Abstract
Acute infections caused by the murine malarial parasite Plasmodium chabaudi adami are resolved by antibody-independent mechanisms of immunity. The fact that athymic nude mice developed high-grade unrelenting malaria and died when infected with this parasite suggested a significant role for T lymphocytes. Using adoptive transfer techniques, we demonstrated that spleen cells from either nonimmune or immune donor BALB/c mice eventually suppressed P. chabaudi adami infections in histocompatible recipient nude mice in a dose-dependent manner. Infections in recipients of "immune" spleen cells were less severe, demonstrating a depressed peak parasitemia and a shortened duration of patent infection, than was observed in recipients of normal spleen cells. Also, when sufficient numbers of immune spleen cells were transferred, the second wave of parasitemia (characteristic of this infection in nonimmune mice) failed to occur. T lymphocytes mediated protection in recipient mice, since T-cell-enriched, but not B-cell-enriched, spleen cell fractions suppressed P. chabaudi adami infections in nude mice. Protection was best achieved with T cells that bore the L3T4 phenotype. Patent parasitemias developed in all recipient mice, suggesting that the grafted cells did not limit parasite growth directly but achieved this end by activating other as yet unidentified inhibiting cell systems.
Full text
PDF

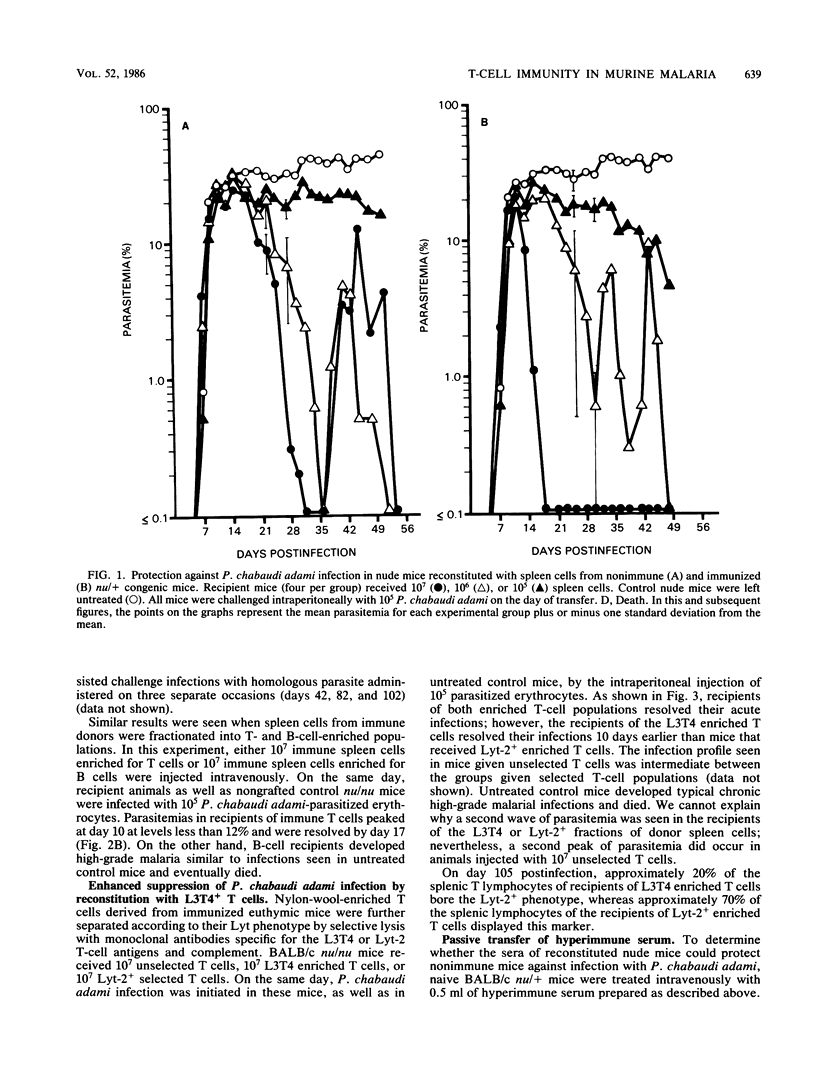
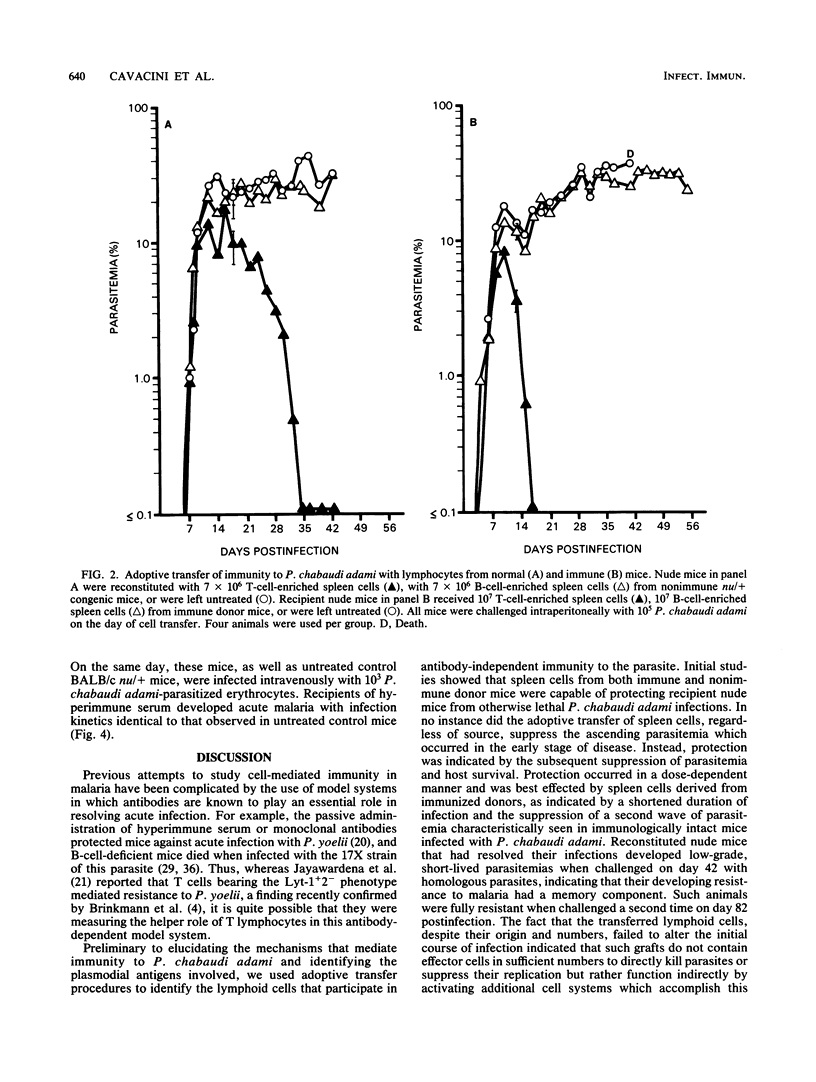
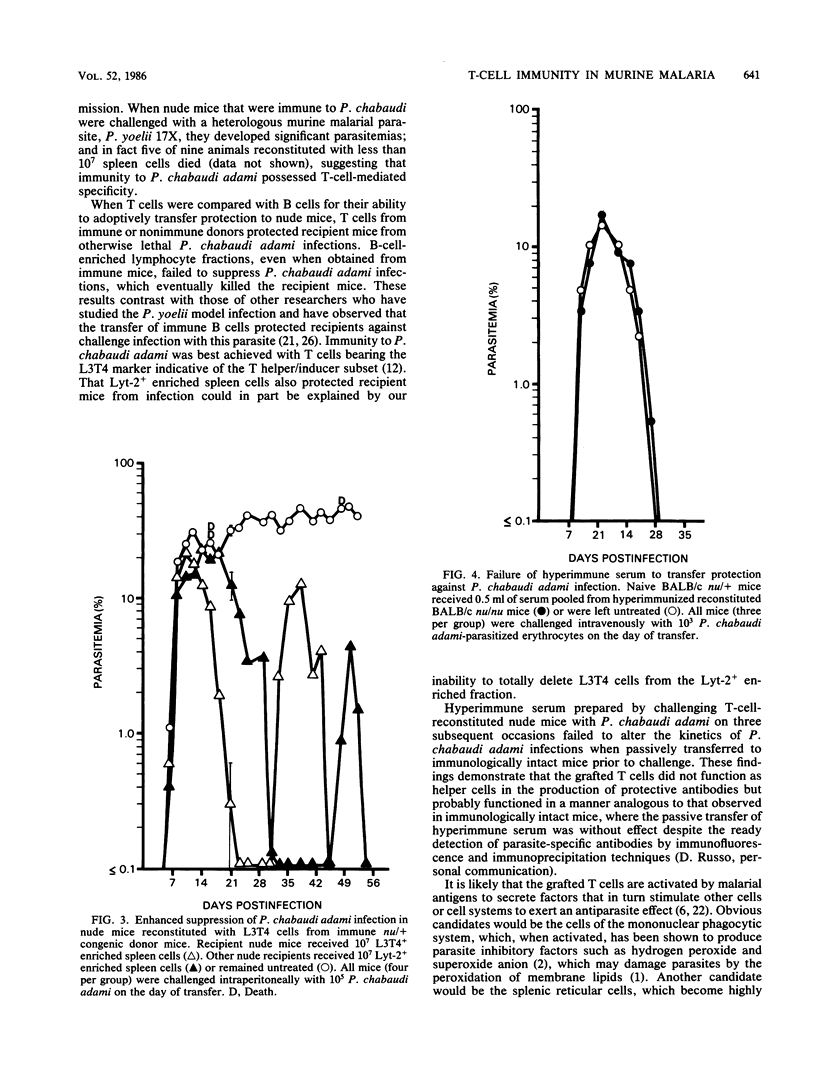


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C. Cellular immunity to malaria and babesia parasites: a personal viewpoint. Contemp Top Immunobiol. 1984;12:463–490. doi: 10.1007/978-1-4684-4571-8_12. [DOI] [PubMed] [Google Scholar]
- Allison A. C., Clark I. A. Specific and non-specific immunity to haemoprotozoa. Am J Trop Med Hyg. 1977 Nov;26(6 Pt 2):216–222. doi: 10.4269/ajtmh.1977.26.216. [DOI] [PubMed] [Google Scholar]
- Allison A. C., Eugui E. M. The role of cell-mediated immune responses in resistance to malaria, with special reference to oxidant stress. Annu Rev Immunol. 1983;1:361–392. doi: 10.1146/annurev.iy.01.040183.002045. [DOI] [PubMed] [Google Scholar]
- Brinkmann V., Kaufmann S. H., Simon M. M. T-cell-mediated immune response in murine malaria: differential effects of antigen-specific Lyt T-cell subsets in recovery from Plasmodium yoelii infection in normal and T-cell-deficient mice. Infect Immun. 1985 Mar;47(3):737–743. doi: 10.1128/iai.47.3.737-743.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown I. N., Phillips R. S. Immunity to Plasmodium berghei in rats: passive serum transfer and role of the spleen. Infect Immun. 1974 Dec;10(6):1213–1218. doi: 10.1128/iai.10.6.1213-1218.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN S., McGREGOR I. A., CARRINGTON S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961 Nov 25;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Allison A. C., Cox F. E. Protection of mice against Babesia and Plasmodium with BCG. Nature. 1976 Jan 29;259(5541):309–311. doi: 10.1038/259309a0. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Cowden W. B., Butcher G. A. Free oxygen radical generators as antimalarial drugs. Lancet. 1983 Jan 29;1(8318):234–234. doi: 10.1016/s0140-6736(83)92603-x. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Hunt N. H. Evidence for reactive oxygen intermediates causing hemolysis and parasite death in malaria. Infect Immun. 1983 Jan;39(1):1–6. doi: 10.1128/iai.39.1.1-6.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. Immunity to malaria. Proc R Soc Lond B Biol Sci. 1979 Jan 15;203(1153):323–345. doi: 10.1098/rspb.1979.0001. [DOI] [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Diggs C. L., Osler A. G. Humoral immunity in rodent malaria. II. Inhibition of parasitemia by serum antibody. J Immunol. 1969 Feb;102(2):298–305. [PubMed] [Google Scholar]
- Grun J. L., Long C. A., Weidanz W. P. Effects of splenectomy on antibody-independent immunity to Plasmodium chabaudi adami malaria. Infect Immun. 1985 Jun;48(3):853–858. doi: 10.1128/iai.48.3.853-858.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grun J. L., Weidanz W. P. Antibody-independent immunity to reinfection malaria in B-cell-deficient mice. Infect Immun. 1983 Sep;41(3):1197–1204. doi: 10.1128/iai.41.3.1197-1204.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grun J. L., Weidanz W. P. Immunity to Plasmodium chabaudi adami in the B-cell-deficient mouse. Nature. 1981 Mar 12;290(5802):143–145. doi: 10.1038/290143a0. [DOI] [PubMed] [Google Scholar]
- Haidaris C. G., Haynes J. D., Meltzer M. S., Allison A. C. Serum containing tumor necrosis factor is cytotoxic for the human malaria parasite Plasmodium falciparum. Infect Immun. 1983 Oct;42(1):385–393. doi: 10.1128/iai.42.1.385-393.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann E. J., Weidanz W. P., Long C. A. Susceptibility of CXB recombinant inbred mice to murine plasmodia. Infect Immun. 1984 Mar;43(3):981–985. doi: 10.1128/iai.43.3.981-985.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder A. A., Freeman R. R. Immunization against blood-stage rodent malaria using purified parasite antigens. Nature. 1981 Nov 26;294(5839):361–364. doi: 10.1038/294361a0. [DOI] [PubMed] [Google Scholar]
- Jayawardena A. N., Murphy D. B., Janeway C. A., Gershon R. K. T cell-mediated immunity in malaria. I. The Ly phenotype of T cells mediating resistance to Plasmodium yoelii. J Immunol. 1982 Jul;129(1):377–381. [PubMed] [Google Scholar]
- Jensen J. B., Boland M. T., Allan J. S., Carlin J. M., Vande Waa J. A., Divo A. A., Akood M. A. Association between human serum-induced crisis forms in cultured Plasmodium falciparum and clinical immunity to malaria in Sudan. Infect Immun. 1983 Sep;41(3):1302–1311. doi: 10.1128/iai.41.3.1302-1311.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J. B., Hoffman S. L., Boland M. T., Akood M. A., Laughlin L. W., Kurniawan L., Marwoto H. A. Comparison of immunity to malaria in Sudan and Indonesia: crisis-form versus merozoite-invasion inhibition. Proc Natl Acad Sci U S A. 1984 Feb;81(3):922–925. doi: 10.1073/pnas.81.3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Majarian W. R., Daly T. M., Weidanz W. P., Long C. A. Passive immunization against murine malaria with an IgG3 monoclonal antibody. J Immunol. 1984 Jun;132(6):3131–3137. [PubMed] [Google Scholar]
- Ockenhouse C. F., Shear H. L. Oxidative killing of the intraerythrocytic malaria parasite Plasmodium yoelii by activated macrophages. J Immunol. 1984 Jan;132(1):424–431. [PubMed] [Google Scholar]
- Rank R. G., Weidanz W. P., Bondi A. Nonsterilizing immunity in avian malaria: an antibody-independent phenomenon. Proc Soc Exp Biol Med. 1976 Feb;151(2):257–259. doi: 10.3181/00379727-151-39186. [DOI] [PubMed] [Google Scholar]
- Roberts D. W., Rank R. G., Weidanz W. P., Finerty J. F. Prevention of recrudescent malaria in nude mice by thymic grafting or by treatment with hyperimmune serum. Infect Immun. 1977 Jun;16(3):821–826. doi: 10.1128/iai.16.3.821-826.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D. W., Weidanz W. P. T-cell immunity to malaria in the B-cell deficient mouse. Am J Trop Med Hyg. 1979 Jan;28(1):1–3. doi: 10.4269/ajtmh.1979.28.1. [DOI] [PubMed] [Google Scholar]
- Rzepczyk C. M., Clark I. A. Failure of bacterial lipopolysaccharide to elicit a cytostatic effect on Plasmodium vinckei petteri in C3H/HeJ mice. Infect Immun. 1982 Jan;35(1):58–63. doi: 10.1128/iai.35.1.58-63.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverne J., Dockrell H. M., Playfair J. H. Endotoxin-induced serum factor kills malarial parasites in vitro. Infect Immun. 1981 Jul;33(1):83–89. doi: 10.1128/iai.33.1.83-89.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. W., Bever C. T., Rollwagen F. M., Evans C. B., Asofsky R. The rodent malaria parasite Plasmodium yoelii lacks both types 1 and 2 T-independent antigens. J Immunol. 1982 Apr;128(4):1854–1859. [PubMed] [Google Scholar]
- Trizio D., Cudkowicz G. Separation of T and B lymphocytes by nylon wool columns: evaluation of efficacy by functional assays in vivo. J Immunol. 1974 Oct;113(4):1093–1097. [PubMed] [Google Scholar]
- Weidanz W. P., Grun J. L. Antibody-independent mechanisms in the development of acquired immunity to malaria. Adv Exp Med Biol. 1983;162:409–423. doi: 10.1007/978-1-4684-4481-0_37. [DOI] [PubMed] [Google Scholar]
- Weinbaum F. I., Evans C. B., Tigelaar R. E. Immunity to Plasmodium Berghei yoelii in mice. I. The course of infection in T cell and B cell deficient mice. J Immunol. 1976 Nov;117(5 PT2):1999–2005. [PubMed] [Google Scholar]


