Abstract
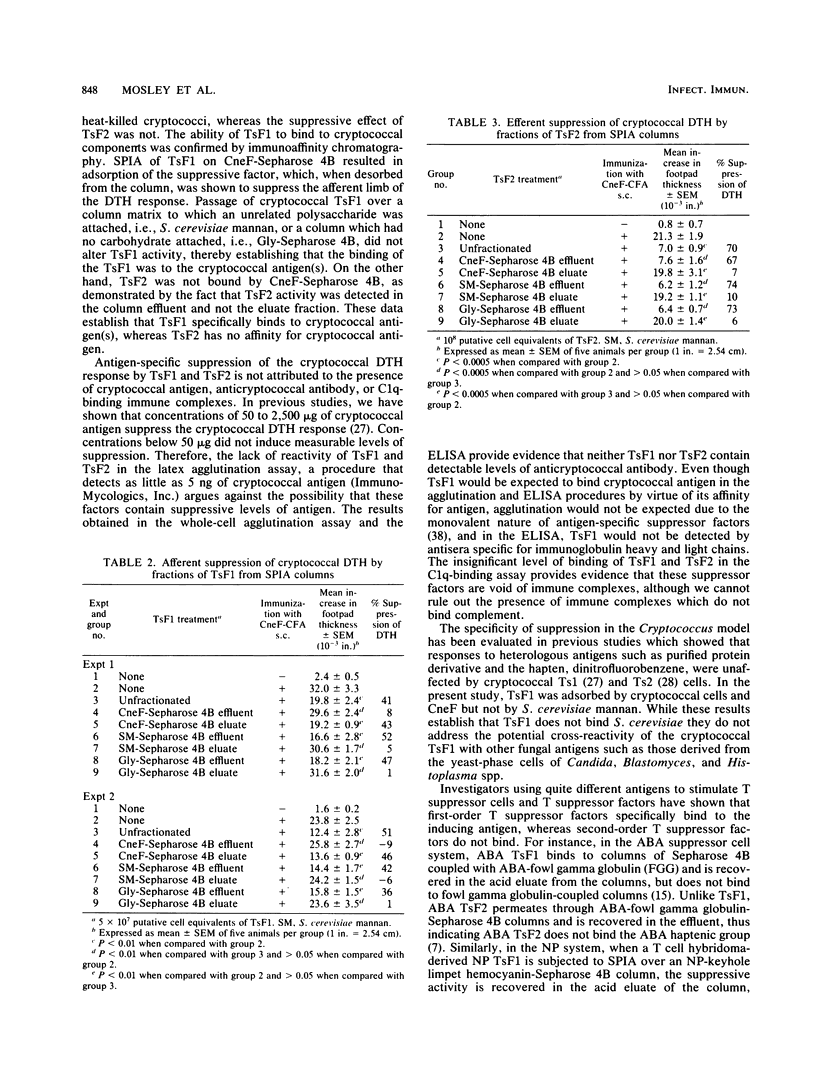
In the murine cryptococcal suppressor cell circuit, two different T-cell suppressor factors, TsF1 and TsF2, have been identified which specifically suppress the delayed-type hypersensitivity (DTH) response to cryptococcal culture filtrate antigen (CneF). TsF1 is produced by a first-order T suppressor (Ts1) cell population and suppresses the afferent limb of the DTH response, whereas TsF2 is produced by a second-order T suppressor (Ts2) cell population and suppresses the efferent limb of the cryptococcal DTH response. The objective of this study was to ascertain whether TsF1 or TsF2 could bind to cryptococcal antigen. To assess this, adsorption of TsF1 and TsF2 was performed with heat-killed Cryptococcus neoformans cells and by solid-phase immunoadsorption (SPIA) on columns containing cryptococcal antigens, i.e., CneF covalently bound to Sepharose 4B. The suppressive effect of TsF1 was removed by adsorption with intact heat-killed cryptococci and by SPIA on CneF-Sepharose 4B. The binding of cryptococcal TsF1 to the cryptococcal SPIA column was shown to be specific since Sepharose 4B columns either coupled with Saccharomyces cerevisiae mannan or blocked with glycine did not adsorb the suppressor activity. In contrast, the suppressive component of TsF2 did not bind to heat-killed cryptococci, CneF-Sepharose 4B, S. cerevisiae mannan-Sepharose 4B, or glycine-Sepharose 4B columns. These results, together with the finding that cryptococcal antigen, anticryptococcal antibody, and C1q-binding immune complexes were not demonstrated in either TsF1 or TsF2, establish that TsF1 and TsF2 can be differentiated on the basis of their affinity for cryptococcal antigen.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman A., Katz D. H. Production and isolation of helper and suppressor factors. J Immunol Methods. 1980;38(1-2):9–41. doi: 10.1016/0022-1759(80)90328-2. [DOI] [PubMed] [Google Scholar]
- Cauley L. K., Murphy J. W. Response of congenitally athymic (nude) and phenotypically normal mice to Cryptococcus neoformans infection. Infect Immun. 1979 Mar;23(3):644–651. doi: 10.1128/iai.23.3.644-651.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claman H. N., Miller S. D., Conlon P. J., Moorhead J. W. Control of experimental contact sensitivity. Adv Immunol. 1980;30:121–157. doi: 10.1016/s0065-2776(08)60195-9. [DOI] [PubMed] [Google Scholar]
- Deepe G. S., Jr, Watson S. R., Bullock W. E. Cellular origins and target cells of immunoregulatory factors in mice with disseminated histoplasmosis. J Immunol. 1984 Apr;132(4):2064–2071. [PubMed] [Google Scholar]
- Deepe G. S., Jr, Watson S. R., Bullock W. E. Generation of disparate immunoregulatory factors in two inbred strains of mice with disseminated histoplasmosis. J Immunol. 1982 Nov;129(5):2186–2191. [PubMed] [Google Scholar]
- Dietz M. H., Sy M. S., Benacerraf B., Nisonoff A., Greene M. I., Germain R. N. Antigen- and receptor-driven regulatory mechanisms. VII. H-2-restricted anti-idiotypic suppressor factor from efferent suppressor T cells. J Exp Med. 1981 Feb 1;153(2):450–463. doi: 10.1084/jem.153.2.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorf M. E., Benacerraf B. Suppressor cells and immunoregulation. Annu Rev Immunol. 1984;2:127–157. doi: 10.1146/annurev.iy.02.040184.001015. [DOI] [PubMed] [Google Scholar]
- Fresno M., Nabel G., McVay-Boudreau L., Furthmayer H., Cantor H. Antigen-specific T lymphocyte clones. I. Characterization of a T lymphocyte clone expressing antigen-specific suppressive activity. J Exp Med. 1981 May 1;153(5):1246–1259. doi: 10.1084/jem.153.5.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goren M. B., Middlebrook G. M. Protein conjugates of polysaccharide from Cryptococcus neoforman s. J Immunol. 1967 May;98(5):901–913. [PubMed] [Google Scholar]
- Gray B. M. ELISA methodology for polysaccharide antigens: protein coupling of polysaccharides for adsorption to plastic tubes. J Immunol Methods. 1979;28(1-2):187–192. doi: 10.1016/0022-1759(79)90340-5. [DOI] [PubMed] [Google Scholar]
- Greene M. I., Bach B. A., Benacerraf B. Mechanisms of regulation of cell-mediated immunity. III. The characterization of azobenzenearsonate-specific suppressor T-cell-derived-suppressor factors. J Exp Med. 1979 May 1;149(5):1069–1083. doi: 10.1084/jem.149.5.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene M. I., Nelles M. J., Sy M. S., Nisonoff A. Regulation of immunity to the azobenzenearsonate hapten. Adv Immunol. 1982;32:253–300. doi: 10.1016/s0065-2776(08)60723-3. [DOI] [PubMed] [Google Scholar]
- Greene M. I., Pierres A., Dorf M. E., Benacerraf B. The I-J subregion codes for determinats on suppressor factor(s) which limit the contact sensitivity response to picryl chloride. J Exp Med. 1977 Jul 1;146(1):293–296. doi: 10.1084/jem.146.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene M. I. The genetic and cellular basis of regulation of the immune response to tumor antigens. Contemp Top Immunobiol. 1980;11:81–116. doi: 10.1007/978-1-4684-3701-0_2. [DOI] [PubMed] [Google Scholar]
- Heusser C., Boesman M., Nordin J. H., Isliker H. Effect of chemical and enzymatic radioiodination on in vitro human Clq activities. J Immunol. 1973 Mar;110(3):820–828. [PubMed] [Google Scholar]
- Liew F. Y., Chan-Liew W. L. Regulation of delayed-type hypersensitivity. II. Specific suppressor factor for delayed-type hypersensitivity to sheep erythrocytes in mice. Eur J Immunol. 1978 Mar;8(3):168–171. doi: 10.1002/eji.1830080305. [DOI] [PubMed] [Google Scholar]
- Minami M., Okuda K., Furusawa S., Benacerraf B., Dorf M. E. Analysis of T cell hybridomas. I. Characterization of H-2 and Igh-restricted monoclonal suppressor factors. J Exp Med. 1981 Nov 1;154(5):1390–1402. doi: 10.1084/jem.154.5.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan M. A., Blackstock R. A., Bulmer G. S., Hall N. K. Modification of macrophage phagocytosis in murine cryptococcosis. Infect Immun. 1983 May;40(2):493–500. doi: 10.1128/iai.40.2.493-500.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W., Cozad G. C. Immunological unresponsiveness induced by cryptococcal capsular polysaccharide assayed by the hemolytic plaque technique. Infect Immun. 1972 Jun;5(6):896–901. doi: 10.1128/iai.5.6.896-901.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W. Effects of first-order Cryptococcus-specific T-suppressor cells on induction of cells responsible for delayed-type hypersensitivity. Infect Immun. 1985 May;48(2):439–445. doi: 10.1128/iai.48.2.439-445.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W., Gregory J. A., Larsh H. W. Skin testing of guinea pigs and footpad testing of mice with a new antigen for detecting delayed hypersensitivity to Cryptococcus neoformans. Infect Immun. 1974 Feb;9(2):404–409. doi: 10.1128/iai.9.2.404-409.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W., Moorhead J. W. Regulation of cell-mediated immunity in cryptococcosis. I. Induction of specific afferent T suppressor cells by cryptococcal antigen. J Immunol. 1982 Jan;128(1):276–283. [PubMed] [Google Scholar]
- Murphy J. W., Mosley R. L., Moorhead J. W. Regulation of cell-mediated immunity in cryptococcosis. II. Characterization of first-order T suppressor cells (Ts1) and induction of second-order suppressor cells. J Immunol. 1983 Jun;130(6):2876–2881. [PubMed] [Google Scholar]
- Murphy J. W., Mosley R. L. Regulation of cell-mediated immunity in cryptococcosis. III. Characterization of second-order T suppressor cells (Ts2). J Immunol. 1985 Jan;134(1):577–584. [PubMed] [Google Scholar]
- Okuda K., Minami M., Sherr D. H., Dorf M. E. Hapten-specific T cell responses to 4-hydroxy-3-nitrophenyl acetyl. XI. Pseudogenetic restrictions of hybridoma suppressor factors. J Exp Med. 1981 Aug 1;154(2):468–479. doi: 10.1084/jem.154.2.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich S. S., Rich R. R. Regulatory mechanisms in cell-mediated immune responses. II. A genetically restricted suppressor of mixed lymphocyte reactions released by alloantigen-activated spleen cells. J Exp Med. 1975 Dec 1;142(6):1391–1402. doi: 10.1084/jem.142.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich S. S., Rich R. R. Regulatory mechanisms in cell-mediated immune responses. IV. Expression of a receptor for mixed lymphocyte reaction suppressor factor on activated T lymphocytes. J Exp Med. 1976 Nov 2;144(5):1214–1226. doi: 10.1084/jem.144.5.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg L., Porath J. Preparation of adsorbents for biospecific affinity chromatography. Attachment of group-containing ligands to insoluble polymers by means of bifuctional oxiranes. J Chromatogr. 1974 Mar 13;90(1):87–98. doi: 10.1016/s0021-9673(01)94777-6. [DOI] [PubMed] [Google Scholar]
- Sy M. S., Dietz M. H., Germain R. N., Benacerraf B., Greene M. I. Antigen- and receptor-driven regulatory mechanisms. IV. Idiotype-bearing I-J+ suppressor T cell factors induce second-order suppressor T cells which express anti-idiotypic receptors. J Exp Med. 1980 May 1;151(5):1183–1195. doi: 10.1084/jem.151.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada T., Okumura K. The role of antigen-specific T cell factors in the immune response. Adv Immunol. 1979;28:1–87. doi: 10.1016/s0065-2776(08)60799-3. [DOI] [PubMed] [Google Scholar]
- Takei F., Levy J. G., Kilburn D. G. Characterization of a soluble factor that specifically suppresses the in vitro generation of cells cytotoxic for syngeneic tumor cells in mice. J Immunol. 1978 Apr;120(4):1218–1224. [PubMed] [Google Scholar]
- Webb D. R., Kapp J. A., Pierce C. W. The biochemistry of antigen-specific T-cell factors. Annu Rev Immunol. 1983;1:423–438. doi: 10.1146/annurev.iy.01.040183.002231. [DOI] [PubMed] [Google Scholar]
- Whitaker R. B., Nepom J. T., Sy M. S., Takaoki M., Gramm C. F., Fox I., Germain R. N., Nelles M. J., Greene M. I., Benacerraf B. Suppressor factor from a T cell hybrid inhibits delayed-type hypersensitivity responses to azobenzenearsonate. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6441–6445. doi: 10.1073/pnas.78.10.6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi K., Chao N., Murphy D. B., Gershon R. K. Molecular composition of an antigen-specific, Ly-1 T suppressor inducer factor. One molecule binds antigen and is I-J-; another is I-J+, does not bind antigen, and imparts an Igh-variable region-linked restriction. J Exp Med. 1982 Mar 1;155(3):655–665. doi: 10.1084/jem.155.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubler R. H., Lange G., Lambert P. H., Miescher P. A. Detection of immune complexes in unheated sera by modified 125I-Clq binding test. Effect of heating on the binding of Clq by immune complexes and application of the test to systemic lupus erythematosus. J Immunol. 1976 Jan;116(1):232–235. [PubMed] [Google Scholar]