Abstract
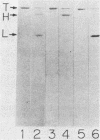
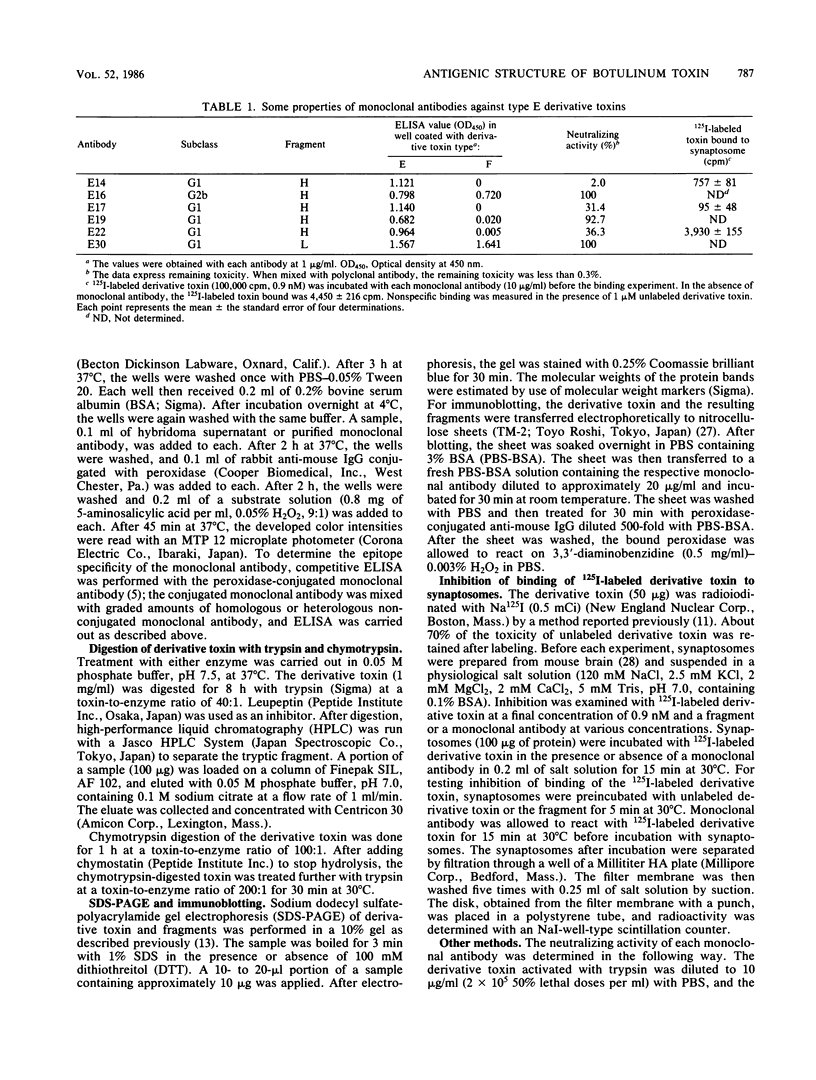
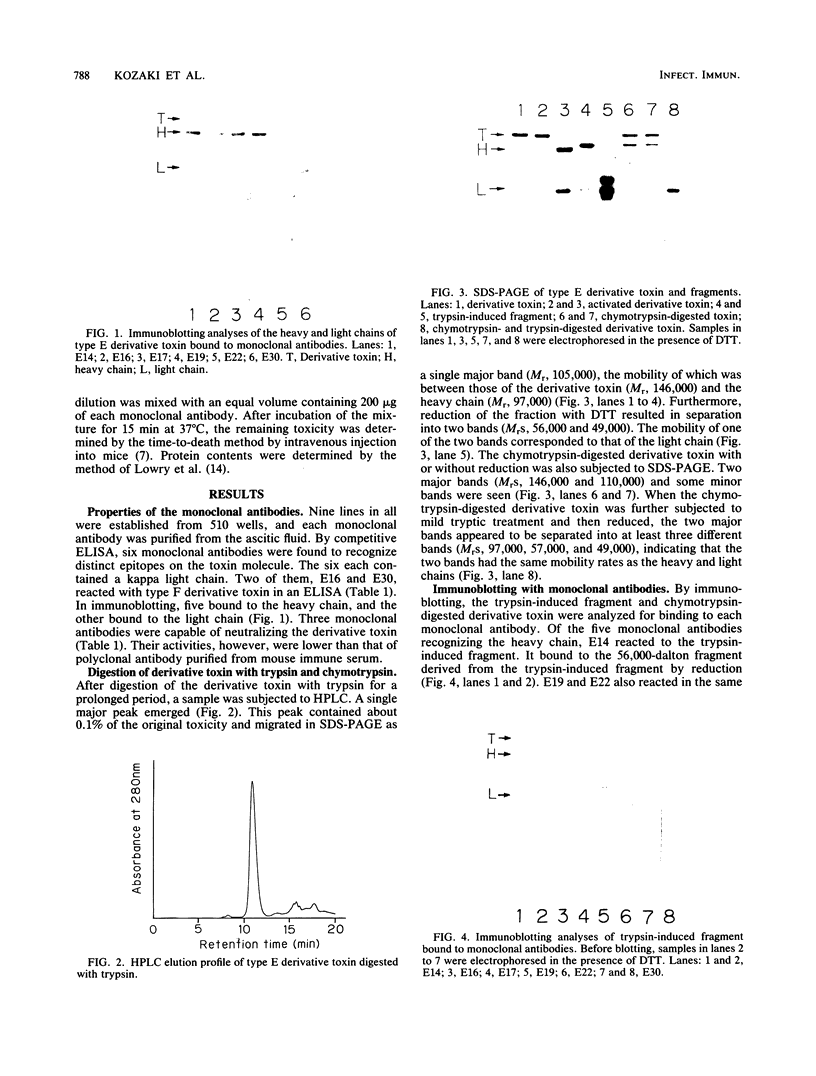
Six monoclonal antibodies against Clostridium botulinum type E derivative toxin were prepared. Three of the five binding to the heavy chain neutralized the derivative toxin; the other one binding to the light chain did not. Immunoblotting analysis with the monoclonal antibodies showed that the fragment obtained by tryptic digestion consisted of the light chain and part of the heavy chain (H-1 fragment) linked together by a disulfide bond(s) and that the antigenic determinants common between type E and F derivative toxins were located on both the heavy and light chains. The fragment induced by chymotrypsin treatment, like the tryptic fragment, bound to four monoclonal antibodies. The mild tryptic treatment and reduction resulted in separation of the chymotryptic fragment into two smaller fragments corresponding to the light chain and H-1 fragment. These results indicate that H-1 fragment contains the amino-terminal portion of the heavy chain. The monoclonal antibody neutralizing the toxin and probably recognizing the epitope on the carboxyl-terminal portion (H-2 fragment) of the heavy chain effectively competed for binding of 125I-labeled derivative toxin to synaptosomes. Of the two monoclonal antibodies neutralizing the toxin and recognizing the epitopes on H-1 fragment, one partially inhibited binding, but the other did not. This suggests that the binding of 125I-labeled derivative toxin depends mainly on the carboxyl-terminal region of the heavy chain and that interference with binding is not the only means of toxin neutralization.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruck C., Portetelle D., Glineur C., Bollen A. One-step purification of mouse monoclonal antibodies from ascitic fluid by DEAE Affi-Gel blue chromatography. J Immunol Methods. 1982 Sep 30;53(3):313–319. doi: 10.1016/0022-1759(82)90178-8. [DOI] [PubMed] [Google Scholar]
- Bulatova T. I., Perova E. V. Antigennaia struktura Cl. botulinum tipov E i F. Zh Mikrobiol Epidemiol Immunobiol. 1970 Apr;47(4):28–32. [PubMed] [Google Scholar]
- DasGupta B. R., Sugiyama H. A common subunit structure in Clostridium botulinum type A, B and E toxins. Biochem Biophys Res Commun. 1972 Jul 11;48(1):108–112. doi: 10.1016/0006-291x(72)90350-6. [DOI] [PubMed] [Google Scholar]
- Kitamura M., Sakaguchi S., Sakaguchi G. Purification and some properties of Clostridium botulinum type-E toxin. Biochim Biophys Acta. 1968 Oct 21;168(2):207–217. doi: 10.1016/0005-2795(68)90144-x. [DOI] [PubMed] [Google Scholar]
- Kondo H., Shimizu T., Kubonoya M., Izumi N., Takahashi M., Sakaguchi G. Titration of botulinum toxins for lethal toxicity by intravenous injection into mice. Jpn J Med Sci Biol. 1984 Jun;37(3):131–135. doi: 10.7883/yoken1952.37.131. [DOI] [PubMed] [Google Scholar]
- Kozaki S. Interaction of botulinum type A, B and E derivative toxins with synaptosomes of rat brain. Naunyn Schmiedebergs Arch Pharmacol. 1979 Jul;308(1):67–70. doi: 10.1007/BF00499721. [DOI] [PubMed] [Google Scholar]
- Kozaki S., Miyazaki S., Sakaguchi G. Development of antitoxin with each of two complementary fragments of Clostridium botulinum type B derivative toxin. Infect Immun. 1977 Dec;18(3):761–766. doi: 10.1128/iai.18.3.761-766.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozaki S., Sakaguchi G. Binding to mouse brain synaptosomes of Clostridium botulinum type E derivative toxin before and after tryptic activation. Toxicon. 1982;20(5):841–846. doi: 10.1016/0041-0101(82)90071-x. [DOI] [PubMed] [Google Scholar]
- Kozaki S., Togashi S., Sakaguchi G. Separation of Clostridium botulinum type A derivative toxin into two fragments. Jpn J Med Sci Biol. 1981 Apr;34(2):61–68. doi: 10.7883/yoken1952.34.61. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Murayama S., Syuto B., Oguma K., Iida H., Kubo S. Comparison of Clostridium botulinum toxins type D and C1 in molecular property, antigenicity and binding ability to rat-brain synaptosomes. Eur J Biochem. 1984 Aug 1;142(3):487–492. doi: 10.1111/j.1432-1033.1984.tb08312.x. [DOI] [PubMed] [Google Scholar]
- Ohishi I., Sakaguchi G. Molecular construction of Clostridium botulinum type F progenitor toxin. Appl Microbiol. 1975 Apr;29(4):444–447. doi: 10.1128/am.29.4.444-447.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi I., Sakaguchi G. Purification of Clostridium botuliunum type F progenitor toxin. Appl Microbiol. 1974 Dec;28(6):923–928. doi: 10.1128/am.28.6.923-928.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi G. Clostridium botulinum toxins. Pharmacol Ther. 1982;19(2):165–194. doi: 10.1016/0163-7258(82)90061-4. [DOI] [PubMed] [Google Scholar]
- Sakaguchi G., Sakaguchi S., Kozaki S., Sugii S., Oishi I. Cross reaction in reversed passive hemagglutination between Clostridium botulinum type A and B toxins and its avoidance by the sue of anti-toxic component immunoglobulin isolated by affinity chromatography. Jpn J Med Sci Biol. 1974 Jun;27(3):161–172. doi: 10.7883/yoken1952.27.161. [DOI] [PubMed] [Google Scholar]
- Sathyamoorthy V., DasGupta B. R. Partial amino acid sequences of the heavy and light chains of botulinum neurotoxin type E. Biochem Biophys Res Commun. 1985 Mar 29;127(3):768–772. doi: 10.1016/s0006-291x(85)80009-7. [DOI] [PubMed] [Google Scholar]
- Sathyamoorthy V., DasGupta B. R. Separation, purification, partial characterization and comparison of the heavy and light chains of botulinum neurotoxin types A, B, and E. J Biol Chem. 1985 Sep 5;260(19):10461–10466. [PubMed] [Google Scholar]
- Shone C. C., Hambleton P., Melling J. Inactivation of Clostridium botulinum type A neurotoxin by trypsin and purification of two tryptic fragments. Proteolytic action near the COOH-terminus of the heavy subunit destroys toxin-binding activity. Eur J Biochem. 1985 Aug 15;151(1):75–82. doi: 10.1111/j.1432-1033.1985.tb09070.x. [DOI] [PubMed] [Google Scholar]
- Simpson L. L. Kinetic studies on the interaction between botulinum toxin type A and the cholinergic neuromuscular junction. J Pharmacol Exp Ther. 1980 Jan;212(1):16–21. [PubMed] [Google Scholar]
- Simpson L. L. The origin, structure, and pharmacological activity of botulinum toxin. Pharmacol Rev. 1981 Sep;33(3):155–188. [PubMed] [Google Scholar]
- Sugiyama H. Clostridium botulinum neurotoxin. Microbiol Rev. 1980 Sep;44(3):419–448. doi: 10.1128/mr.44.3.419-448.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syuto B., Kubo S. Separation and characterization of heavy and light chains from Clostridium botulinum type C toxin and their reconstitution. J Biol Chem. 1981 Apr 25;256(8):3712–3717. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITTAKER V. P. The isolation and characterization of acetylcholine-containing particles from brain. Biochem J. 1959 Aug;72:694–706. doi: 10.1042/bj0720694. [DOI] [PMC free article] [PubMed] [Google Scholar]