Abstract
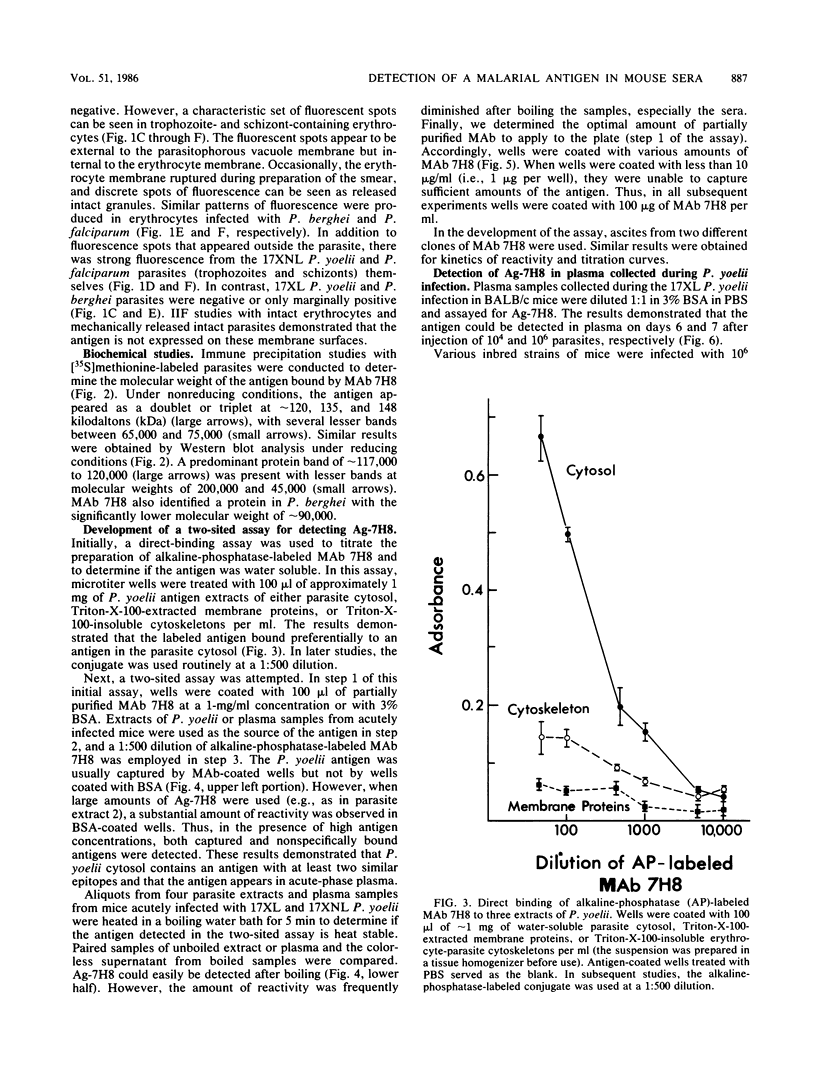
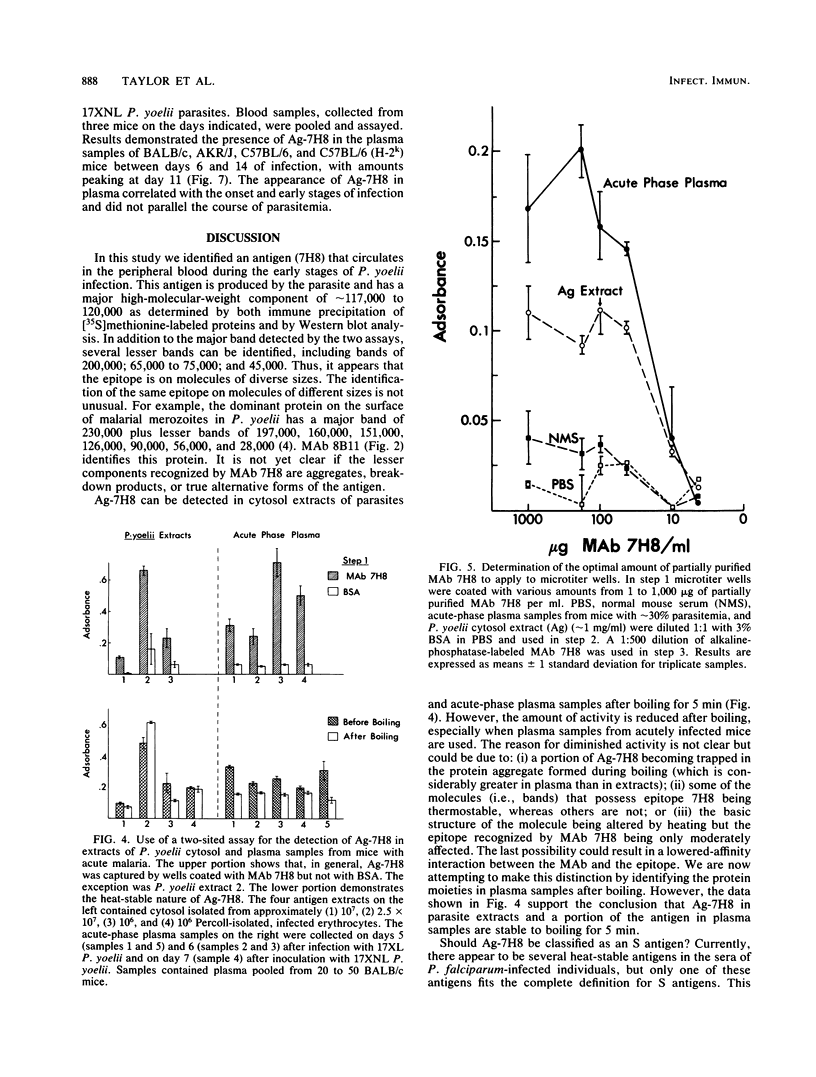
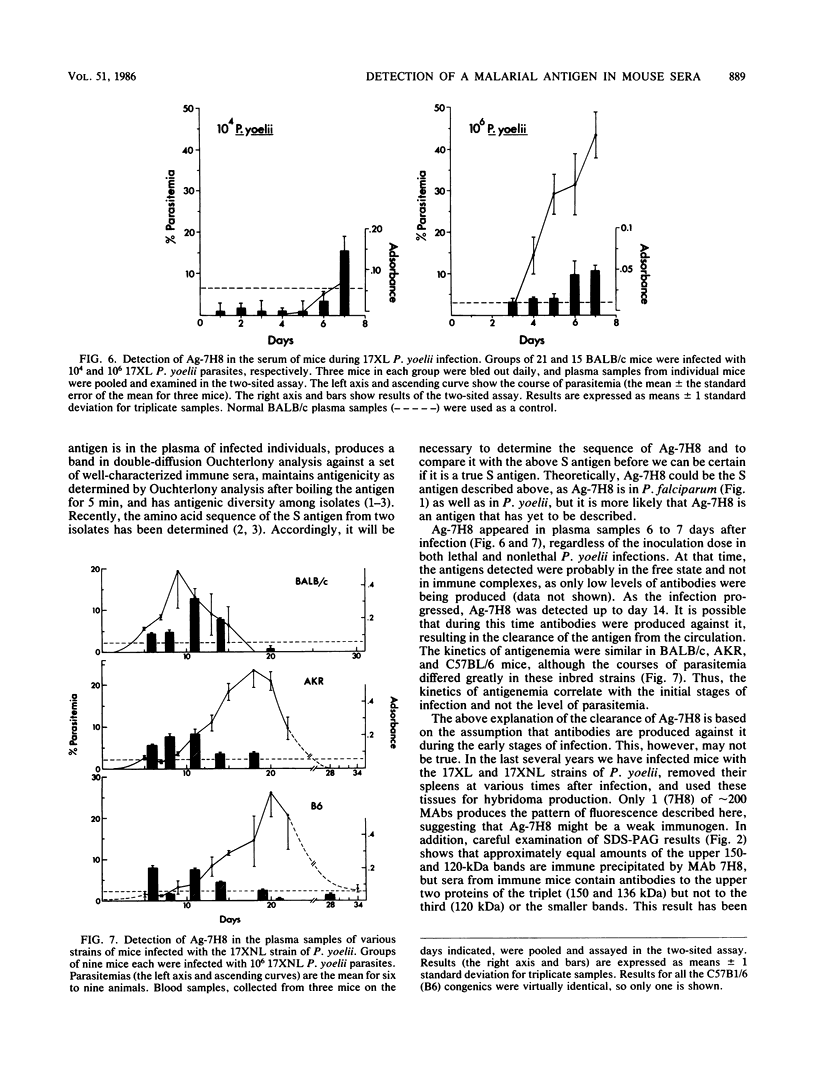
Antigens, circulating in the blood during malarial infections, have been implicated in immune protection, immunosuppression, and immune-complex formation. We used a monoclonal antibody (MAb 7H8) to identify an antigen (Ag-7H8) in the sera of mice infected with Plasmodium yoelii. The major form of the antigen has a molecular weight of approximately 120,000 in P. yoelii, with minor components of 220,000; 65,000 to 75,000; and 45,000. Ag-7H8 remains antigenic after boiling for 5 min. A two-sited assay was developed with MAb 7H8 that demonstrated that the Ag-7H8 has at least two similar epitopes per molecule. The two-sited assay was used to follow Ag-7H8 in the blood of mice during lethal (strain 17XL) and nonlethal (strain 17XNL) P. yoelii infections. Ag-7H8 appeared on days 6 and 7 after infection with 10(6) and 10(4) 17XL P. yoelii parasites, respectively, and remained until the animals died. It was in plasma samples between days 6 and 14 after 17XNL P. yoelii injections in several inbred strains of mice, regardless of the course of parasitemia. Thus, the kinetics of antigenemia correspond with early stages of infection and not with the number of circulating parasites. Indirect immunofluorescence assays demonstrated that MAb 7H8 detects a cross-reactive antigen in other malarial parasites, including Plasmodium berghei and Plasmodium falciparum. Thus, this two-sited assay may have general application for the serodiagnosis of malaria and may be beneficial in determining the relationship of circulating antigens to malarial immunity.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anders R. F., Brown G. V., Edwards A. Characterization of an S antigen synthesized by several isolates of Plasmodium falciparum. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6652–6656. doi: 10.1073/pnas.80.21.6652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppel R. L., Cowman A. F., Lingelbach K. R., Brown G. V., Saint R. B., Kemp D. J., Anders R. F. Isolate-specific S-antigen of Plasmodium falciparum contains a repeated sequence of eleven amino acids. Nature. 1983 Dec 22;306(5945):751–756. doi: 10.1038/306751a0. [DOI] [PubMed] [Google Scholar]
- Cowman A. F., Saint R. B., Coppel R. L., Brown G. V., Anders R. F., Kemp D. J. Conserved sequences flank variable tandem repeats in two S-antigen genes of Plasmodium falciparum. Cell. 1985 Apr;40(4):775–783. doi: 10.1016/0092-8674(85)90337-x. [DOI] [PubMed] [Google Scholar]
- Holder A. A., Freeman R. R. Immunization against blood-stage rodent malaria using purified parasite antigens. Nature. 1981 Nov 26;294(5839):361–364. doi: 10.1038/294361a0. [DOI] [PubMed] [Google Scholar]
- Houba V. Immunopathology of nephropathies associated with malaria. Bull World Health Organ. 1975;52(2):199–207. [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McGregor I. A. Immunology of malarial infection and its possible consequences. Br Med Bull. 1972 Jan;28(1):22–27. doi: 10.1093/oxfordjournals.bmb.a070886. [DOI] [PubMed] [Google Scholar]
- McGregor I. A., Turner M. W., Williams K., Hall P. Soluble antigens in the blood of African patients with severe plasmodium falciparum malaria. Lancet. 1968 Apr 27;1(7548):881–884. doi: 10.1016/s0140-6736(68)90237-7. [DOI] [PubMed] [Google Scholar]
- Saul A., Myler P., Schofield L., Kidson C. A high molecular weight antigen in Plasmodium falciparum recognized by inhibitory monoclonal antibodies. Parasite Immunol. 1984 Jan;6(1):39–50. doi: 10.1111/j.1365-3024.1984.tb00780.x. [DOI] [PubMed] [Google Scholar]
- Taylor D. W., Kim K. J., Munoz P. A., Evans C. B., Asofsky R. Monoclonal antibodies to stage-specific, species-specific, and cross-reactive antigens of the rodent malarial parasite, Plasmodium yoelii. Infect Immun. 1981 May;32(2):563–570. doi: 10.1128/iai.32.2.563-570.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. W., Munoz P. A., Kim K. J., Evans C. B., Asofsky R. Plasmodium yoelii: comparison of indirect immunofluorescence and radioimmunoassay for detecting monoclonal antibodies to malaria. Exp Parasitol. 1982 Jun;53(3):362–370. doi: 10.1016/0014-4894(82)90079-0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOLLER A. FLUORESCENT ANTIBODY METHODS AND THEIR USE IN MALARIA RESEARCH. Bull World Health Organ. 1964;30:343–354. [PMC free article] [PubMed] [Google Scholar]
- Wilson R. J., McGregor I. A., Hall P., Williams K., Bartholomew R. Antigens associated with Plasmodium falciparum infections in man. Lancet. 1969 Jul 26;2(7613):201–205. doi: 10.1016/s0140-6736(69)91437-8. [DOI] [PubMed] [Google Scholar]
- Wilson R. J. Serotyping Plasmodium falciparum malaria with S-antigens. Nature. 1980 Apr 3;284(5755):451–452. doi: 10.1038/284451a0. [DOI] [PubMed] [Google Scholar]
- Winchell E. J., Ling I. T., Wilson R. J. Metabolic labelling and characterisation of S-antigens, the heat-stable, strain-specific antigens of Plasmodium falciparum. Mol Biochem Parasitol. 1984 Mar;10(3):287–296. doi: 10.1016/0166-6851(84)90027-6. [DOI] [PubMed] [Google Scholar]