google Scholar]
- Rüchel R. On the role of proteinases from Candida albicans in the pathogenesis of acronecrosis. Zentralbl Bakteriol Mikrobiol Hyg A. 1983 Nov;255(4):524–536. [PubMed] [Google Scholar]
- Rüchel R. Properties of a purified proteinase from the yeast Candida albicans. Biochim Biophys Acta. 1981 May 14;659(1):99–113. doi: 10.1016/0005-2744(81)90274-6. [DOI] [PubMed] [Google Scholar]
- Rüchel R., Uhlemann K., Böning B. Secretion of acid proteinases by different species of the genus Candida. Zentralbl Bakteriol Mikrobiol Hyg A. 1983 Nov;255(4):537–548. [PubMed] [Google Scholar]
- Staib F., Mishra S. K., Tompak B., Grosse G., Abel T., Blisse A., Folkens U., Fröhlich B. Pathogenic yeast-like fungi in meat products. Zentralbl Bakteriol A. 1980;248(3):422–429. [PubMed] [Google Scholar]
- Staib F. Proteolysis and pathogenicity of Candida albicans strains. Mycopathol Mycol Appl. 1969 May 28;37(4):345–348. doi: 10.1007/BF02129881. [DOI] [PubMed] [Google Scholar]
- Staib F. Serum-Protein-Agar-pH 4,2 und 5,0 für Sprosspilze. Zentralbl Bakteriol Orig. 1964 Dec;195(2):265–267. [PubMed] [Google Scholar]
- Sundsmo J. S., Götze O. Human monocyte spreading induced by factor B of the alternative pathway of complement activation. Cell Immunol. 1980 Jun;52(1):1–17. doi: 10.1016/0008-8749(80)90395-0. [DOI] [PubMed] [Google Scholar]