Abstract
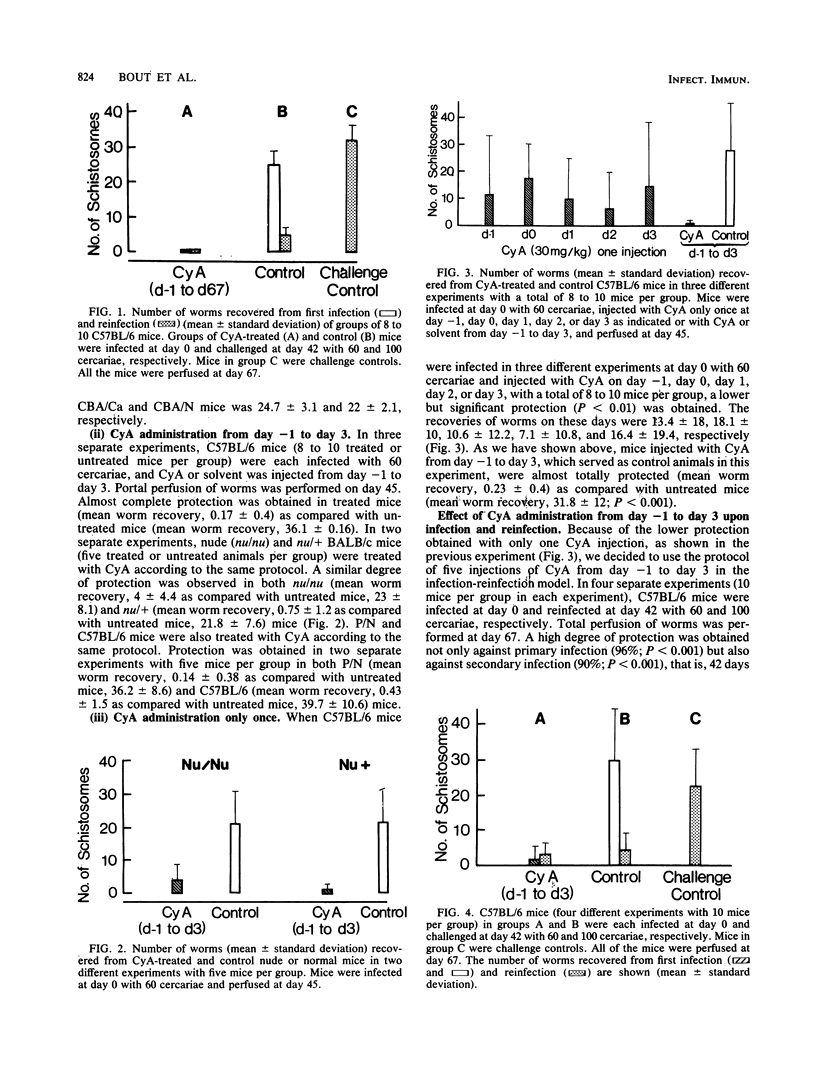
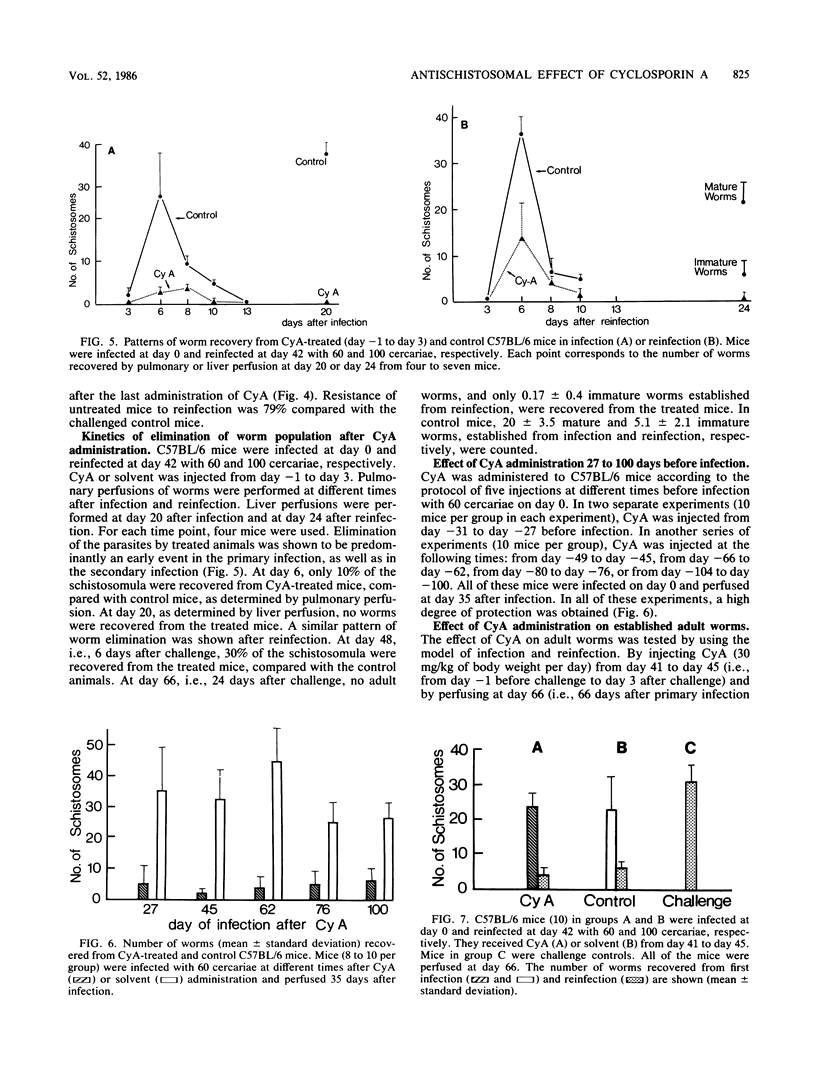
C57BL/6 mice infected with Schistosoma mansoni at day 0 and injected with cyclosporin A (CyA) either daily or from day -1 to day 3 were protected against schistosomiasis mansoni as indicated by a decrease in the number of worms recovered from the liver 45 days after infection. CyA treatment also protected rats and strains of mice with known immunity defects (nu/nu, P/N, CBA/N). Protection was evident against both primary and secondary infection in mice infected at day 0, reinfected at day 42, and treated daily with CyA either during the course of the experiment or only from day -1 to day 3, as indicated by the worm burden at day 67. In such an experiment of infection and reinfection, the immature worms were shown to be the target of CyA. Administration of the drug 27, 45, 62, or 100 days before infection confirmed the long-term protective effect of CyA. This drug did not evoke the killing of adult worms in vivo. These data confirm and define the curative and preventive effect of CyA against schistosomiasis mansoni.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer S. The chemotherapy of schistosomiasis. Annu Rev Pharmacol Toxicol. 1985;25:485–508. doi: 10.1146/annurev.pa.25.040185.002413. [DOI] [PubMed] [Google Scholar]
- Belosevic M., Faubert G. M., MacLean J. D. The effects of cyclosporin A on the course of infection with Giardia muris in mice. Am J Trop Med Hyg. 1986 May;35(3):496–500. doi: 10.4269/ajtmh.1986.35.496. [DOI] [PubMed] [Google Scholar]
- Borel J. F., Feurer C., Magnée C., Stähelin H. Effects of the new anti-lymphocytic peptide cyclosporin A in animals. Immunology. 1977 Jun;32(6):1017–1025. [PMC free article] [PubMed] [Google Scholar]
- Bout D. T., Deslee D., Capron A. R. Protection against schistosomiasis produced by cyclosporin A. Am J Trop Med Hyg. 1984 Jan;33(1):185–186. doi: 10.4269/ajtmh.1984.33.185. [DOI] [PubMed] [Google Scholar]
- Bout D., Haque A., Capron A. Filaricidal effects of cyclosporin-A against Dipetalonema viteae in Mastomys natalensis. Trans R Soc Trop Med Hyg. 1984;78(5):670–671. doi: 10.1016/0035-9203(84)90236-0. [DOI] [PubMed] [Google Scholar]
- Bueding E., Hawkins J., Cha Y. N. Antischistosomal effects of cyclosporin A. Agents Actions. 1981 Jul;11(4):380–383. doi: 10.1007/BF01982474. [DOI] [PubMed] [Google Scholar]
- Cohen D. J., Loertscher R., Rubin M. F., Tilney N. L., Carpenter C. B., Strom T. B. Cyclosporine: a new immunosuppressive agent for organ transplantation. Ann Intern Med. 1984 Nov;101(5):667–682. doi: 10.7326/0003-4819-101-5-667. [DOI] [PubMed] [Google Scholar]
- Doenhoff M., Bickle Q., Long E., Bain J., McGregor A. Factors affecting the acquisition of resistance against Schistosoma mansoni in the mouse. I. Demonstration of resistance to reinfection using a model system that involves perfusion of mice within three weeks of challenge. J Helminthol. 1978 Sep;52(3):173–186. doi: 10.1017/s0022149x00005344. [DOI] [PubMed] [Google Scholar]
- James S. L., Skamene E., Meltzer M. S. Macrophages as effector cells of protective immunity in murine schistosomiasis. V. Variation in macrophage schistosomulacidal and tumoricidal activities among mouse strains and correlation with resistance to reinfection. J Immunol. 1983 Aug;131(2):948–953. [PubMed] [Google Scholar]
- Mack D. G., McLeod R. New micromethod to study the effect of antimicrobial agents on Toxoplasma gondii: comparison of sulfadoxine and sulfadiazine individually and in combination with pyrimethamine and study of clindamycin, metronidazole, and cyclosporin A. Antimicrob Agents Chemother. 1984 Jul;26(1):26–30. doi: 10.1128/aac.26.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe R. E., Remington J. S., Araujo F. G. In vivo and in vitro effects of cyclosporin A on Trypanosoma cruzi. Am J Trop Med Hyg. 1985 Sep;34(5):861–865. doi: 10.4269/ajtmh.1985.34.861. [DOI] [PubMed] [Google Scholar]
- Nickell S. P., Scheibel L. W., Cole G. A. Inhibition by cyclosporin A of rodent malaria in vivo and human malaria in vitro. Infect Immun. 1982 Sep;37(3):1093–1100. doi: 10.1128/iai.37.3.1093-1100.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson L. A., Lindblad R., Olling S., Ouchterlony O. The effect of cyclosporin A on the course of murine infection by Schistosoma mansoni. Parasite Immunol. 1985 Jan;7(1):19–27. doi: 10.1111/j.1365-3024.1985.tb00476.x. [DOI] [PubMed] [Google Scholar]
- Sher A., Mackenzie P., Smithers S. R. Decreased recovery of invading parasites from the lungs as a parameter of acquired immunity to schistosomiasis in the mouse. J Infect Dis. 1974 Dec;130(6):626–633. doi: 10.1093/infdis/130.6.626. [DOI] [PubMed] [Google Scholar]
- Smithers S. R., Terry R. J. Resistance to experimental infection with Schistosoma mansoni in rhesus monkeys induced by the transfer of adult worms. Trans R Soc Trop Med Hyg. 1967;61(4):517–533. doi: 10.1016/0035-9203(67)90102-2. [DOI] [PubMed] [Google Scholar]
- Smithers S. R., Terry R. J. The infection of laboratory hosts with cercariae of Schistosoma mansoni and the recovery of the adult worms. Parasitology. 1965 Nov;55(4):695–700. doi: 10.1017/s0031182000086248. [DOI] [PubMed] [Google Scholar]
- Thommen-Scott K. Antimalarial activity of cyclosporin A. Agents Actions. 1981 Dec;11(6-7):770–773. doi: 10.1007/BF01978803. [DOI] [PubMed] [Google Scholar]
- Thomson A. W., Whiting P. H., Simpson J. G. Cyclosporine: immunology, toxicity and pharmacology in experimental animals. Agents Actions. 1984 Oct;15(3-4):306–327. doi: 10.1007/BF01972366. [DOI] [PubMed] [Google Scholar]


