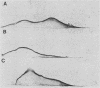
Abstract
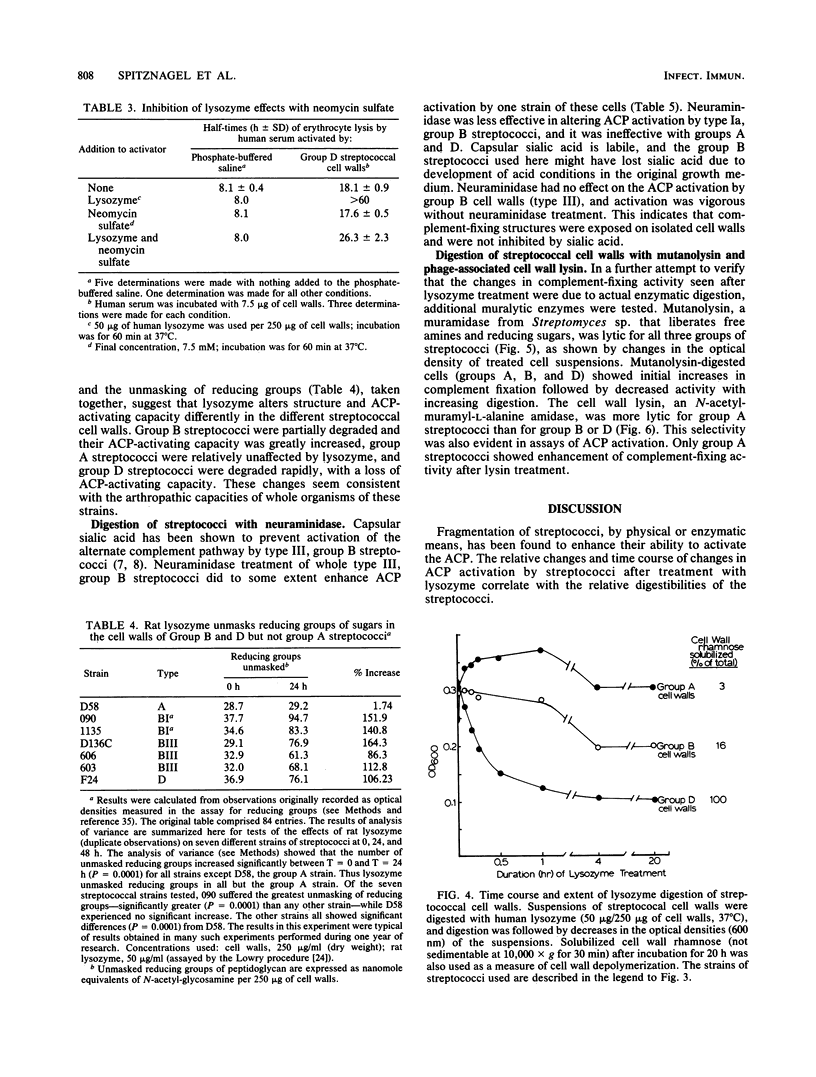
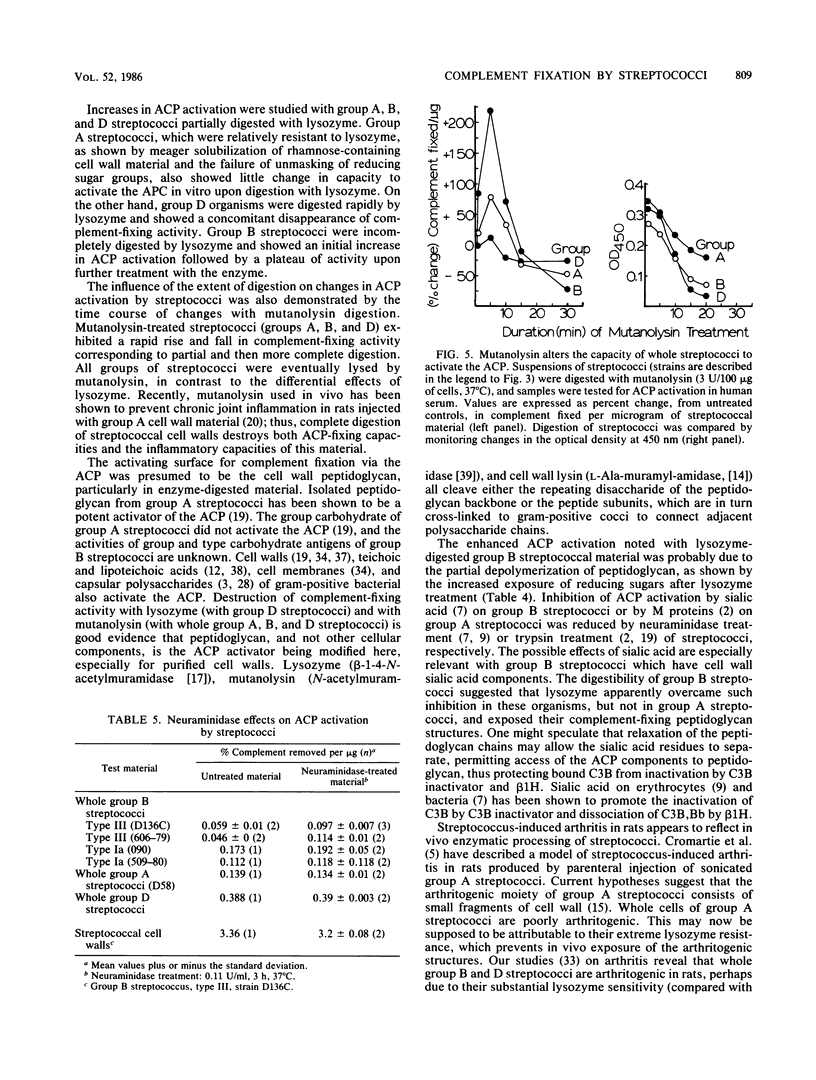
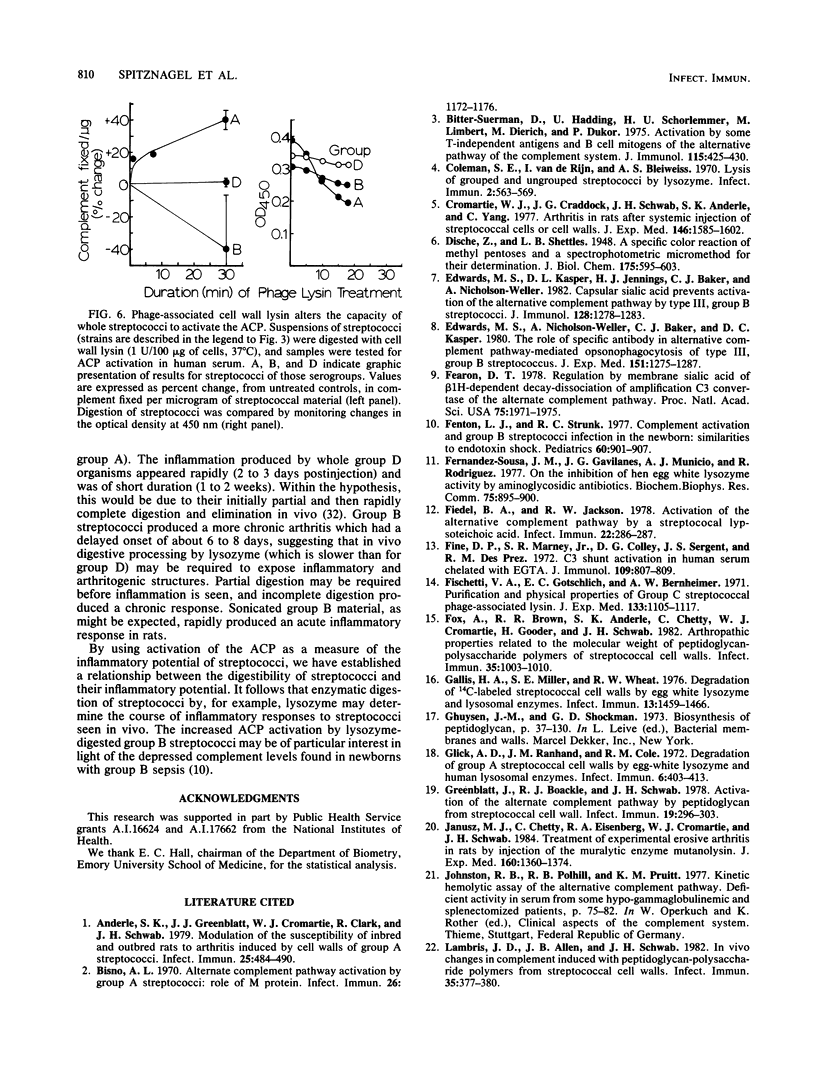
Streptococci and streptococcal cell wall fragments induce arthritis in rats, with the severity and duration depending on the capacity of the cells or cell fragments to resist degradation by tissue enzymes. Their phlogogenic effects are apparently related to their ability to activate the alternate complement pathway (ACP). The in vitro activation of the ACP by lysozyme-treated cells and cell walls of group A, B, and D streptococci suggests that both rat and human lysozyme can modulate this activity, i.e., increasing it, decreasing it, or doing both in that order. The effects of the lysozymes also correlated with the degree to which they can unmask the aminosugar-reducing groups detectable in a given amount of cell wall, which suggests that partial depolymerization of the cell wall is critical for ACP activation. The effects of mutanolysin and C phage lysin on ACP activation were found to be correlated with their action on streptococcal cell walls. Neuraminidase had relatively little effect on ACP activation by most streptococcal strains tested. We conclude that the participation of tissue enzymes, including but not necessarily limited to lysozyme, is an important determinant for the clinical arthritis induced by group A, B, or D streptococci. Experimental arthritis induced in rats with whole (or disrupted) streptococci may depend both on the capacities of the cell walls to activate the ACP and on the capacities of the host tissue enzymes to modulate this activation. Great severity and long durations of the disease were determined by the capacity of the enzymes to degrade cell wall antigens to a degree sufficient to ensure efficient activation of the ACP without completely degrading the material so that it no longer activates complement. In this model, the limited resistance of group B peptidoglycan to lysozyme was a critical pathogenic factor.
Full text
PDF

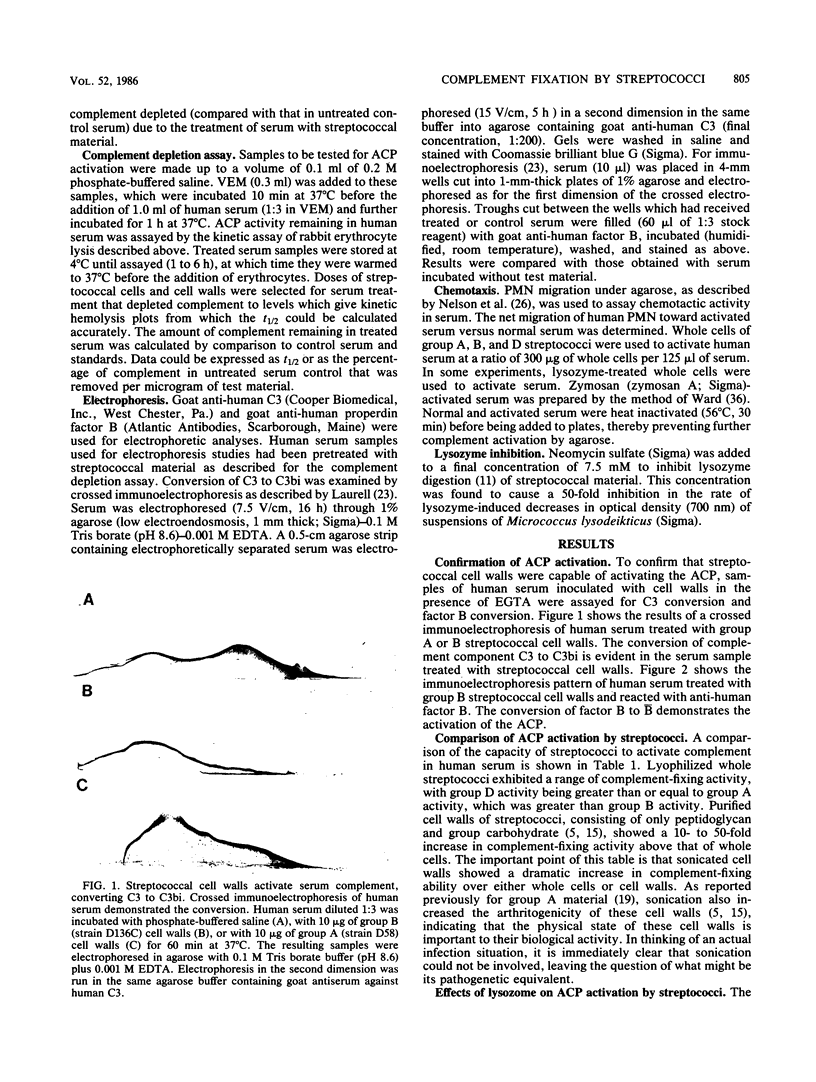
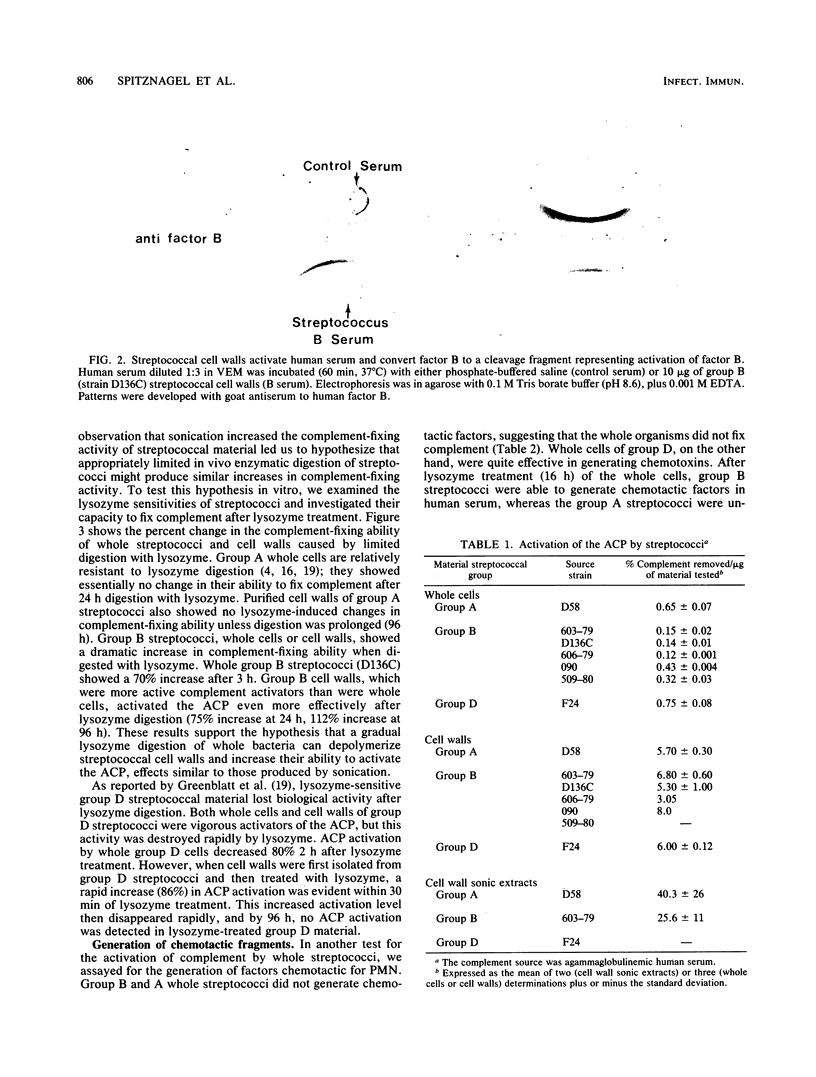
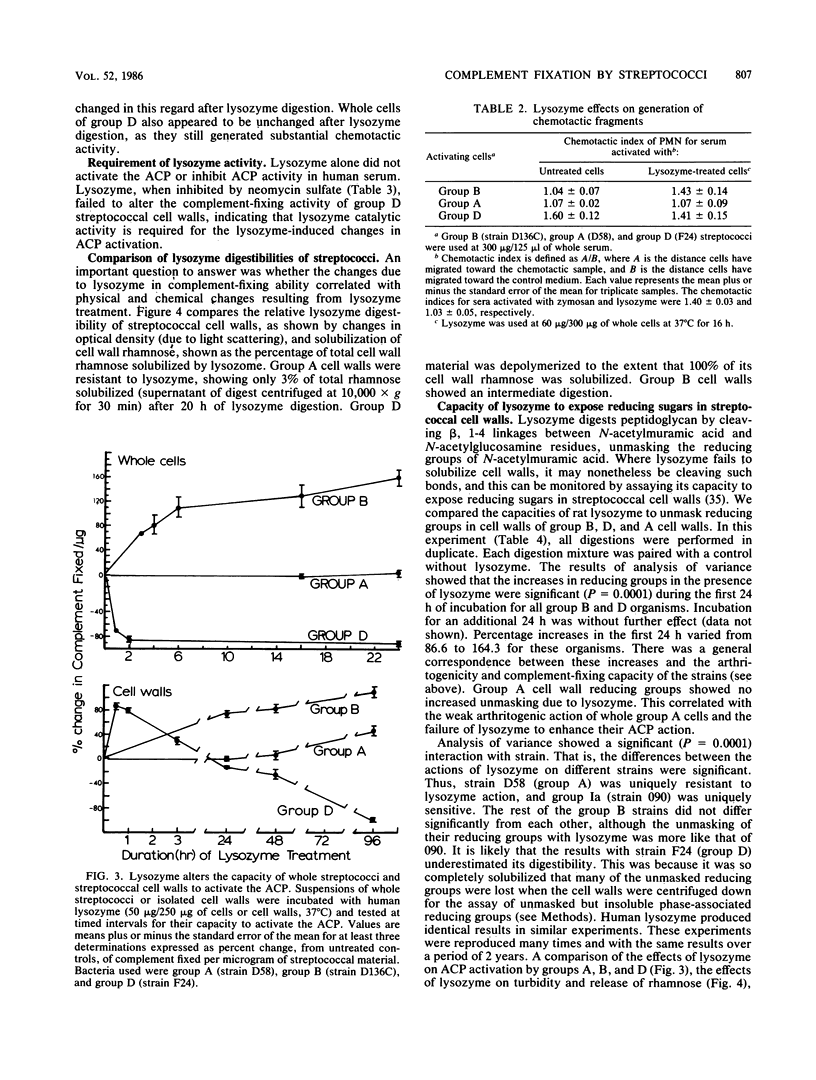

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderle S. K., Greenblatt J. J., Cromartie W. J., Clark R., Schwab J. H. Modulation of the susceptibility of inbred and outbred rats to arthritis induced by cell walls of group A streptococci. Infect Immun. 1979 Aug;25(2):484–490. doi: 10.1128/iai.25.2.484-490.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisno A. L. Alternate complement pathway activation by group A streptococci: role of M-protein. Infect Immun. 1979 Dec;26(3):1172–1176. doi: 10.1128/iai.26.3.1172-1176.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitter-Suermann D., Hadding U., Schorlemmer H. U., Limbert M., Dierich M., Dukor P. Activation by some T-independent antigens and B cell mitogens of the alternative pathway of the complement system. J Immunol. 1975 Aug;115(2):425–430. [PubMed] [Google Scholar]
- Coleman S. E., van de Rijn I., Bleiweis A. S. Lysis of grouped and ungrouped streptococci by lysozyme. Infect Immun. 1970 Nov;2(5):563–569. doi: 10.1128/iai.2.5.563-569.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromartie W. J., Craddock J. G., Schwab J. H., Anderle S. K., Yang C. H. Arthritis in rats after systemic injection of streptococcal cells or cell walls. J Exp Med. 1977 Dec 1;146(6):1585–1602. doi: 10.1084/jem.146.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DISCHE Z., SHETTLES L. B. A specific color reaction of methylpentoses and a spectrophotometric micromethod for their determination. J Biol Chem. 1948 Sep;175(2):595–603. [PubMed] [Google Scholar]
- Edwards M. S., Kasper D. L., Jennings H. J., Baker C. J., Nicholson-Weller A. Capsular sialic acid prevents activation of the alternative complement pathway by type III, group B streptococci. J Immunol. 1982 Mar;128(3):1278–1283. [PubMed] [Google Scholar]
- Edwards M. S., Nicholson-Weller A., Baker C. J., Kasper D. L. The role of specific antibody in alternative complement pathway-mediated opsonophagocytosis of type III, group B Streptococcus. J Exp Med. 1980 May 1;151(5):1275–1287. doi: 10.1084/jem.151.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T. Regulation by membrane sialic acid of beta1H-dependent decay-dissociation of amplification C3 convertase of the alternative complement pathway. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1971–1975. doi: 10.1073/pnas.75.4.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton L. J., Strunk R. C. Complement activation and group B streptococcal infection in the newborn: similarities to endotoxin shock. Pediatrics. 1977 Dec;60(6):901–907. [PubMed] [Google Scholar]
- Fernández-Sousa J. M., Gavilanes J. G., Municio A. M., Rodriguez R. On the inhibition of hen egg-white lysozyme activity by aminoglycosidic antibiotics. Biochem Biophys Res Commun. 1977 Apr 25;75(4):895–900. doi: 10.1016/0006-291x(77)91466-8. [DOI] [PubMed] [Google Scholar]
- Fiedel B. A., Jackson R. W. Activation of the alternative complement pathway by a streptococcal lipoteichoic acid. Infect Immun. 1978 Oct;22(1):286–287. doi: 10.1128/iai.22.1.286-287.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine D. P., Marney S. R., Jr, Colley D. G., Sergent J. S., Des Prez R. M. C3 shunt activation in human serum chelated with EGTA. J Immunol. 1972 Oct;109(4):807–809. [PubMed] [Google Scholar]
- Fischetti V. A., Gotschlich E. C., Bernheimer A. W. Purification and physical properties of group C streptococcal phage-associated lysin. J Exp Med. 1971 May 1;133(5):1105–1117. doi: 10.1084/jem.133.5.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A., Brown R. R., Anderle S. K., Chetty C., Cromartie W. J., Gooder H., Schwab J. H. Arthropathic properties related to the molecular weight of peptidoglycan-polysaccharide polymers of streptococcal cell walls. Infect Immun. 1982 Mar;35(3):1003–1010. doi: 10.1128/iai.35.3.1003-1010.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallis H. A., Miller S. E., Wheat R. W. Degradation of 14C-labeled streptococcal cell walls by egg white lysozyme and lysosomal enzymes. Infect Immun. 1976 May;13(5):1459–1466. doi: 10.1128/iai.13.5.1459-1466.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick A. D., Ranhand J. M., Cole R. M. Degradation of group A streptococcal cell walls by egg-white lysozyme and human lysosomal enzymes. Infect Immun. 1972 Sep;6(3):403–413. doi: 10.1128/iai.6.3.403-413.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt J., Boackle R. J., Schwab J. H. Activation of the alternate complement pathway by peptidoglycan from streptococcal cell wall. Infect Immun. 1978 Jan;19(1):296–303. doi: 10.1128/iai.19.1.296-303.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janusz M. J., Chetty C., Eisenberg R. A., Cromartie W. J., Schwab J. H. Treatment of experimental erosive arthritis in rats by injection of the muralytic enzyme mutanolysin. J Exp Med. 1984 Nov 1;160(5):1360–1374. doi: 10.1084/jem.160.5.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lambris J. D., Allen J. B., Schwab J. H. In vivo changes in complement induced with peptidoglycan-polysaccharide polymers from streptococcal cell walls. Infect Immun. 1982 Jan;35(1):377–380. doi: 10.1128/iai.35.1.377-380.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrzakowski M. C., Cooney M. H., Martin L. E., Spitznagel J. K. Bactericidal activity of fractionated granule contents from human polymorphonuclear leukocytes. Infect Immun. 1979 Mar;23(3):587–591. doi: 10.1128/iai.23.3.587-591.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. D., Quie P. G., Simmons R. L. Chemotaxis under agarose: a new and simple method for measuring chemotaxis and spontaneous migration of human polymorphonuclear leukocytes and monocytes. J Immunol. 1975 Dec;115(6):1650–1656. [PubMed] [Google Scholar]
- Ohanian S. H., Schwab J. H. Persistence of group a streptococcal cell walls related to chronic inflammation of rabbit dermal connective tissue. J Exp Med. 1967 Jun 1;125(6):1137–1148. doi: 10.1084/jem.125.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PILLEMER L., SCHOENBERG M. D., BLUM L., WURZ L. Properdin system and immunity. II. Interaction of the properdin system with polysaccharides. Science. 1955 Sep 23;122(3169):545–549. doi: 10.1126/science.122.3169.545. [DOI] [PubMed] [Google Scholar]
- Platts-Mills T. A., Ishizaka K. Activation of the alternate pathway of human complements by rabbit cells. J Immunol. 1974 Jul;113(1):348–358. [PubMed] [Google Scholar]
- Schwab J. H. Biological properties of streptococcal cell-wall particles. I. Determinants of the chronic nodular lesion of connective tissue. J Bacteriol. 1965 Nov;90(5):1405–1411. doi: 10.1128/jb.90.5.1405-1411.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab J. H., Ohanian S. H. Degradation of streptococcal cell wall antigens in vivo. J Bacteriol. 1967 Nov;94(5):1346–1352. doi: 10.1128/jb.94.5.1346-1352.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitznagel J. K., Goodrum K. J., Warejcka D. J. Rat arthritis due to whole group B streptococci. Clinical and histopathologic features compared with groups A and D. Am J Pathol. 1983 Jul;112(1):37–47. [PMC free article] [PubMed] [Google Scholar]
- Tauber J. W., Polley M. J., Zabriskie J. B. Nonspecific complement activation by streptococcal structures. II. Properdin-independent initiation of the alternate pathway. J Exp Med. 1976 Jun 1;143(6):1352–1366. doi: 10.1084/jem.143.6.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. S., Shockman G. D. A modification of the Park and Johnson reducing sugar determination suitable for the assay of insoluble materials: its application to bacterial cell walls. Anal Biochem. 1968 Feb;22(2):260–268. doi: 10.1016/0003-2697(68)90315-1. [DOI] [PubMed] [Google Scholar]
- Ward P. A. Chemotoxis of mononuclear cells. J Exp Med. 1968 Nov 1;128(5):1201–1221. doi: 10.1084/jem.128.5.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelstein J. A., Tomasz A. Activation of the alternative complement pathway by pneumococcal cell wall teichoic acid. J Immunol. 1978 Jan;120(1):174–178. [PubMed] [Google Scholar]
- Winkelstein J. A., Tomasz A. Activation of the alternative pathway by pneumococcal cell walls. J Immunol. 1977 Feb;118(2):451–454. [PubMed] [Google Scholar]