Abstract
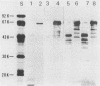
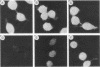
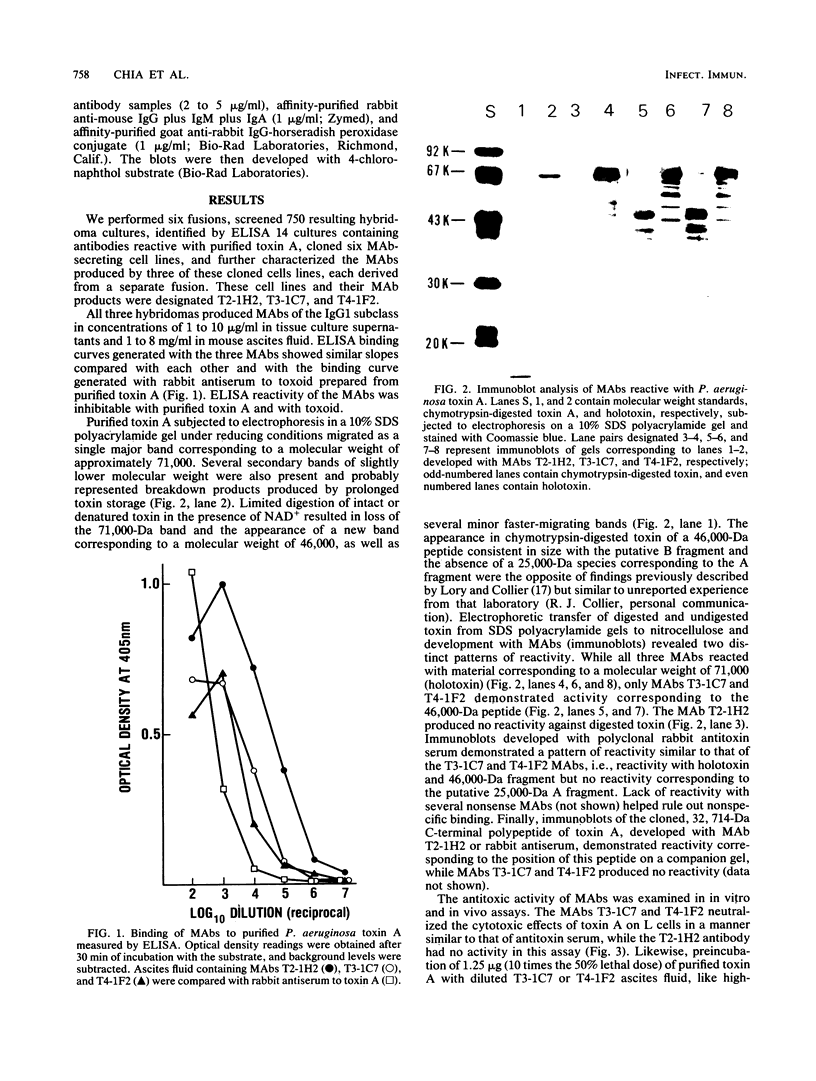
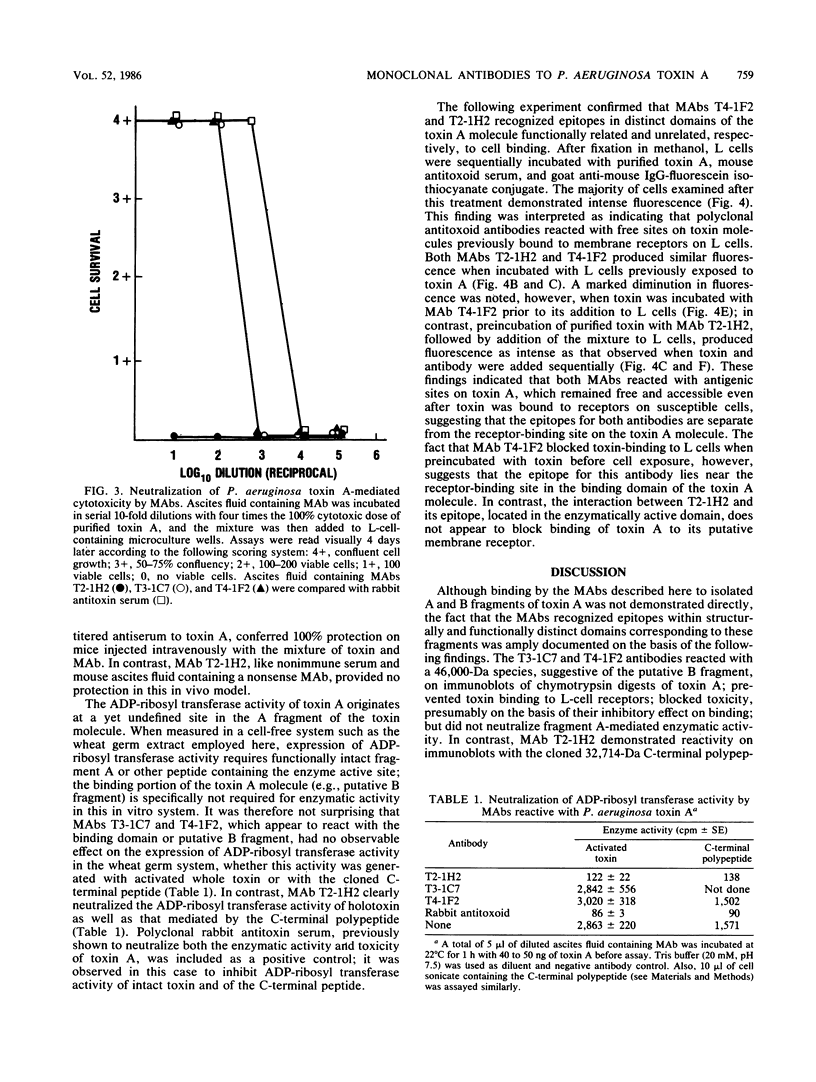
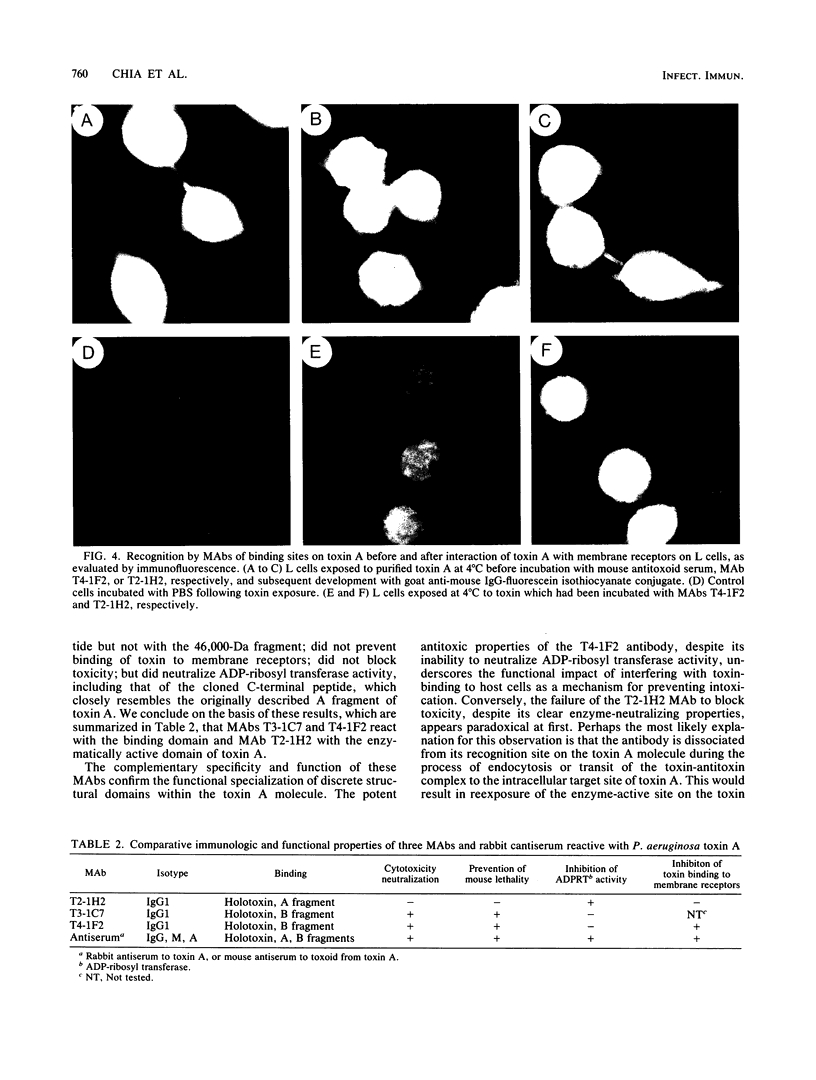
Monoclonal antibodies (MAbs) are described which react with two discrete structural domains of Pseudomonas aeruginosa toxin A and which have two distinct functional profiles. The MAbs designated T3-1C7 and T4-1F2 reacted with a 46,000-dalton peptide similar to the putative B or binding fragment of toxin A. These antibodies neutralized the cytotoxic and lethal properties of toxin but had no effect on its ADP-ribosyl transferase activity. T4-1F2 interfered with the binding of toxin A to membrane receptors on mouse fibroblasts (L cells), although the epitope for the antibody appears to be distinct from the actual receptor binding site. The MAb designated T2-1H2 reacted with intact toxin A and with a cloned, enzymatically active carboxy-terminal polypeptide similar to the toxin A fragment. This MAb neutralized the ADP-ribosyl transferase activity of activated holotoxin and of the cloned peptide, but inhibited neither binding of toxin to membrane receptors nor its cytotoxic and lethal actions. The complementary specificity and function of these MAbs confirm the functional specialization of discrete structural domains within the toxin A molecule. Our findings suggest the greater antitoxic potential of antibodies that block binding, compared with those which inhibit the enzymatic activity of toxin A.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Callahan L. T., 3rd Purification and characterization of Pseudomonas aeruginosa exotoxin. Infect Immun. 1974 Jan;9(1):113–118. doi: 10.1128/iai.9.1.113-118.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier R. J., Kandel J. Structure and activity of diphtheria toxin. I. Thiol-dependent dissociation of a fraction of toxin into enzymically active and inactive fragments. J Biol Chem. 1971 Mar 10;246(5):1496–1503. [PubMed] [Google Scholar]
- Cross A. S., Sadoff J. C., Iglewski B. H., Sokol P. A. Evidence for the role of toxin A in the pathogenesis of infection with Pseudomonas aeruginosa in humans. J Infect Dis. 1980 Oct;142(4):538–546. doi: 10.1093/infdis/142.4.538. [DOI] [PubMed] [Google Scholar]
- Galloway D. R., Hedstrom R. C., Pavlovskis O. R. Production and characterization of monoclonal antibodies to exotoxin A from Pseudomonas aeruginosa. Infect Immun. 1984 May;44(2):262–267. doi: 10.1128/iai.44.2.262-267.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G. L., Smith D. H., Baldridge J. S., Harkins R. N., Vasil M. L., Chen E. Y., Heyneker H. L. Cloning, nucleotide sequence, and expression in Escherichia coli of the exotoxin A structural gene of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 1984 May;81(9):2645–2649. doi: 10.1073/pnas.81.9.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglewski B. H., Liu P. V., Kabat D. Mechanism of action of Pseudomonas aeruginosa exotoxin Aiadenosine diphosphate-ribosylation of mammalian elongation factor 2 in vitro and in vivo. Infect Immun. 1977 Jan;15(1):138–144. doi: 10.1128/iai.15.1.138-144.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinger K. W., Shuster C. W. A macromolecular structure produced by Pseudomonas aeruginosa is recognized by antibody to exotoxin A. Infect Immun. 1984 Mar;43(3):912–919. doi: 10.1128/iai.43.3.912-919.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leppla S. H. Large-scale purification and characterization of the exotoxin of Pseudomonas aeruginosa. Infect Immun. 1976 Oct;14(4):1077–1086. doi: 10.1128/iai.14.4.1077-1086.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P. V. Exotoxins of Pseudomonas aeruginosa. I. Factors that influence the production of exotoxin A. J Infect Dis. 1973 Oct;128(4):506–513. doi: 10.1093/infdis/128.4.506. [DOI] [PubMed] [Google Scholar]
- Liu P. V., Yoshii S., Hsieh H. Exotoxins of Pseudomonas aeruginosa. II. Concentration, purification, and characterization of exotoxin A. J Infect Dis. 1973 Oct;128(4):514–519. doi: 10.1093/infdis/128.4.514. [DOI] [PubMed] [Google Scholar]
- Lory S., Collier R. J. Expression of enzymic activity by exotoxin A from Pseudomonas aeruginosa. Infect Immun. 1980 May;28(2):494–501. doi: 10.1128/iai.28.2.494-501.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappenheimer A. M., Jr, Uchida T., Harper A. A. An immunological study of the diphtheria toxin molecule. Immunochemistry. 1972 Sep;9(9):891–906. doi: 10.1016/0019-2791(72)90163-2. [DOI] [PubMed] [Google Scholar]
- Pavlovskis O. R., Gordon F. B. Pseudomonas aeruginosa exotoxin: effect on cell cultures. J Infect Dis. 1972 Jun;125(6):631–636. doi: 10.1093/infdis/125.6.631. [DOI] [PubMed] [Google Scholar]
- Pavlovskis O. R., Iglewski B. H., Pollack M. Mechanism of action of Pseudomonas aeruginosa exotoxin A in experimental mouse infections: adenosine diphosphate ribosylation of elongation factor 2. Infect Immun. 1978 Jan;19(1):29–33. doi: 10.1128/iai.19.1.29-33.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovskis O. R., Pollack M., Callahan L. T., 3rd, Iglewski B. H. Passive protection by antitoxin in experimental Pseudomonas aeruginosa burn infections. Infect Immun. 1977 Dec;18(3):596–602. doi: 10.1128/iai.18.3.596-602.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack M., Callahan L. T., 3rd, Taylor N. S. Neutralizing antibody to Pseudomonas aeruginosa exotoxin in human sera: evidence for in vivo toxin production during infection. Infect Immun. 1976 Oct;14(4):942–947. doi: 10.1128/iai.14.4.942-947.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack M., Huang A. I., Prescott R. K., Young L. S., Hunter K. W., Cruess D. F., Tsai C. M. Enhanced survival in Pseudomonas aeruginosa septicemia associated with high levels of circulating antibody to Escherichia coli endotoxin core. J Clin Invest. 1983 Dec;72(6):1874–1881. doi: 10.1172/JCI111150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack M., Prescott R. K. Toxoid from exotoxin A of Pseudomonas aeruginosa: preparation and characterization. J Infect Dis. 1982 May;145(5):688–698. doi: 10.1093/infdis/145.2.688. [DOI] [PubMed] [Google Scholar]
- Pollack M. Pseudomonas aeruginosa exotoxin A. N Engl J Med. 1980 Jun 12;302(24):1360–1362. doi: 10.1056/NEJM198006123022410. [DOI] [PubMed] [Google Scholar]
- Pollack M., Taylor N. S., Callahan L. T., 3rd Exotoxin production by clinical isolates of pseudomonas aeruginosa. Infect Immun. 1977 Mar;15(3):776–780. doi: 10.1128/iai.15.3.776-780.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack M., Young L. S. Protective activity of antibodies to exotoxin A and lipopolysaccharide at the onset of Pseudomonas aeruginosa septicemia in man. J Clin Invest. 1979 Feb;63(2):276–286. doi: 10.1172/JCI109300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saelinger C. B., Snell K., Holder I. A. Experimental studies on the pathogenesis of infections due to Pseudomonas aeruginosa: direct evidence for toxin production during Pseudomonas infection of burned skin tissues. J Infect Dis. 1977 Oct;136(4):555–561. doi: 10.1093/infdis/136.4.555. [DOI] [PubMed] [Google Scholar]
- Snell K., Holder I. A., Leppla S. A., Saelinger C. B. Role of exotoxin and protease as possible virulence factors in experimental infections with Pseudomonas aeruginosa. Infect Immun. 1978 Mar;19(3):839–845. doi: 10.1128/iai.19.3.839-845.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor N. S., Pollack M. Purification of Pseudomonas aeruginosa exotoxin by affinity chromatography. Infect Immun. 1978 Jan;19(1):66–70. doi: 10.1128/iai.19.1.66-70.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasil M. L., Kabat D., Iglewski B. H. Structure-activity relationships of an exotoxin of Pseudomonas aeruginosa. Infect Immun. 1977 Apr;16(1):353–361. doi: 10.1128/iai.16.1.353-361.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. E., Cryz S. J., Friedman R. L., Iglewski B. H. Contribution of toxin A and elastase to virulence of Pseudomonas aeruginosa in chronic lung infections of rats. Infect Immun. 1982 Jun;36(3):1223–1228. doi: 10.1128/iai.36.3.1223-1228.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]