Abstract
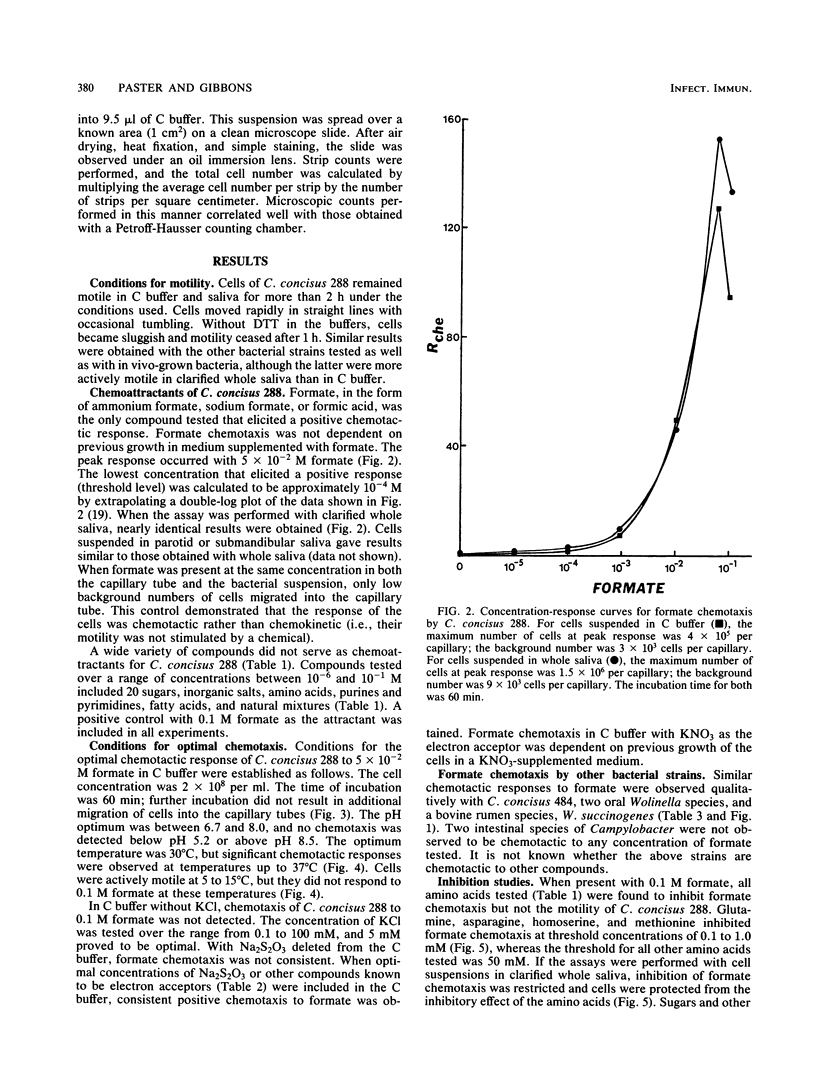
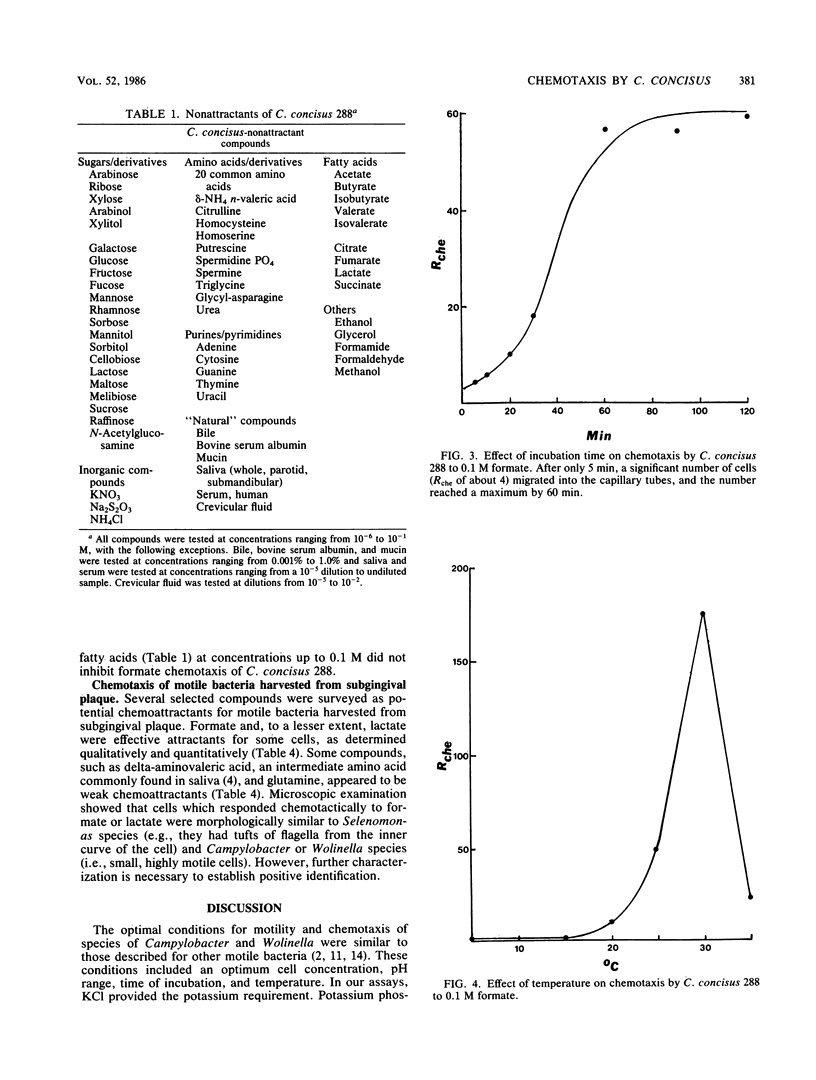
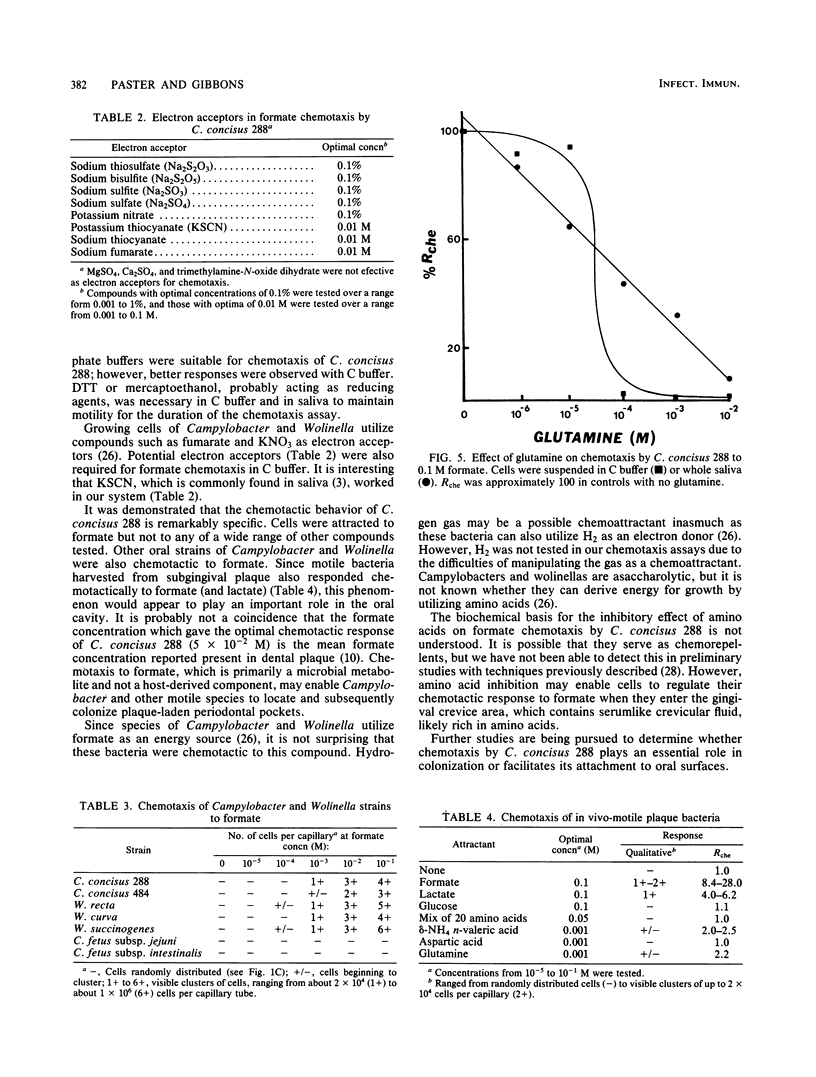
Cells of Campylobacter concisus 288 were chemotactic toward formate, but not to any other compound tested. Chemicals that were not chemoattractants included 20 sugars, inorganic salts, amino acids, and their derivatives, purines and pyrimidines, fatty acids, and natural mixtures such as saliva, serum, crevicular fluid, and mucin. Chemotaxis was measured quantitatively by a modification of the capillary method. Cells were suspended in 0.01 M Tris buffer, pH 7.5, supplemented with 5 mM KCl, 0.1% Na2S2O3, and 0.1 mM dithiothreitol. Whole, parotid, and submandibular salivas were also suitable as chemotaxis buffers. Optimum response (0.4 X 10(6) to 1.5 X 10(6) cells per capillary) for chemotaxis occurred with 5 X 10(-2) M formate at 30 degrees C for 60 min. At 5 to 15 degrees C, cells were motile, but chemotaxis was not detected. Glutamine, asparagine, homoserine, and methionine inhibited formate chemotaxis at threshold concentrations of 0.1 to 1.0 mM, whereas the threshold for all other amino acids tested was 50 mM. Sugars and fatty acids at concentrations up to 0.1 M did not inhibit formate chemotaxis. C. concisus 484, Wolinella recta 371, W. curva VPI 9584, and W. succinogenes 602 were also chemotactic to formate, but C. fetus subsp. jejuni VPI H641 and C. fetus subsp. intestinalis VPI 1176 were not. Motile bacteria harvested directly from subgingival plaque were also chemotactic to formate and, to a lesser extent, lactate. Selected sugars, other fatty acids, and amino acids did not serve as chemoattractants for these plaque bacteria.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. A method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis by Escherichia coli. J Gen Microbiol. 1973 Jan;74(1):77–91. doi: 10.1099/00221287-74-1-77. [DOI] [PubMed] [Google Scholar]
- Adler J. Chemotaxis in bacteria. Annu Rev Biochem. 1975;44:341–356. doi: 10.1146/annurev.bi.44.070175.002013. [DOI] [PubMed] [Google Scholar]
- Freter R., Allweiss B., O'Brien P. C., Halstead S. A., Macsai M. S. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: in vitro studies. Infect Immun. 1981 Oct;34(1):241–249. doi: 10.1128/iai.34.1.241-249.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R., O'Brien P. C., Macsai M. S. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: in vivo studies. Infect Immun. 1981 Oct;34(1):234–240. doi: 10.1128/iai.34.1.234-240.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R., O'Brien P. C. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: chemotactic responses of Vibrio cholerae and description of motile nonchemotactic mutants. Infect Immun. 1981 Oct;34(1):215–221. doi: 10.1128/iai.34.1.215-221.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R., O'Brien P. C. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: fitness and virulence of nonchemotactic Vibrio cholerae mutants in infant mice. Infect Immun. 1981 Oct;34(1):222–233. doi: 10.1128/iai.34.1.222-233.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Greenberg E. P., Canale-Parola E. Relationship between cell coiling and motility of spirochetes in viscous environments. J Bacteriol. 1977 Sep;131(3):960–969. doi: 10.1128/jb.131.3.960-969.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guentzel M. N., Berry L. J. Motility as a virulence factor for Vibrio cholerae. Infect Immun. 1975 May;11(5):890–897. doi: 10.1128/iai.11.5.890-897.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. J., Lawless J. G. Role of chemotaxis in the ecology of denitrifiers. Appl Environ Microbiol. 1985 Jan;49(1):109–114. doi: 10.1128/aem.49.1.109-114.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshland D. E., Jr Biochemistry of sensing and adaptation in a simple bacterial system. Annu Rev Biochem. 1981;50:765–782. doi: 10.1146/annurev.bi.50.070181.004001. [DOI] [PubMed] [Google Scholar]
- LOESCHE W. J., GIBBONS R. J., SOCRANSKY S. S. BIOCHEMICAL CHARACTERISTICS OF VIBRIO SPUTORUM AND RELATIONSHIP TO VIBRIO BUBULUS AND VIBRIO FETUS. J Bacteriol. 1965 Apr;89:1109–1116. doi: 10.1128/jb.89.4.1109-1116.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listgarten M. A., Helldén L. Relative distribution of bacteria at clinically healthy and periodontally diseased sites in humans. J Clin Periodontol. 1978 May;5(2):115–132. doi: 10.1111/j.1600-051x.1978.tb01913.x. [DOI] [PubMed] [Google Scholar]
- Listgarten M. A. Structure of the microbial flora associated with periodontal health and disease in man. A light and electron microscopic study. J Periodontol. 1976 Jan;47(1):1–18. doi: 10.1902/jop.1976.47.1.1. [DOI] [PubMed] [Google Scholar]
- Mandel I. D., Wotman S. The salivary secretions in health and disease. Oral Sci Rev. 1976;(8):25–47. [PubMed] [Google Scholar]
- Mesibov R., Adler J. Chemotaxis toward amino acids in Escherichia coli. J Bacteriol. 1972 Oct;112(1):315–326. doi: 10.1128/jb.112.1.315-326.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morooka T., Umeda A., Amako K. Motility as an intestinal colonization factor for Campylobacter jejuni. J Gen Microbiol. 1985 Aug;131(8):1973–1980. doi: 10.1099/00221287-131-8-1973. [DOI] [PubMed] [Google Scholar]
- Moulton R. C., Montie T. C. Chemotaxis by Pseudomonas aeruginosa. J Bacteriol. 1979 Jan;137(1):274–280. doi: 10.1128/jb.137.1.274-280.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paster B. J., Canale-Parola E. Physiological diversity of rumen spirochetes. Appl Environ Microbiol. 1982 Mar;43(3):686–693. doi: 10.1128/aem.43.3.686-693.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J. Subgingival microflora and periodontal disease. J Clin Periodontol. 1979 Oct;6(5):351–382. doi: 10.1111/j.1600-051x.1979.tb01935.x. [DOI] [PubMed] [Google Scholar]
- Socransky S. S. Microbiology of periodontal disease -- present status and future considerations. J Periodontol. 1977 Sep;48(9):497–504. doi: 10.1902/jop.1977.48.9.497. [DOI] [PubMed] [Google Scholar]
- Stanton T. B., Savage D. C. Motility as a factor in bowel colonization by Roseburia cecicola, an obligately anaerobic bacterium from the mouse caecum. J Gen Microbiol. 1984 Jan;130(1):173–183. doi: 10.1099/00221287-130-1-173. [DOI] [PubMed] [Google Scholar]
- Tanner A. C., Haffer C., Bratthall G. T., Visconti R. A., Socransky S. S. A study of the bacteria associated with advancing periodontitis in man. J Clin Periodontol. 1979 Oct;6(5):278–307. doi: 10.1111/j.1600-051x.1979.tb01931.x. [DOI] [PubMed] [Google Scholar]
- Tso W. W., Adler J. Negative chemotaxis in Escherichia coli. J Bacteriol. 1974 May;118(2):560–576. doi: 10.1128/jb.118.2.560-576.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]



