Abstract
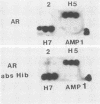
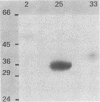
DNA from Haemophilus influenzae type b was cloned in Escherichia coli with a vector lambda gt11 Amp1. Clones producing antigens reactive with hyperimmune rabbit antisera were identified by colony radioimmune assay. A second screening with hyperimmune serum adsorbed with intact H. influenzae type b bacteria was used to identify those clones producing surface-exposed outer membrane proteins. The proteins expressed in E. coli were coupled to Sepharose and used to affinity purify antibodies which were tested for reactivity with outer membrane vesicles. It was found by Western blotting that the clones were producing antigens corresponding to Mr 49,000, 39,500, and 35,000 major outer membrane protein or antigens of H. influenzae type b. Additional clones could be detected by human serum, but their reactivity was not removed when serum was adsorbed with intact bacteria. One of these studied in more detail was found to produce an antigen present in H. influenzae type b lysate but not in outer membrane vesicle preparations.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNS K. I., THOMAS C. A., Jr ISOLATION OF HIGH MOLECULAR WEIGHT DNA FROM HEMOPHILUS INFLUENZAE. J Mol Biol. 1965 Mar;11:476–490. doi: 10.1016/s0022-2836(65)80004-3. [DOI] [PubMed] [Google Scholar]
- Barenkamp S. J., Munson R. S., Jr, Granoff D. M. Subtyping isolates of Haemophilus influenzae type b by outer-membrane protein profiles. J Infect Dis. 1981 May;143(5):668–676. doi: 10.1093/infdis/143.5.668. [DOI] [PubMed] [Google Scholar]
- Bowtell D. D., Saint R. B., Rickard M. D., Mitchell G. F. Expression of Taenia taeniaeformis antigens in Escherichia coli. Mol Biochem Parasitol. 1984 Oct;13(2):173–185. doi: 10.1016/0166-6851(84)90111-7. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Erwin A. L., Kenny G. E. Haemophilus influenzae type b isolates show antigenic variation in a major outer membrane protein. Infect Immun. 1984 Nov;46(2):570–577. doi: 10.1128/iai.46.2.570-577.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskola J., Käyhty H., Peltola H., Karanko V., Mäkelä P. H., Samuelson J., Gordon L. K. Antibody levels achieved in infants by course of Haemophilus influenzae type B polysaccharide/diphtheria toxoid conjugate vaccine. Lancet. 1985 May 25;1(8439):1184–1186. doi: 10.1016/s0140-6736(85)92863-6. [DOI] [PubMed] [Google Scholar]
- Gulig P. A., McCracken G. H., Jr, Frisch C. F., Johnston K. H., Hansen E. J. Antibody response of infants to cell surface-exposed outer membrane proteins of Haemophilus influenzae type b after systemic Haemophilus disease. Infect Immun. 1982 Jul;37(1):82–88. doi: 10.1128/iai.37.1.82-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. J., Frisch C. F., Johnston K. H. Detection of antibody-accessible proteins on the cell surface of Haemophilus influenzae type b. Infect Immun. 1981 Sep;33(3):950–953. doi: 10.1128/iai.33.3.950-953.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. J., Robertson S. M., Gulig P. A., Frisch C. F., Haanes E. J. Immunoprotection of rats against Haemophilus influenzae type B disease mediated by monoclonal antibody against a haemophilus outer-membrane protein. Lancet. 1982 Feb 13;1(8268):366–368. doi: 10.1016/s0140-6736(82)91394-0. [DOI] [PubMed] [Google Scholar]
- Hunter W. M. Radioimmunoassay and saturation analysis. Preparation and assessment of radioactive tracers. Br Med Bull. 1974 Jan;30(1):18–23. doi: 10.1093/oxfordjournals.bmb.a071161. [DOI] [PubMed] [Google Scholar]
- Kemp D. J., Coppel R. L., Cowman A. F., Saint R. B., Brown G. V., Anders R. F. Expression of Plasmodium falciparum blood-stage antigens in Escherichia coli: detection with antibodies from immune humans. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3787–3791. doi: 10.1073/pnas.80.12.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A., Gulig P. A., McCracken G. H., Jr, Loftus T. A., Hansen E. J. A minor high-molecular-weight outer membrane protein of Haemophilus influenzae type b is a protective antigen. Infect Immun. 1985 Jan;47(1):253–259. doi: 10.1128/iai.47.1.253-259.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loeb M. R., Smith D. H. Outer membrane protein composition in disease isolates of Haemophilus influenzae: pathogenic and epidemiological implications. Infect Immun. 1980 Dec;30(3):709–717. doi: 10.1128/iai.30.3.709-717.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Munson R. S., Jr, Shenep J. L., Barenkamp S. J., Granoff D. M. Purification and comparison of outer membrane protein P2 from Haemophilus influenzae type b isolates. J Clin Invest. 1983 Aug;72(2):677–684. doi: 10.1172/JCI111017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltola H., Käyhty H., Virtanen M., Mäkelä P. H. Prevention of Hemophilus influenzae type b bacteremic infections with the capsular polysaccharide vaccine. N Engl J Med. 1984 Jun 14;310(24):1561–1566. doi: 10.1056/NEJM198406143102404. [DOI] [PubMed] [Google Scholar]
- Singer A., Hodes R. J. Mechanisms of T cell-B cell interaction. Annu Rev Immunol. 1983;1:211–241. doi: 10.1146/annurev.iy.01.040183.001235. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]