Abstract
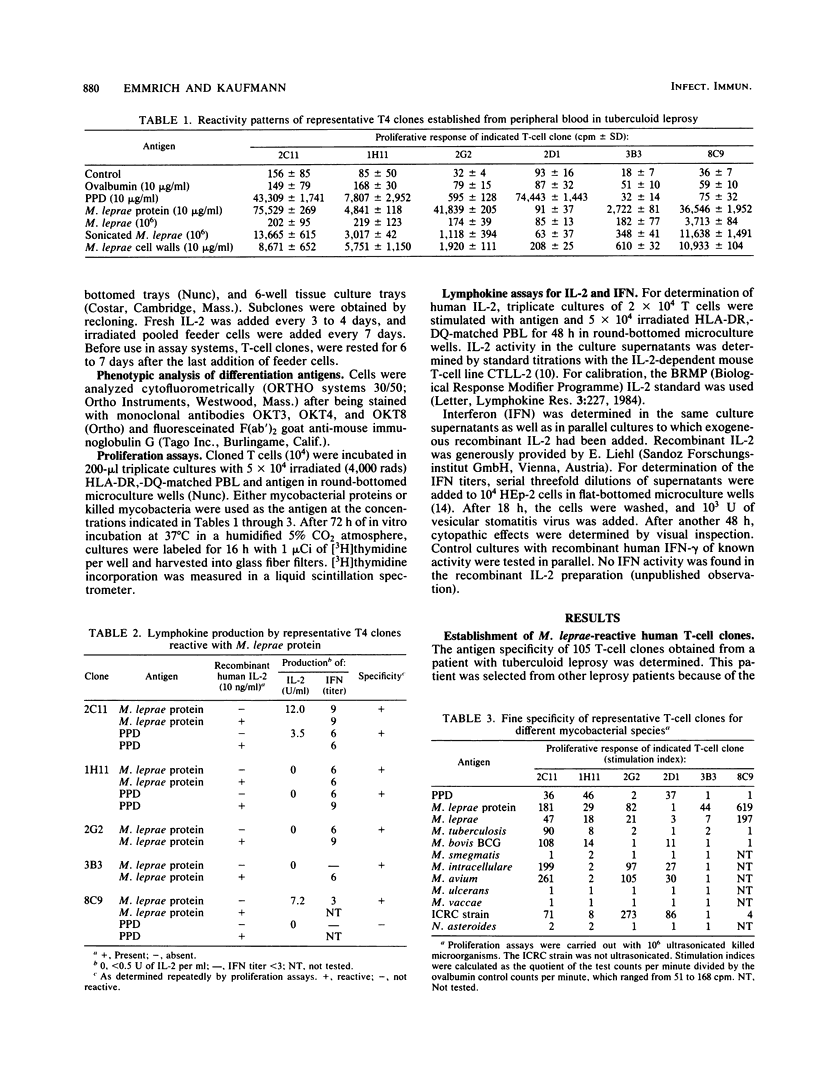
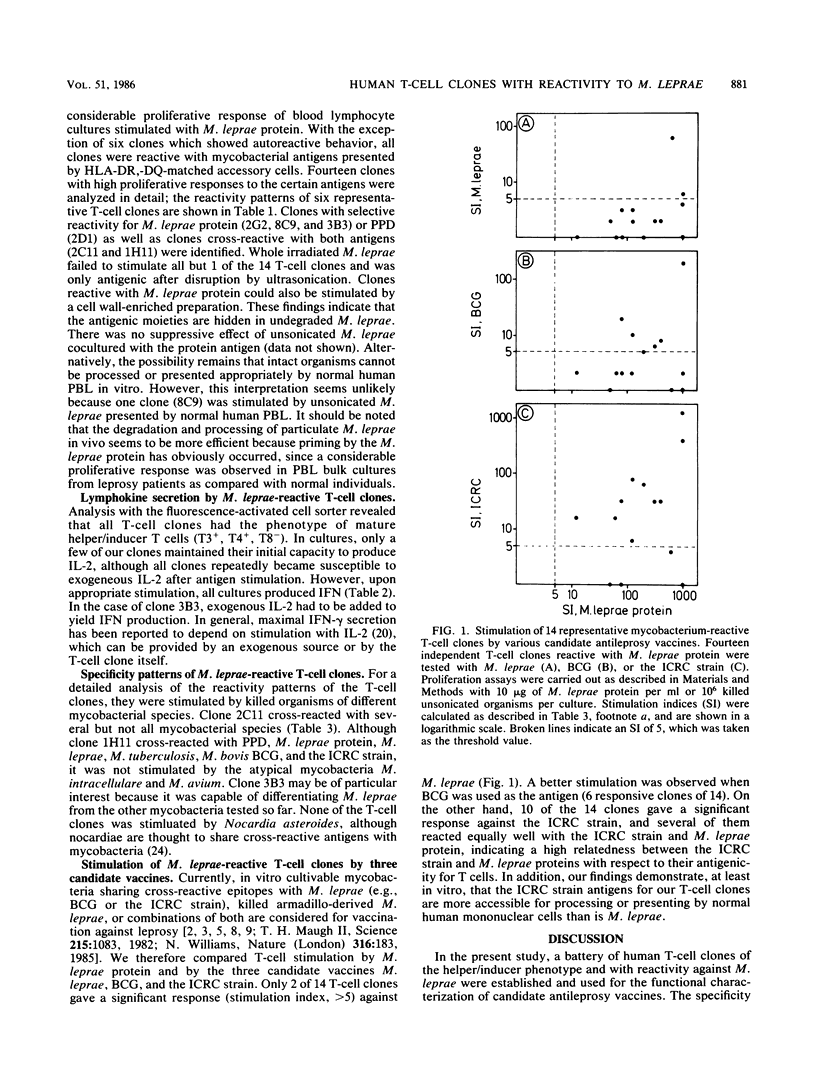
T-cell clones with the T4 phenotype were established from patients with tuberculoid leprosy. The antigen reactivity of these clones ranged from stringent specificity for Mycobacterium leprae to broad cross-reactivity with other mycobacteria. Killed M. leprae had a weak stimulatory capacity which could be enhanced by ultrasonication. Among the three candidate antileprosy vaccines, M. leprae, M. bovis BCG, and the ICRC (Indian Cancer Research Center) strain, the last was superior in stimulating cross-reactive T4 clones. This finding argues for a differential masking of similar or identical membrane antigens in various mycobacterial species. T-cell clones with defined reactivity patterns for mycobacterial antigens could be helpful tools for the characterization of an antileprosy vaccine.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bechelli L. M., Garbajosa P. G., Gyi M. M., Uemura K., Sundaresan T., Martínez Domínguez V., Matejka M., Tamondong C., Quagliato R., Engler V. BCG vaccination of children against leprosy: seven-year findings of the controlled WHO trial in Burma. Bull World Health Organ. 1973;48(3):323–334. [PMC free article] [PubMed] [Google Scholar]
- Bloom B. R., Godal T. Selective primary health care: strategies for control of disease in the developing world. V. Leprosy. Rev Infect Dis. 1983 Jul-Aug;5(4):765–780. doi: 10.1093/clinids/5.4.765. [DOI] [PubMed] [Google Scholar]
- Brown J. A., Stone M. M., Sutherland I. B.C.G. vaccination of children against leprosy in Uganda: results at end of second follow-up. Br Med J. 1968 Jan 6;1(5583):24–27. doi: 10.1136/bmj.1.5583.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Curtiss J. E., Jacobs W. R., Docherty M. A., Ritchie L. R., Curtiss R., 3rd Molecular analysis of DNA and construction of genomic libraries of Mycobacterium leprae. J Bacteriol. 1985 Mar;161(3):1093–1102. doi: 10.1128/jb.161.3.1093-1102.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Convit J., Aranzazu N., Ulrich M., Pinardi M. E., Reyes O., Alvarado J. Immunotherapy with a mixture of Mycobacterium leprae and BCG in different forms of leprosy and in Mitsuda-negative contacts. Int J Lepr Other Mycobact Dis. 1982 Dec;50(4):415–424. [PubMed] [Google Scholar]
- Deo M. G., Bapat C. V., Bhalerao V., Chaturvedi R. M., Bhatki W. S., Chulawala R. G. Antileprosy potentials of ICRC vaccine. A study in patients and healthy volunteers. Int J Lepr Other Mycobact Dis. 1983 Dec;51(4):540–549. [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Godal T., Myrvang B., Froland S. S., Shao J., Melaku G. Evidence that the mechanism of immunological tolerance ("central failure") is operative in the lack of host resistance in lepromatous leprosy. Scand J Immunol. 1972;1(4):311–321. doi: 10.1111/j.1365-3083.1972.tb03296.x. [DOI] [PubMed] [Google Scholar]
- Hahn H., Kaufmann S. H. The role of cell-mediated immunity in bacterial infections. Rev Infect Dis. 1981 Nov-Dec;3(6):1221–1250. doi: 10.1093/clinids/3.6.1221. [DOI] [PubMed] [Google Scholar]
- Haregewoin A., Godal T., Mustafa A. S., Belehu A., Yemaneberhan T. T-cell conditioned media reverse T-cell unresponsiveness in lepromatous leprosy. Nature. 1983 May 26;303(5915):342–344. doi: 10.1038/303342a0. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Brinkmann V. Attempts to characterize the T-cell population and lymphokine involved in the activation of macrophage oxygen metabolism in murine listeriosis. Cell Immunol. 1984 Oct 15;88(2):545–550. doi: 10.1016/0008-8749(84)90186-2. [DOI] [PubMed] [Google Scholar]
- Kirchheimer W. F., Sanchez R. M., Shannon E. J. Effect of specific vaccine on cell-mediated immunity of armadillos against M. leprae. Int J Lepr Other Mycobact Dis. 1978 Jul-Dec;46(3-4):353–357. [PubMed] [Google Scholar]
- Mehra V., Bloom B. R. Induction of cell-mediated immunity to Mycobacterium leprae in guinea pigs. Infect Immun. 1979 Mar;23(3):787–794. doi: 10.1128/iai.23.3.787-794.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra V., Brennan P. J., Rada E., Convit J., Bloom B. R. Lymphocyte suppression in leprosy induced by unique M. leprae glycolipid. Nature. 1984 Mar 8;308(5955):194–196. doi: 10.1038/308194a0. [DOI] [PubMed] [Google Scholar]
- Nogueira N., Kaplan G., Levy E., Sarno E. N., Kushner P., Granelli-Piperno A., Vieira L., Colomer Gould V., Levis W., Steinman R. Defective gamma interferon production in leprosy. Reversal with antigen and interleukin 2. J Exp Med. 1983 Dec 1;158(6):2165–2170. doi: 10.1084/jem.158.6.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel P. J., Lefford M. J. Induction of cell-mediated immunity to Mycobacterium leprae in mice. Infect Immun. 1978 Jan;19(1):87–93. doi: 10.1128/iai.19.1.87-93.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reem G. H., Yeh N. H. Interleukin 2 regulates expression of its receptor and synthesis of gamma interferon by human T lymphocytes. Science. 1984 Jul 27;225(4660):429–430. doi: 10.1126/science.6429853. [DOI] [PubMed] [Google Scholar]
- Schultz R. M., Kleinschmidt W. J. Functional identity between murine gamma interferon and macrophage activating factor. Nature. 1983 Sep 15;305(5931):239–240. doi: 10.1038/305239a0. [DOI] [PubMed] [Google Scholar]
- Shepard C. C., Walker L. L., van Landingham R. Heat stability of Mycobacterium leprae immunogenicity. Infect Immun. 1978 Oct;22(1):87–93. doi: 10.1128/iai.22.1.87-93.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. L., Mackett M., Moss B. Infectious vaccinia virus recombinants that express hepatitis B virus surface antigen. Nature. 1983 Apr 7;302(5908):490–495. doi: 10.1038/302490a0. [DOI] [PubMed] [Google Scholar]
- Van Voorhis W. C., Kaplan G., Sarno E. N., Horwitz M. A., Steinman R. M., Levis W. R., Nogueira N., Hair L. S., Gattass C. R., Arrick B. A. The cutaneous infiltrates of leprosy: cellular characteristics and the predominant T-cell phenotypes. N Engl J Med. 1982 Dec 23;307(26):1593–1597. doi: 10.1056/NEJM198212233072601. [DOI] [PubMed] [Google Scholar]
- Young R. A., Mehra V., Sweetser D., Buchanan T., Clark-Curtiss J., Davis R. W., Bloom B. R. Genes for the major protein antigens of the leprosy parasite Mycobacterium leprae. Nature. 1985 Aug 1;316(6027):450–452. doi: 10.1038/316450a0. [DOI] [PubMed] [Google Scholar]


