Abstract
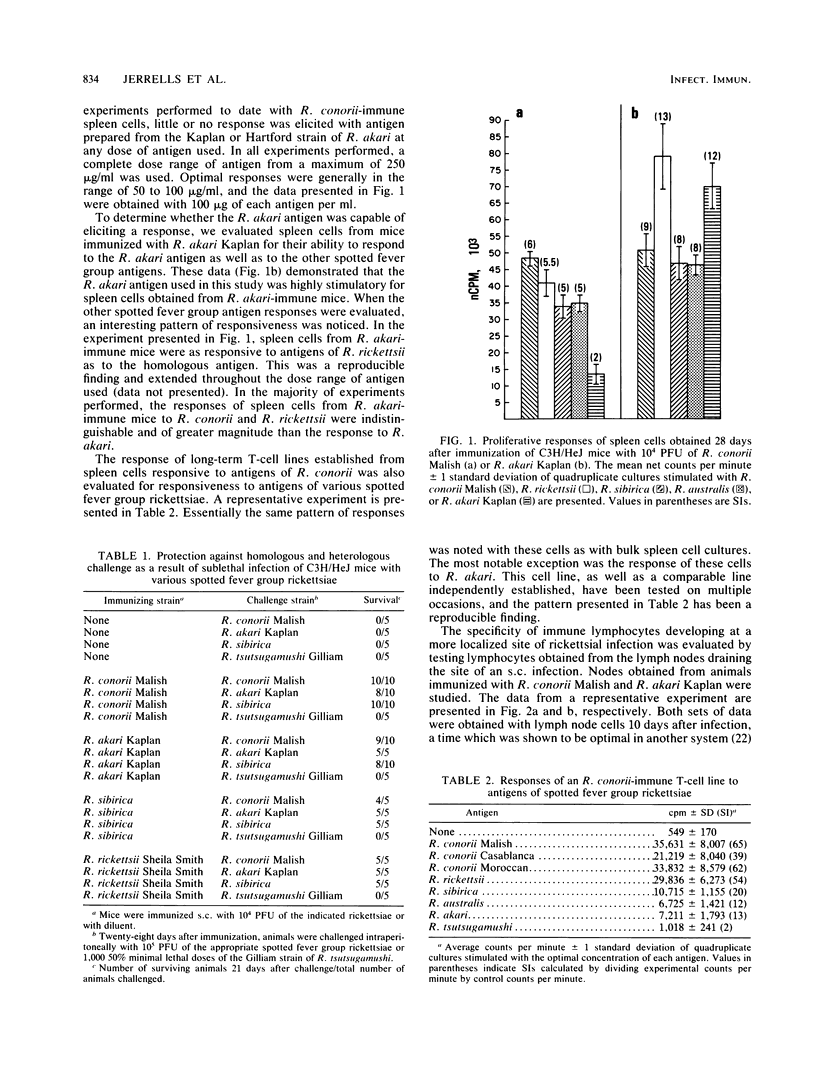
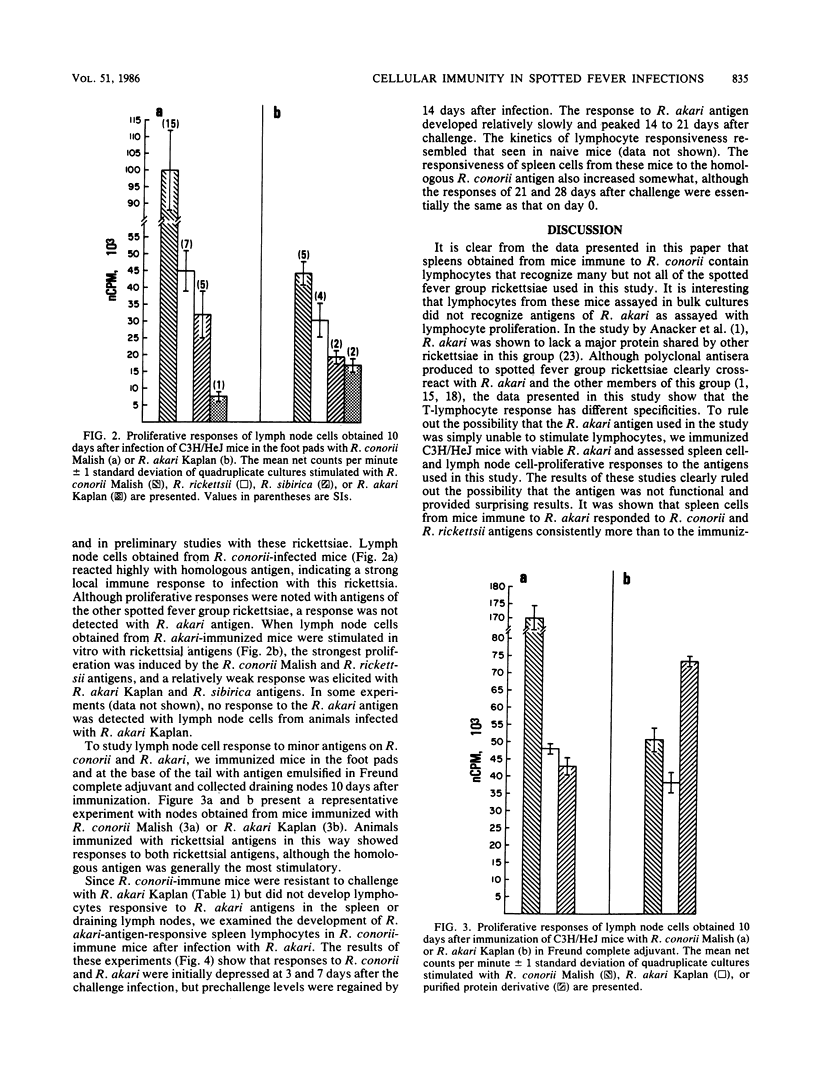
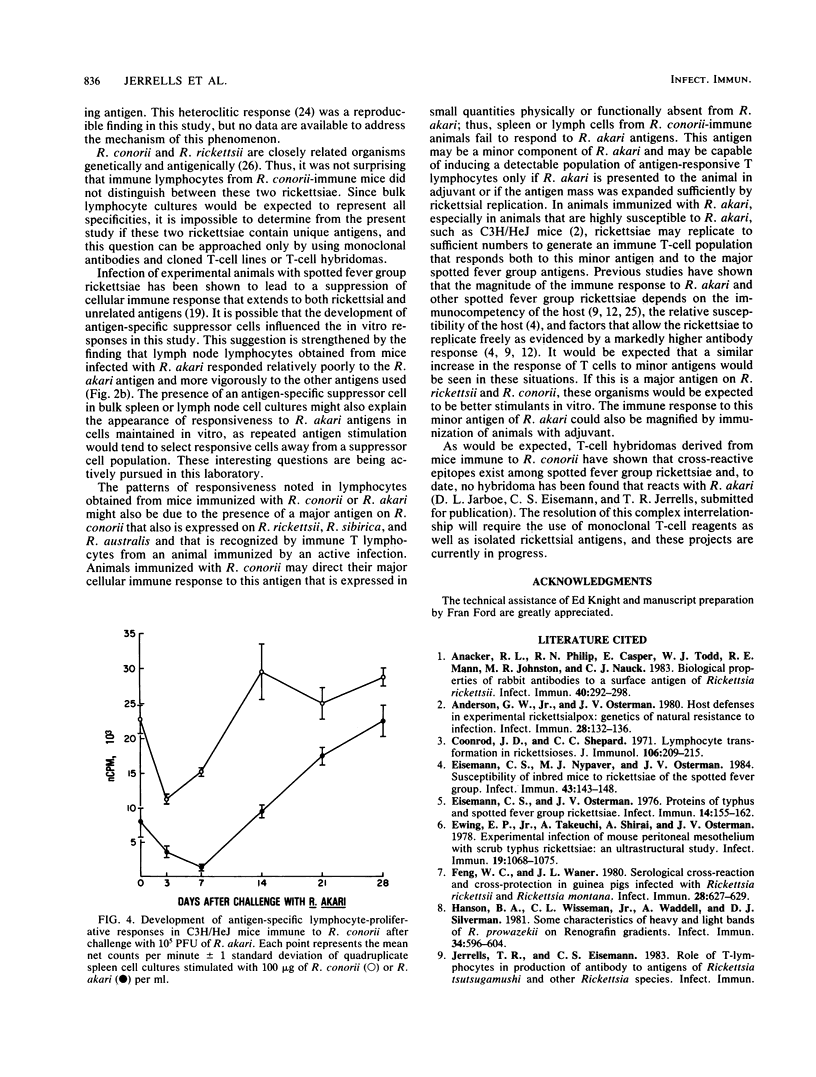
Lymphocyte proliferation in response to antigens on spotted fever group rickettsiae was used as a method to investigate the group-specific protective immunity to rechallenge characteristic of this group of rickettsiae at the T-cell receptor level. Spleen cells from Rickettsia conorii-immune C3H/HeJ mice proliferated in response to R. rickettsii Sheila Smith, R. sibirica 246, R. australis, and all tested strains of R. conorii (Casablanca, Moroccan, and Malish). Spleen cells from these mice, however, responded poorly or not at all to antigens prepared from the Kaplan or Hartford strain of R. akari. Proliferation of immune T cells maintained as in vitro cell lines showed a similar pattern of reactivity to these antigens; however, response to R. akari was consistently demonstrable. Spleen cells from C3H/HeJ mice immunized with R. akari responded to R. akari and R. conorii antigens as well as antigens from the other spotted fever group rickettsiae. Lymphocytes obtained from lymph nodes draining foot pads infected with R. conorii or R. akari demonstrated cross-reactivity similar to that found with immune spleen cells. If immunization was accomplished with R. conorii antigen emulsified in Freund complete adjuvant, the resulting lymph node cells were able to respond to R. akari antigens. These data suggest that infection with R. conorii induces a population of T lymphocytes that recognize an antigen(s) that also is found on other spotted fever rickettsiae and that may be responsible for cross-protective immunity. This antigen probably is not a major antigen on R. akari.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anacker R. L., Philip R. N., Casper E., Todd W. J., Mann R. E., Johnston M. R., Nauck C. J. Biological properties of rabbit antibodies to a surface antigen of Rickettsia rickettsii. Infect Immun. 1983 Apr;40(1):292–298. doi: 10.1128/iai.40.1.292-298.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson G. W., Jr, Osterman J. V. Host defenses in experimental rickettsialpox: genetics of natural resistance to infection. Infect Immun. 1980 Apr;28(1):132–136. doi: 10.1128/iai.28.1.132-136.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coonrod J. D., Shepard C. C. Lymphocyte transformation in rickettsioses. J Immunol. 1971 Jan;106(1):209–216. [PubMed] [Google Scholar]
- Eisemann C. S., Nypaver M. J., Osterman J. V. Susceptibility of inbred mice to rickettsiae of the spotted fever group. Infect Immun. 1984 Jan;43(1):143–148. doi: 10.1128/iai.43.1.143-148.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisemann C. S., Osterman J. V. Proteins of typhus and spotted fever group rickettsiae. Infect Immun. 1976 Jul;14(1):155–162. doi: 10.1128/iai.14.1.155-162.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing E. P., Jr, Takeuchi A., Shirai A., Osterman J. V. Experimental infection of mouse peritoneal mesothelium with scrub typhus rickettsiae: an ultrastructural study. Infect Immun. 1978 Mar;19(3):1068–1075. doi: 10.1128/iai.19.3.1068-1075.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W. C., Waner J. L. Serological cross-reaction and cross-protection in guinea pigs infected with Rickettsia rickettsii and Rickettsia montana. Infect Immun. 1980 May;28(2):627–629. doi: 10.1128/iai.28.2.627-629.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson B. A., Wisseman C. L., Jr, Waddell A., Silverman D. J. Some characteristics of heavy and light bands of Rickettsia prowazekii on Renografin gradients. Infect Immun. 1981 Nov;34(2):596–604. doi: 10.1128/iai.34.2.596-604.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrells T. R., Eisemann C. S. Role of T-lymphocytes in production of antibody to antigens of Rickettsia tsutsugamushi and other Rickettsia species. Infect Immun. 1983 Aug;41(2):666–674. doi: 10.1128/iai.41.2.666-674.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrells T. R., Osterman J. V. Development of specific and cross-reactive lymphocyte proliferative responses during chronic immunizing infections with Rickettsia tsutsugamushi. Infect Immun. 1983 Apr;40(1):147–156. doi: 10.1128/iai.40.1.147-156.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon R. H., Ascher M. S., Kishimoto R. A., Pedersen C. E., Jr In vitro guinea pig leukocyte reactions to Rickettsia rickettsii. Infect Immun. 1977 Dec;18(3):840–846. doi: 10.1128/iai.18.3.840-846.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon R. H., Pedersen C. E., Jr Immune responses to Rickettsia akari infection in congenitally athymic nude mice. Infect Immun. 1980 May;28(2):310–313. doi: 10.1128/iai.28.2.310-313.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimoto M., Fathman C. G. Antigen-reactive T cell clones. I. Transcomplementing hybrid I-A-region gene products function effectively in antigen presentation. J Exp Med. 1980 Oct 1;152(4):759–770. doi: 10.1084/jem.152.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokorin I. N., Kabanova E. A., Shirokova E. M., Abrosimova G. E., Rybkina N. N., Pushkareva V. i. Role of T lymphocytes in Rickettsia conorii infection. Acta Virol. 1982 Jan;26(1-2):91–97. [PubMed] [Google Scholar]
- Kokorin I. N., Kabanova E. A., Shirokova E. M. Role of macrophages in infection with Rickettsia conorii. Acta Virol. 1980 Mar;24(2):137–143. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Oaks S. C., Jr, Osterman J. V., Hetrick F. M. Plaque assay and cloning of scrub typhus rickettsiae in irradiated L-929 cells. J Clin Microbiol. 1977 Jul;6(1):76–80. doi: 10.1128/jcm.6.1.76-80.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormsbee R., Peacock M., Philip R., Casper E., Plorde J., Gabre-Kidan T., Wright L. Antigenic relationships between the typhus and spotted fever groups of rickettsiae. Am J Epidemiol. 1978 Jul;108(1):53–59. [PubMed] [Google Scholar]
- Oster C. N., Kenyon R. H., Pedersen C. E., Jr Suppression of cellular immune responses in guinea pigs infected with spotted fever group rickettsiae. Infect Immun. 1978 Nov;22(2):411–417. doi: 10.1128/iai.22.2.411-417.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterman J. V., Eisemann C. S. Rickettsial indirect hemagglutination test: isolation of erythrocyte-sensitizing substance. J Clin Microbiol. 1978 Aug;8(2):189–196. doi: 10.1128/jcm.8.2.189-196.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterman J. V., Eisemann C. S. Surface proteins of typhus and spotted fever group rickettsiae. Infect Immun. 1978 Sep;21(3):866–873. doi: 10.1128/iai.21.3.866-873.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer B. A., Hetrick F. M., Jerrells T. R. Gamma interferon production in response to homologous and heterologous strain antigens in mice chronically infected with Rickettsia tsutsugamushi. Infect Immun. 1984 Oct;46(1):237–244. doi: 10.1128/iai.46.1.237-244.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen C. E., Jr, Walters V. D. Comparative electrophoresis of spotted fever group rickettsial proteins. Life Sci. 1978 Feb;22(7):583–587. doi: 10.1016/0024-3205(78)90337-5. [DOI] [PubMed] [Google Scholar]
- Solinger A. M., Ultee M. E., Margoliash E., Schwartz R. H. T-lymphocyte response to cytochrome c. I. Demonstration of a T-cell heteroclitic proliferative response and identification of a topographic antigenic determinant on pigeon cytochrome c whose immune recognition requires two complementing major histocompatibility complex-linked immune response genes. J Exp Med. 1979 Oct 1;150(4):830–848. doi: 10.1084/jem.150.4.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. H., Henderson F. W. Effect of immunosuppression on Rickettsia rickettsii infection in guinea pigs. Infect Immun. 1978 Apr;20(1):221–227. doi: 10.1128/iai.20.1.221-227.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. H., Montenegro M. R., Hegarty B. C., Tringali G. R. Rocky Mountain spotted fever vaccine: a regional need. South Med J. 1984 Apr;77(4):447–449. doi: 10.1097/00007611-198404000-00009. [DOI] [PubMed] [Google Scholar]
- Weiss E., Coolbaugh J. C., Williams J. C. Separation of viable Rickettsia typhi from yolk sac and L cell host components by renografin density gradient centrifugation. Appl Microbiol. 1975 Sep;30(3):456–463. doi: 10.1128/am.30.3.456-463.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


