Abstract
It is generally believed that dividing cells gain complex features of differentiation only after exiting the cell cycle because cell division and differentiation are both under such tight regulation that their coexistence is deemed unlikely. As the major proliferating cell type in the mammalian CNS, NG2 glial cells (NG2 cells) account for 5–8% of the glial cell population and form synaptic contacts with neurons. Here we report that NG2 cells divide while maintaining their differentiation, including morphological features, such as the elaboration of multiple complex cellular processes and physiological features including active glutamatergic and GABAergic synaptic responses. Not only do NG2 cells continue to receive excitatory and inhibitory synaptic inputs as they undergo mitosis, a subpopulation of dividing NG2 cells can fire action potentials upon depolarization, thereby revealing that these dividing NG2 cells retain voltage-gated ion channels as well as transmitter receptors for signal processing. These findings provide a clear counterexample of the widely perceived incompatibility between cell division and differentiation.
Keywords: action potential, differentiation, NG2 cells, proliferation, mitosis
Glial cells constitute 90% of the cells in our brain and have been classified as astrocytes, oligodendrocytes, and microglia (1). NG2 glial cells (NG2 cells), previously known as “protoplasmic” astrocytes on the basis of their morphology in the adult CNS (2), have also been described as oligodendrocyte-type-2 astrocytes (O2A) (3), oligodendrocyte precursor cells (4), “complex glia” (5), or polydendrocytes (6). NG2 cells are the only glial cells that express the chondroitin sulfate proteoglycan NG2, which not only provides the namesake but also serves as a cell marker for NG2 cells (2, 7). Whereas some NG2-expressing cells can generate oligodendrocytes and astrocytes at early developmental stages in vivo (8), there are many NG2 cells in the adult brain after the completion of myelination (4). Moreover, NG2 expression in the adult brain does not overlap with the expression of markers for other glial cell types (9) nor do NG2 cells express immature cell markers including nestin and DCX. Importantly, NG2 cells receive synaptic inputs from neurons (10–14), and the neuron-NG2 glia synapses can undergo activity-dependent modification analogous to long-term potentiation (11), a hallmark of the cellular mechanism underlying learning and memory (15). NG2 cells also make contact with nodes of Ranvier (16) in the white matter and hence are capable of responding to or modulating neuronal activity in a manner perhaps similar to the function of astrocytes (17). Given the unique morphological, physiological, and functional properties of NG2 cells, which are widely distributed in the adult brain, they are now recognized as a distinct, differentiated, macroglial cell population (18, 19).
NG2 cells retain the ability to proliferate throughout life (9). They represent the main population of dividing cells and account for ≈70% of BrdU incorporating cells in the adult CNS (9, 20). The propensity for cell division of NG2 cells, which display highly differentiated morphological and electrophysiological features that are suggestive of functional significance in the adult brain, presents an opportunity to examine whether any of the features of NG2 cell differentiation is retained during cell division.
The generally perceived incompatibility between differentiation and proliferation has recently been challenged. Ajioka et al. (21) found that cancer cells derived from horizontal interneurons via inactivation of Rb and p130 proliferate while maintaining neurites and synapses characteristic of differentiated neurons, thus revealing that, at least under pathological conditions, proliferating cells may remain differentiated. To test whether proliferation could be compatible with differentiation of normal cell types, we examined NG2 cells in healthy brains of adult rodents and tested the hypothesis that these cells can enter the cell cycle while maintaining their highly differentiated features, including complex cell morphology and synaptic inputs.
Results
NG2 Cells Divide While Retaining Differentiated Morphology.
NG2 cells in the rodent brain are known to retain the ability to proliferate throughout the postnatal life (4). Indeed, when cell proliferation was examined by BrdU incorporation in mouse brains double-labeled with the antibody against BrdU and antibody specific for NG2 cells, we found BrdU+NG2+ cells in 3-week-old to 5-month-old mice (Fig. 1). BrdU+NG2+ cells reside in many brain regions (Fig. 1 A–D). Of 164 BrdU+ cells examined in non-subventricular zone (non-SVZ) regions from P25 brain, 95.7% (157 of 164) of these BrdU+ cells were NG2+. However, there were few BrdU+NG2+ cells in SVZ, a region known for neurogenesis and gliogenesis (22, 23). Of 63 BrdU+ cells examined in the SVZ region (within ≈30 μm from the edge), only one was NG2+ (1.6%). Additionally, there were only 4 BrdU+NG2+ cells located within 50 μm from the edge bordering the ventricle (Fig. 1 D and E). Thus, NG2 cells likely account for the majority of proliferating cells outside the SVZ in the postnatal brain.
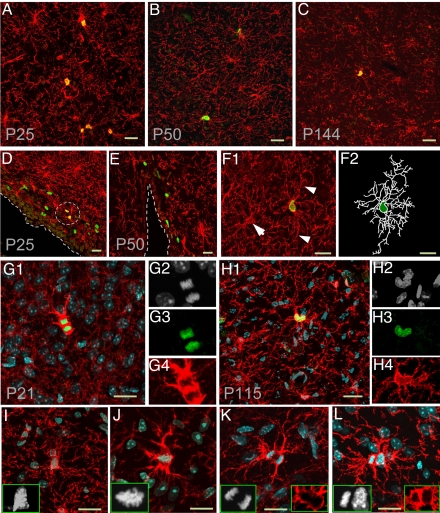
Fig. 1.
Dividing NG2 cells with differentiated morphology in adult brains. (A–C) Immunostaining with antibodies against NG2 (red) and BrdU (green) in cerebral cortex from P25 (A), P50 (B), and P144 (C) mice. (D and E) Immunostaining with antibodies against NG2 (red) and BrdU (green) in SVZ region from P25 (D) and P50 (E) mouse brains. NG2+BrdU+ cells are circled. (F) Example micrograph showing the differentiated morphology of 1 NG2+BrdU+ cell from a P50 mouse brain 2 h after BrdU pulse-labeling. Arrowheads point at 2 long processes of the BrdU+ cell, and the arrow points at a BrdU− cells. The processes and cell body of the dividing NG2 cell were outlined with soft Neurolucida (F2). (G and H) Immunostaining with antibodies against NG2 (red) and Ki67 (green) in the cortex of P21 (G1–G4) and P115 (H1–H4) mouse brain, respectively. Nuclei of dividing cells were strongly labeled by DAPI (white, G2 and H2) and Ki67 (G3 and H3). The Ki67+ cells in telophase (G1) and prophase (H1) both bear multiple processes. (I--L) Four dividing NG2 cells (red) labeled by DAPI (white) and anti-NG2 (red) in different mitotic stages: prometaphase (I), metaphase (J), anaphase (K), and telophase (L). Note the difference between the shape and density of DAPI signal in dividing cells and nondividing cells (I–L). Note NG2 expression was up-regulated during mitosis (G, J, and K). All scale bars are 20 μm.
The NG2 cell morphological differentiation is characterized by the presence of multiple thin, branched processes extending radically from the cell body (Fig. S1). What happens to these processes when a NG2 cell enters the cell cycle? To address this question, we used BrdU to label dividing cells. BrdU is incorporated into newly synthesized DNA during the S phase of the cell cycle and is included in the DNA thereafter. Thus, after BrdU pulse labeling, the BrdU+ cells include those cells in S, G2, and M phase each with a single BrdU+ nucleus, and the daughter cells shortly after exit of the cell cycle, thus giving the appearance of pairs of adjacent BrdU+ nuclei (24). We found that almost all of the BrdU+NG2+ cells appeared as isolated individual cells with multiprocess morphology 2 h after the first BrdU injection (98.9%, 90 of 91 cells) (Fig. 1F), whereas pairs of double-positive cells (NG2+BrdU+) were very common 8 h after pulse labeling, with one-third of BrdU+ cells [36.0%, 58 of 161; postnatal days 25 and 50 (P25 and P50)] appearing as pairs of double-positive (NG2+BrdU+) cells with similar morphological differentiation and a short distance between their nuclei (Fig. S2). Most of the pairs of double-positive cells examined displayed similar morphological differentiation (NG2+BrdU+, n = 30) (Fig. S2), and none exhibited the bipolar/unipolar morphology characteristic of NG2-cell divisions induced by injury (25). This result suggests that NG2 cells are likely to maintain their multiple-branched processes during cell division.
To examine the cellular morphology during mitosis, we used DAPI to mark the DNA, thereby rendering those cells at metaphase (Fig. 1J Inset) easily distinguishable from cells at telophase (Fig. 1 G2 and L). These mitotic NG2 cells maintained the multiple processes (Fig. 1 I–L). Extensive morphological differentiation was also evident in dividing cells triple-labeled with DAPI, antibody specific for NG2, and antibody against the proliferating cell nuclear protein Ki67 (Fig. 1 G–H). These observations indicate that NG2 cells can divide while exhibiting their differentiated morphology.
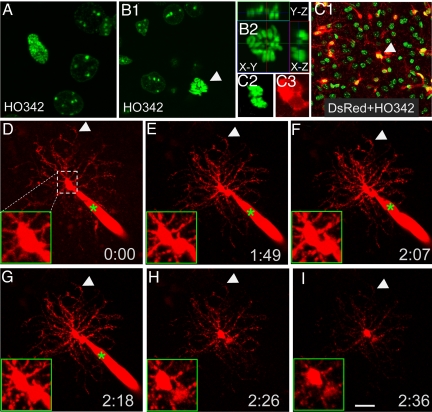
To determine whether NG2 cells can maintain their complex processes during division rather than having to retract then regrow them, we used time-lapse microscopy to monitor the division of a cell loaded via the whole-cell patch-clamp electrode with Alexa Fluor 568/594 dyes, which allowed visualization of very thin processes emanating from the cell body in the acute brain slice (26). NG2 cells can be identified based on their distinct electrophysiological properties as described previously (11, 26). In some of the experiments (see Figs. 3–5), NG2 cells were identified by their red fluorescence in the NG2DsRedBAC transgenic mice generated by the Nishiyama lab (8) (Fig. 2C). To label the dividing nuclei, acute brain slices were incubated with Hoechst 33342 (HO342), a cell-permeable DNA-binding dye (Fig. 2 A and B). Alexa Fluor 568/594 dyes were then loaded into the dividing cells exhibiting NG2 cell electrophysiological features. Whereas BrdU incorporation studies revealed that only one-third of the dividing cells would yield 2 separate daughter cells within 8 h, one example was captured during time lapse imaging for over 2 h: The mother cell divided into 2 daughter cells, with the soma of the mother cell splitting into 2, while all of the processes remained unretracted (Fig. 2 D–I). This result strongly supports the idea that NG2 cells can retain their differentiated morphology during mitosis.
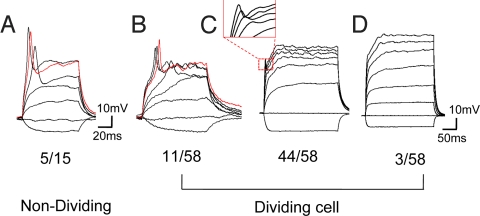
Fig. 3.
Dividing NG2 cells with action potentials. (A) An example of nondividing cells (5 of 15 cells) in visual cortex with all-or-none action potential. (B) An example of dividing cells (11 of 58 cells) with all-or-none action potential. (C) An example of dividing NG2 cells (44 of 58 cells) with graded spikes that have greater amplitudes at stronger depolarization. (D) An example of dividing NG2 cells (3 of 58 cells) without any spike even when the cell was depolarized to 0 mV.
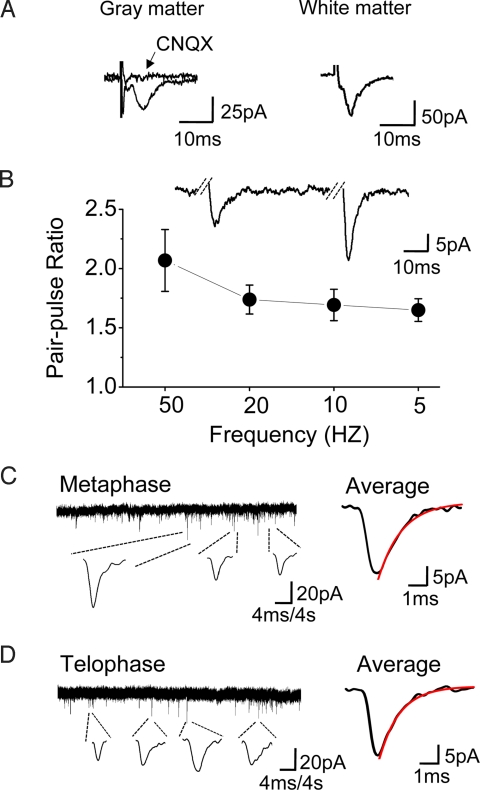
Fig. 4.
Dividing NG2 cells with functional synapses. (A) Evoked synaptic currents recorded from the dividing cells in gray matter (Left) and white matter (Right). (Left) The synaptic response was blocked by AMPAR antagonist CNQX (10 μM). (B) PPF in dividing NG2 cells at various frequency (n = 3–5 cells for each condition). (Inset) An example trace of pair-pulse at 20 Hz. (C and D) Spontaneous postsynaptic currents recorded in two dividing cells (low Cl−, 15 mM). Continuous traces represent the current recorded from the cells in metaphase (C) and telophase (D), respectively. Traces below show example responses in higher magnification. The average of individual responses in Left is shown in Right (C, n = 18; D, n = 13). The red line is a single exponential fit to the decay. C, rise time (10–90%), 0.80 ms; τ decay (90–37%), 1.50 ms; D, rise time, 0.70 ms; τ decay, 1.30 ms. All of the recordings were from a holding potential of −60 mV.
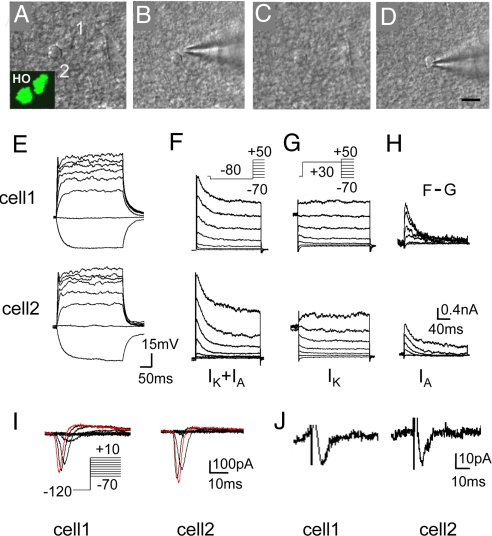
Fig. 5.
NG2 cells divide symmetrically. (A–D) Example micrograph of the procedure for recording from 2 daughter cells at telophase. (Inset) HO342 signal of daughter cells. (Scale bar, 10 μm.) (E–H) Superimposed membrane potential changes (E) in 2 daughter NG2 cells of late-telophase (current injection, 300 ms, 40 pA steps from −40 to +240 pA). (F and G) Superimposed current recordings from a typical dividing NG2 cell under voltage clamp at different voltages (200 ms, 20 mV steps from −70 to +50 mV) preceded by a prepulse conditioning potential of −80 mV (F) or +20 mV (G) for 200 ms. Note that with a prepulse of −80 mV, an initial transient outward currents (IA) followed by a sustained outward current (IK) was recorded (F), whereas only IK could be recorded with a prepulse of +20 mV that inactivates IA (G). (H) Isolated IA by subtracting F from G. (I and J) An example of 2 daughter cells in late-telophase both exhibiting sodium currents (I) and synaptic inputs (J) with similar amplitude. Superimposed membrane currents (I) recorded in the presence of K+ channel blockers at different voltages (100 ms, 10 mV steps from −70 to +10 mV) preceded by a prepulse conditioning potential of −120 mV for 200 ms.
Fig. 2.
Monitering NG2 cell division while retaining differentiated morphology. (A and B) The dividing cells in prophase (A) and metaphase (B1 and B2) labeled by HO342 in acute brain slices. (B2) 9.6 μm Z stack with orthogonal views at the point of dividing nucleus. (C1–C3) A dividing cell of telophase (C2) in an acute slice from a P13 NG2DsRedBac transgenic mouse. (C2) HO342. (C3) DsRed. (B and C) Arrows point at the dividing cells. (D–I) Time-lapse recording of a dividing cell (red) filled with Alexa Fluor 568 from recording pipette (green star). Note the long multiple processes that remained unretracted during dividing. (D–I) Arrows indicate 1 process remaining at the same place during division. (D–I, Insets) Higher magnification views of the somatic region of the cell.(Scale bar, 20 μm.)
A Subpopulation of Dividing NG2 Cells Can Generate Action Potentials.
Action potential has long been considered the unique feature of neurons and other excitable cells, such as cardiac myocytes, although one class of NG2 cells can generate action potentials (27, 28). In the visual cortex, we found that one-third of the nondividing NG2 cells (5 of 15 cells, P9–P11) can fire action potentials (Fig. 3A). To test whether dividing NG2 cells retain such electrophysiological properties, we performed whole-cell patch-clamp recordings of dividing NG2 cells in acute brain slices. Interestingly, 18.9% of the dividing cells (11 of 58, among them 2 cells in metaphase, 1 cell in anaphase, and 8 cells in telophase) generated all-or-none action potentials when depolarized to −30 mV (threshold: −30.6 ± 1.9 mV, n = 8) (Fig. 3B). The threshold was higher than that in neurons (−46.6 ± 0.6 mV, n = 5, from cortex). Most of the other dividing cells (75.9%, 44 of 58) also generated spikes, which grew in size as the depolarization was gradually increased [spike amplitude at different membrane potential (Vm): 0.46 ± 0.17 mV at Vm = −30 mV, n = 39; 3.35 ± 0.64 mV at Vm = −20 mV, n = 37; 8.09 ± 0.96 mV at Vm = −10 mV, n = 33; and 14.0 ± 1.58 mV at Vm = 0 mV, n = 2] (Fig. 3C); although different from the all-or-none feature of action potentials, these graded spikes nonetheless are indicative of the presence of voltage-gated ion channels. Only a small fraction of the dividing NG2 cells (5.2%, 3 of 58) didn't produce any spike, even when they were depolarized to 0 mV (Fig. 3D). Having found that some of the dividing NG2 cells are able to fire action potentials, we went on to ask whether dividing NG2 cells that have retained their morphological differentiation could also respond to synaptic inputs with changes in membrane potential.
Dividing NG2 Cells Receive Functional Synaptic Inputs.
NG2 cells in several brain regions receive synaptic inputs from neurons (10–14). To determine whether dividing NG2 cells still respond to synaptic inputs, we recorded dividing NG2 cells while stimulating nerve fibers ≈150 μm from the dividing cell via a bipolar stimulating electrode. In the presence of the GABAA receptor antagonist bicuculline (10 μM), evoked excitatory postsynaptic currents (EPSCs) were detected in ≈86% (12 of 14) of the dividing NG2 cells. The EPSCs were eliminated by the AMPA receptor (AMPAR) antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) (10 μM, n = 3) (Fig. 4A). Dividing NG2 cells also received synaptic inputs in the white matter, such as the corpus callosum (n = 5) (Fig. 4A). Analogous to the paired pulse facilitation (PPF) of EPSCs observed in neurons, dividing NG2 cells demonstrated synaptic facilitation that varied with the frequency of stimulation (5–50 Hz) (Fig. 4B), comparable to the PPF of nondividing NG2 cells in the hippocampus (11).
Given that both glutamatergic and GABAergic synapses have been found in nondividing NG2 cells (29), the attempt was made to record GABAergic synaptic responses by using high KCl-based recording solutions ([Cl−]o = 130 mM; ECl− = ≈1 mV). In the presence of CNQX (10 μM), GABAergic responses were observed in dividing cells (n = 3) and could be blocked by bicuculline (10 μM) (data not shown), in agreement with a recent report (30).
Remarkably, spontaneous synaptic responses could be recorded in some dividing NG2 cells (9.9%, 8 of 81 cells; [Cl−]o = 15 mM, ECl− = −55mV). Spontaneous postsynaptic currents were observed at different mitotic stages of NG2 cells including prometaphase (n = 1), metaphase (n = 4), and telophase (n = 3). The currents had rapid rise and decay time (606 ± 56 μs to rise from 10% to 90% peak amplitude; decay time constant, 1.34 ± 0.18 ms; average peak amplitude, 25.3 ± 2.1 pA, n = 8 cells) (Fig. 4 C and D). The kinetics was very similar to those of EPSCs in nondividing NG2 cells (10, 11), indicating that the responses were mediated by glutamatergic synapses formed before cell division. These results demonstrate that dividing NG2 cells maintain functional synapses during mitosis.
A Subpopulation of NG2 Cells Divide Symmetrically.
NG2 cells can divide asymmetrically after injury or while these cells are maintained in a culture (25, 31). To examine the pattern of NG2 cell division in brain slices, the morphological and electrophysiological properties of both daughter cells were compared at or shortly after late-telophase/cytokinesis. After 8 h of pulse-labeling with BrdU, about one-third of the dividing NG2 cells appeared as pairs of daughter cells (n = 58/161). These pairs of BrdU+NG2+ cells shared similar morphology with respect to the size and the number of processes (Fig. 1L, Fig. S2, and Table S1). Moreover, whole-cell recordings of 2 daughter cells emerging from a dividing cell in telophase (Fig. 5 A–D) revealed that the 2 daughter cells showed comparable potassium currents (n = 9 pairs) (Fig. 5 F–H and Table S1), sodium currents (n = 2 pairs) (Fig. 5I), and synaptic inputs (n = 2 pairs) (Fig. 5J). To demonstrate that the 2 daughter cells are fully separated, we verified the completion of cytokinesis by showing that the dye loaded into one daughter cell did not reach the other daughter cell (Fig. S3). These observations strongly suggest that NG2 cells primarily divide symmetrically in vivo.
Discussion
This study demonstrates that, under normal condition in the brain from 9-day to 20 week-old adult mice, highly differentiated glial cells with multiple, branched processes and functional synapses can enter the cell cycle and undergo cell division without losing these features of differentiation. Moreover, dividing NG2 cells still maintain their intrinsic excitability during proliferation. The study further shows that NG2 cells can divide symmetrically yielding 2 daughter cells with similar electrophysiological properties. A recent report (30) documented similar symmetric division of NG2 cells while exhibiting inhibitory synaptic responses in mice 7–12 days after birth. Whereas this phenomenon in young mice suggests that maintaining synaptic inputs during division may facilitate colonization and myelination of the developing brain (30), this study shows that NG2 cells in the adult brain still retain morphological and physiological differentiation during mitosis. These findings provide a counterexample to the notion that cell division is incompatible with differentiation.
It is generally believed that cell division and differentiation are not mutually compatible because differentiated cells typically undergo apoptosis when they are forced to enter the cell cycle (32). Before differentiated cells reenter the cell cycle, they typically undergo cellular, molecular, and morphological dedifferentiation (33). However, recent studies reveal that differentiation and proliferation are compatible in cancer cells derived from misregulated interneurons (21), in NG2 cells in the developing brain (30), as well as NG2 cells in the adult brain, as shown in our study. This surprising finding has interesting implications in health and in disease.
Gliomas are thought to arise from either preexisting neural stem cells in the adult brain or proliferating cells that are dedifferentiated from glial cell types, such as astrocytes or oligodendrocytes due to oncogenic mutations (34). It remains to be elucidated as to how abnormalities in the cell cycle or other cellular processes might trigger glial transformation for gliomagenesis. This study's finding that NG2 cells proliferate while retaining their differentiated properties raises the question of whether these cells may be transformed to oligodendrogliomas or astrocytomas without dedifferentiation because they lose control of cell cycle regulation.
Synaptic Input Function in NG2 Cell Proliferation.
The electrical activity is important for immature NG2 cell proliferation during development (35) because neurons can release growth factors including neuregulins (NRGs), which are responsible for the proliferation of NG2 cells (36). After transection of developing optic nerves or intraocular injection of tetrodotoxin (TTX) to block neuronal activity, proliferation of NG2 cells decrease by 90% (35). Maintenance of differentiated properties including synaptic connections with neurons during division allows NG2 cells to continuously sense neuronal signals, which may be important for NG2 cells to retain the proliferation capability throughout life. Synaptic connections between neurons and NG2 cells have been found in both gray matter and white matter (10–14); however, direct evidence on the functional significance of synaptic inputs to NG2 cells is still lacking. Consistent with the presence of calcium-permeable GluR2-lacking AMPARs in NG2 cells (10, 11), activation of the AMPARs mediates calcium influx during synaptic transmission or glutamate application (11, 17). Calcium elevation plays a critical role in the long-term synaptic plasticity of synapses formed between neurons and glia (11) and in glial proliferation, growth, and differentiation (37). In this study, we found that the great majority of dividing NG2 cells display some form of excitability indicative of functional voltage-gated ion channels, with ≈20% of these dividing NG2 cells firing all-or-none action potentials on depolarization. With depolarization induced by synaptic activation, the dividing NG2 cells may fire action potentials in vivo (28), resulting in activation of voltage-gated calcium channels. Calcium influx from AMPARs or voltage-gated calcium channels may contribute to signaling, including the regulation of gene expression that controls the proliferation of NG2 cells.
Symmetric Versus Asymmetric NG2 Cell Division.
There are 2 types of cell division: symmetric and asymmetric (38). During asymmetric division, the 2 daughter cells acquire a different fate. NG2 cells can divide symmetrically or asymmetrically in vitro (31, 39). According to experiments involving retroviral infection, BrdU tracing, or a Pdgfra-CreER(T2)/Rosa26-YFP double-transgenic line, a small proportion of cycling NG2 cells can give rise to oligodendrocytes over a period of 10 days to 8 months (9, 40, 41). This observation suggests that a low percentage of proliferating NG2 cells undergo asymmetric division in vivo, although more direct evidence is still lacking for NG2 cell asymmetrical division in the normal adult brain.
In our 8-h BrdU tracing experiment, we did not find any pair of BrdU-containing NG2 cells with unipolar or bipolar morphology as described in the NG2 cell division model under injury (25). We found that the 2 daughter cells in cytokinesis share similar morphology and similar electrophysiological properties, including sodium current (INa), transient potassium current (IA), delay rectifying potassium current (IK), and synaptic responses. Due to the technical difficulty of this experiment, we obtained only limited data from 11 pairs of daughter cells. Hence, we cannot exclude the possibility that a low percentage of dividing cells undergo asymmetric division. Because we could only record from the daughter cells right after their exit from the cell cycle, we also cannot be certain whether these daughter cells will change their properties later.
Methods
BrdU Tracing and Immunocytochemistry.
For BrdU labeling, mice were injected i.p. with BrdU at 100 μg per gram of body weight (Sigma) twice at 0 and 1 h and killed by perfusion with 4% paraformaldehyde in 1× PBS at 2 h or 8 h after the first BrdU injection. Brains were cut into 15- to 60-μm-think sections with a cryostat (model CM3050S; Leica). To detect BrdU, sections were pretreated with 2 M HCl at 37 °C for 30 min and neutralized in 0.1 M sodium borate buffer, pH 8.5. Sections were permeabilized with 0.5% Triton X-100 in 1× PBS and then blocked by 5% BSA (Sigma) and 3% normal goat serum (Jackson ImmunoResearch) with 0.2% Triton X-100 in 1× PBS. Primary antibodies for BrdU (1:200, monoclonal; Roche), NG2 (1:300, Rabbit anti-NG2, ployclonal; Millipore), or Ki67 (1:200, mouse anti-Ki67; BD PharMingen) were diluted with 0.2% Triton X-100 in 1× PBS and applied to sections, alone or in combination, and allowed to incubate for 48 h at 4 °C. Secondary antibodies conjugated with Alexa Fluor 568 or 488 (1:800; Invitrogen) were used. DAPI was introduced to stain the nuclei. Sections were mounted on slides and covered with coverslips by using the antifade mounting medium Fluoromount G (Southern Biotech).
Slice Preparation.
Slices were prepared as described previously (11, 42). The use and care of animals follows the guidelines of the Institutional Animal Care and Use Committee at University of California, San Francisco. In brief, mice (C57BL/6; P9–P18, except otherwise noted) were anesthetized with isoflurane (1%–5%). After decapitation, the whole brain was dissected rapidly and placed in an ice-cold oxygenated (95% O2 and 5% CO2) solution containing 119 mM NaCl, 2.5 mM KCl, 2.5 mM CaCl2, 1.3 mM MgSO4, 1 mM NaH2Po4, 26.2 mM NaHCO3, and 11 mM glucose. Transverse slices (300 μm thick) were cut with a vibratome (model VT-1000S; Leica) and maintained in an incubation chamber for at least 1 h at room temperature (RT; 22–25°C) before loading or recording. For K+ current recordings, 1 μM TTX was added to the above solution. Solution for Na+ current recording (only in Fig. 5F) contained 89 mM NaCl, 10 mM CsCl, 2.5 mM KCl, 2.5 mM CaCl2, 1.3 mM MgCl2, 1 mM NaH2Po4, 20 mM tetraethylammonium chloride, 5 mM 4-aminopyridine, 1 mM BaCl2, 26.2 mM NaHCO3, and 11 mM glucose.
Electrophysiology and Live Cell Nucleus Labeling.
Whole-cell patch recordings from cells in the brain slice were carried out with the aid of markers to identify NG2 cells and dividing cells. NG2 cells were identified by loading Alexa Fluor 568/594 with recording pipette as previously described (11, 26). Sometimes, NG2 cells were identified by DsRed fluorescence by using NG2DsRedBAC mice as described (8). For nuclei labeling, slices were incubated with HO342 (Invitrogen) diluted to 2 μg/ml in articifical cerebrospinal fluid at RT for 30 min before recording and imaging. Recording pipettes were routinely filled with a solution containing 125 mM potassium gluconate, 15 mM KCl, 10 mM Hepes, 3 mM MgATP, 0.3 mM NaGTP, 5 mM sodium phosphocreatine, and 0.2 mM EGTA (pH 7.2–7.4, 290–300 mOsm). For INa recordings and synaptic currents in Fig. 5, pipette solution contained 125 mM cesium gluconate, 5 mM CsCl, 10 mM Hepes, 3 mM MgATP, 0.3 mM NaGTP, 0.2 mM EGTA, 5 mM sodium phosphocreatine, 10 mM tetraethylammonium chloride, and 2 mM BaCl2 (pH 7.2–7.4, 290–300 mOsm). The membrane potential was held at −60 mV for dividing NG2 cells (except otherwise noted). Current pulses (20–60 μA, 100 μS, 0.05 Hz) were applied through extracellular bipolar electrodes (Micro Probe Inc.) placed at ≈150 μm from the cell being recorded to induce synaptic currents.
Time-lapse Imaging of the Dividing Cells.
Video recording of glial cells loaded with 100 μM Alexa Fluor 568/594 (Molecular Probes) through the recording pipette was obtained via a custom-made, 2-photon laser scanning microscopy. The details are described in previous studies (26). Cells were scanned with XYZ mode (1-μm Z step for each optical slice). Images were projected under the maximum through-depth contrast protocol in Image-Pro Plus5.0 (Media Cybernetics). Three-dimensional reconstruction was carried out by using 3D Constructor, a plug-in toolbox for IPP5.0.
Supplementary Material
Acknowledgments.
We thank Dr. Akiko Nishiyama (Department of Physiology and Neurobiology, University of Connecticut) for providing the NG2DsRedBAC transgenic line; F. Huang, L.-J. Wu, W. Huang, and Y.-q. Zhu for critical reading of the manuscript; Y. Xiang, R. Yang, and B. Ye for critical comments on this work; S.-B. Yang for assistance with confocal imaging and genotyping; and Dr. S. Duan and members from Jan and Duan laboratories for valuable discussions. This work was supported by a National Institute of Mental Health grant, National Natural Science Foundation of China Grants 30700215 and 60025514, and National High-Tech Research and Development Program of China 863 Program 2006AA02Z343. Woo-Ping Ge was the recipient of a long-term fellowship of Human Frontier Science Program. Lily Yeh Jan and Yuh Nung Jan are Howard Hughes Medical Institute investigators.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0811353106/DCSupplemental.
References
- 1.Haydon PG. GLIA: Listening and talking to the synapse. Nat Rev Neurosci. 2001;2:185–193. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- 2.Levine JM, Card JP. Light and electron microscopic localization of a cell surface antigen (NG2) in the rat cerebellum: Association with smooth protoplasmic astrocytes. J Neurosci. 1987;7:2711–2720. doi: 10.1523/JNEUROSCI.07-09-02711.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raff MC, Miller RH, Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983;303:390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- 4.Levine JM, Reynolds R, Fawcett JW. The oligodendrocyte precursor cell in health and disease. Trends Neurosci. 2001;24:39–47. doi: 10.1016/s0166-2236(00)01691-x. [DOI] [PubMed] [Google Scholar]
- 5.Schools GP, Zhou M, Kimelberg HK. Electrophysiologically “complex” glial cells freshly isolated from the hippocampus are immunopositive for the chondroitin sulfate proteoglycan NG2. J Neurosci Res. 2003;73:765–777. doi: 10.1002/jnr.10680. [DOI] [PubMed] [Google Scholar]
- 6.Nishiyama A, Watanabe M, Yang Z, Bu J. Identity, distribution, and development of polydendrocytes: NG2-expressing glial cells. J Neurocytol. 2002;31:437–455. doi: 10.1023/a:1025783412651. [DOI] [PubMed] [Google Scholar]
- 7.Stallcup WB. The NG2 antigen, a putative lineage marker: immunofluorescent localization in primary cultures of rat brain. Dev Biol. 1981;83:154–165. doi: 10.1016/s0012-1606(81)80018-8. [DOI] [PubMed] [Google Scholar]
- 8.Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development. 2008;135:145–157. doi: 10.1242/dev.004895. [DOI] [PubMed] [Google Scholar]
- 9.Dawson MR, Polito A, Levine JM, Reynolds R. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci. 2003;24:476–488. doi: 10.1016/s1044-7431(03)00210-0. [DOI] [PubMed] [Google Scholar]
- 10.Bergles DE, Roberts JD, Somogyi P, Jahr CE. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature. 2000;405:187–191. doi: 10.1038/35012083. [DOI] [PubMed] [Google Scholar]
- 11.Ge WP, et al. Long-term potentiation of neuron-glia synapses mediated by Ca2+-permeable AMPA receptors. Science. 2006;312:1533–1537. doi: 10.1126/science.1124669. [DOI] [PubMed] [Google Scholar]
- 12.Ziskin JL, Nishiyama A, Rubio M, Fukaya M, Bergles DE. Vesicular release of glutamate from unmyelinated axons in white matter. Nat Neurosci. 2007;10:321–330. doi: 10.1038/nn1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kukley M, Capetillo-Zarate E, Dietrich D. Vesicular glutamate release from axons in white matter. Nat Neurosci. 2007;10:311–320. doi: 10.1038/nn1850. [DOI] [PubMed] [Google Scholar]
- 14.Mangin JM, Kunze A, Chittajallu R, Gallo V. Satellite NG2 progenitor cells share common glutamatergic inputs with associated interneurons in the mouse dentate gyrus. J Neurosci. 2008;28:7610–7623. doi: 10.1523/JNEUROSCI.1355-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bliss TV, Collingridge GL. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 16.Butt AM, et al. Cells expressing the NG2 antigen contact nodes of Ranvier in adult CNS white matter. Glia. 1999;26:84–91. [PubMed] [Google Scholar]
- 17.Wigley R, Hamilton N, Nishiyama A, Kirchhoff F, Butt AM. Morphological and physiological interactions of NG2-glia with astrocytes and neurons. J Anat. 2007;210:661–670. doi: 10.1111/j.1469-7580.2007.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butt AM, Hamilton N, Hubbard P, Pugh M, Ibrahim M. Synantocytes: The fifth element. J Anat. 2005;207:695–706. doi: 10.1111/j.1469-7580.2005.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishiyama A. Polydendrocytes: NG2 cells with many roles in development and repair of the CNS. Neuroscientist. 2007;13:62–76. doi: 10.1177/1073858406295586. [DOI] [PubMed] [Google Scholar]
- 20.Horner PJ, et al. Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J Neurosci. 2000;20:2218–2228. doi: 10.1523/JNEUROSCI.20-06-02218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ajioka I, et al. Differentiated horizontal interneurons clonally expand to form metastatic retinoblastoma in mice. Cell. 2007;131:378–390. doi: 10.1016/j.cell.2007.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levison SW, Goldman JE. Both oligodendrocytes and astrocytes develop from progenitors in the subventricular zone of postnatal rat forebrain. Neuron. 1993;10:201–212. doi: 10.1016/0896-6273(93)90311-e. [DOI] [PubMed] [Google Scholar]
- 23.Alvarez-Buylla A, Garcia-Verdugo JM. Neurogenesis in adult subventricular zone. J Neurosci. 2002;22:629–634. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polito A, Reynolds R. NG2-expressing cells as oligodendrocyte progenitors in the normal and demyelinated adult central nervous system. J Anat. 2005;207:707–716. doi: 10.1111/j.1469-7580.2005.00454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dawson MR, Levine JM, Reynolds R. NG2-expressing cells in the central nervous system: are they oligodendroglial progenitors? J Neurosci Res. 2000;61:471–479. doi: 10.1002/1097-4547(20000901)61:5<471::AID-JNR1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 26.Zhou W, Ge WP, Zeng S, Duan S, Luo Q. Identification and two-photon imaging of oligodendrocyte in CA1 region of hippocampal slices. Biochem Biophys Res Commun. 2007;352:598–602. doi: 10.1016/j.bbrc.2006.11.048. [DOI] [PubMed] [Google Scholar]
- 27.Chittajallu R, Aguirre A, Gallo V. NG2-positive cells in the mouse white and grey matter display distinct physiological properties. J Physiol. 2004;561:109–122. doi: 10.1113/jphysiol.2004.074252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Káradóttir R, Hamilton NB, Bakiri Y, Attwell D. Spiking and nonspiking classes of oligodendrocyte precursor glia in CNS white matter. Nat Neurosci. 2008;11:450–456. doi: 10.1038/nn2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin SC, Bergles DE. Synaptic signaling between GABAergic interneurons and oligodendrocyte precursor cells in the hippocampus. Nat Neurosci. 2004;7:24–32. doi: 10.1038/nn1162. [DOI] [PubMed] [Google Scholar]
- 30.Kukley M, et al. Glial cells are born with synapses. FASEB J. 2008;22:2957–2969. doi: 10.1096/fj.07-090985. [DOI] [PubMed] [Google Scholar]
- 31.Wren D, Wolswijk G, Noble M. In vitro analysis of the origin and maintenance of O-2Aadult progenitor cells. J Cell Biol. 1992;116:167–176. doi: 10.1083/jcb.116.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Y, Geldmacher DS, Herrup K. DNA replication precedes neuronal cell death in Alzheimer's disease. J Neurosci. 2001;21:2661–2668. doi: 10.1523/JNEUROSCI.21-08-02661.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fischer AJ, McGuire CR, Dierks BD, Reh TA. Insulin and fibroblast growth factor 2 activate a neurogenic program in Muller glia of the chicken retina. J Neurosci. 2002;22:9387–9398. doi: 10.1523/JNEUROSCI.22-21-09387.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ichimura K, Schmidt EE, Goike HM, Collins VP. Human glioblastomas with no alterations of the CDKN2A (p16INK4A, MTS1) and CDK4 genes have frequent mutations of the retinoblastoma gene. Oncogene. 1996;13:1065–1072. [PubMed] [Google Scholar]
- 35.Barres BA, Raff MC. Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature. 1993;361:258–260. doi: 10.1038/361258a0. [DOI] [PubMed] [Google Scholar]
- 36.Canoll PD, et al. GGF/neuregulin is a neuronal signal that promotes the proliferation and survival and inhibits the differentiation of oligodendrocyte progenitors. Neuron. 1996;17:229–243. doi: 10.1016/s0896-6273(00)80155-5. [DOI] [PubMed] [Google Scholar]
- 37.Verkhratsky A, Orkand RK, Kettenmann H. Glial calcium: Homeostasis and signaling function. Physiol Rev. 1998;78:99–141. doi: 10.1152/physrev.1998.78.1.99. [DOI] [PubMed] [Google Scholar]
- 38.Jan YN, Jan LY. Asymmetric cell division. Nature. 1998;392:775–778. doi: 10.1038/33854. [DOI] [PubMed] [Google Scholar]
- 39.Temple S, Raff MC. Clonal analysis of oligodendrocyte development in culture: evidence for a developmental clock that counts cell divisions. Cell. 1986;44:773–779. doi: 10.1016/0092-8674(86)90843-3. [DOI] [PubMed] [Google Scholar]
- 40.Levison SW, Young GM, Goldman JE. Cycling cells in the adult rat neocortex preferentially generate oligodendroglia. J Neurosci Res. 1999;57:435–446. [PubMed] [Google Scholar]
- 41.Rivers LE, et al. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci. 2008;11:1392–1401. doi: 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ge WP, Duan S. Persistent enhancement of neuron-glia signaling mediated by increased extracellular K+ accompanying long-term synaptic potentiation. J Neurophysiol. 2007;97:2564–2569. doi: 10.1152/jn.00146.2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.