Abstract
A crucial problem for most animals is how to deal with multiple types of predator, which differ in their sensory capabilities and methods of prey detection. For animals capable of rapid colour change, one potential strategy is to change their appearance in relation to the threat posed by different predators. Here, we show that the dwarf chameleon, Bradypodion taeniabronchum, exhibits different colour responses to two predators that differ in their visual capabilities. Using a model of animal colour perception to gain a ‘predator's eye view’, we show that chameleons showed better background colour matching in response to birds than snakes, yet they appear significantly more camouflaged to the snake visual system because snakes have poorer colour discrimination.
Keywords: crypsis, predation, visual ecology, lizard
1. Introduction
Most animals face the important challenge of dealing with multiple predators, which differ in their sensory systems, means of prey detection and level of threat. One solution is for prey to adjust their defensive behaviour or appearance in response to these different predators (Hopper 2001; Templeton & Shriner 2004; Stuart-Fox et al. 2006; Langridge et al. 2007; Rundus et al. 2007). For example, ground squirrels (Spermophilus beecheyi) augment infrared emission only when performing deterrent displays to infrared sensitive predators and not others (Rundus et al. 2007) and juvenile cuttlefish (Sepia officinalis) show threatening colour patterns to deter visual, but not chemosensory, predators (Langridge et al. 2007). Here, we test whether dwarf chameleons (Bradypodion taeniabronchum) show different colour responses to two predators (a bird and a snake) that differ in their visual systems.
The ability to rapidly change colour has evolved independently in numerous invertebrate and vertebrate groups, from octopuses to frogs and fish (Stuart-Fox & Moussalli 2008). Colour change not only enables animals to match different backgrounds but also enables prey to change their appearance to different predators (Langridge et al. 2007) and potentially adjust their camouflage depending on the predators' visual capabilities. Dwarf chameleons are ideally suited to testing for such facultative camouflage because they are capable of rapid colour change, rely on stationary background matching as their primary anti-predator strategy and have predators, primarily birds and snakes, which differ greatly in their visual capabilities. Like humans, diurnal snakes are trichromats, having three different types of visual pigment (Sillman et al. 1997), while birds are tetrachromats (Hart & Hunt 2007) and therefore have superior colour discrimination.
We measured the colour responses of dwarf chameleons to a model bird and snake predator in field trials. We used two common predators, the boomslang, Dispholidus typus, a visually hunting, diurnal snake and the fiscal shrike, Lanius collaris, known for impaling chameleons on thorns (electronic supplementary material, figure S1). To test for facultative camouflage, we estimated the detectability of chameleon colour responses to the visual systems of these two predators using a model of animal colour perception (Vorobyev & Osorio 1998; Siddiqi et al. 2004). Although camouflage via background matching requires that both the colour and pattern of the animal represent a random sample of the colour and pattern of the background (Endler 1978), here we focus on the chameleons' capacity for colour matching (electronic supplementary material, figures S1b and S2).
2. Material and methods
(a) Behavioural trials
We captured chameleons (n=8 adults of each sex) at night (field site: 33° 53′ 9.9″ S, 25° 15′ 51.5″ E) and conducted behavioural trials in the field the next day. We placed chameleons on a perch (a natural branch) close to vegetation and presented them with two model predators, both from varying angles, in random order. Their predator models were a stuffed fiscal shrike (L. collaris) and a model boomslang snake (D. typus) made of resin from a cast of a dead boomslang and painted to resemble an adult male by a professional model maker. As soon as chameleons showed specific anti-predator behavioural responses (electronic supplementary material, figure S1b), which we recorded, we took reflectance measurements (electronic supplementary material, figure S3) of three body regions (top, middle and bottom flank) in random order. Chameleons only react to the model predators and not to a moving branch presented as a control stimulus (Stuart-Fox et al. 2006). Protocols for behavioural trials and reflectance measurements are the same as in Stuart-Fox et al. (2006). To measure background colour, we took reflectance readings of the natural perch used in behavioural trials and used the median of these readings (n=10) in subsequent models of colour perception (electronic supplementary material, figure S3). We also took irradiance (illumination) measurements (electronic supplementary material, figure S3) using an SD2000 spectrometer and a calibrated, cosine-corrected irradiance probe (CC-3-DA, Ocean Optics).
(b) Predator visual systems
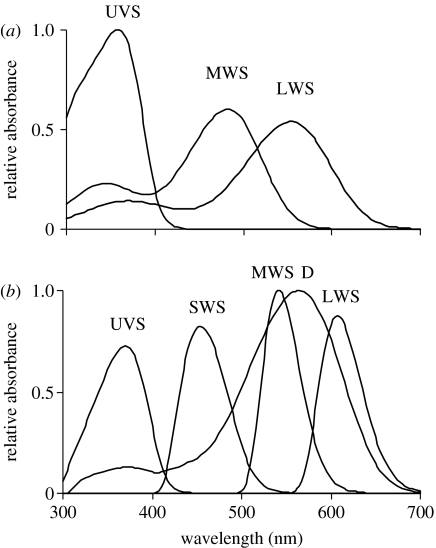
Based on its phylogenetic position, the fiscal shrike is likely to have an ultraviolet sensitive (UVS) visual system like most other passerines (Hart & Hunt 2007). Therefore, we used average photoreceptor spectral sensitivities for the UVS avian visual system from Endler & Mielke (2005), which are already corrected for filtering by ocular media and oil droplets (figure 1b). We used visual pigment absorbance functions (snakes do not have coloured oil droplets) for the garter snake Thamnophis sirtalis from Sillman et al. (1997; figure 1a). This is the only diurnal colubrid snake for which detailed data are available. Although the visual systems of very few snakes have been studied, visual pigment spectral sensitivities appear to be conserved among diurnal snakes (Sillman et al. 1997; E. Loew 2007, personal communication).
Figure 1.
Photoreceptor spectral sensitivities (relative absorbances) for (a) the colubrid snake, T. sirtalis, and (b) ultraviolet sensitive avian visual system. UVS, ultraviolet sensitive; SWS, short-wavelength sensitive; MWS, medium-wavelength sensitive; LWS, long-wavelength sensitive; D, double cone. Absorbance spectra of the single cones are normalized to equal area under the curve and the bird double cone is normalized to a maximum of 1.
(c) Visual modelling
To calculate a predator's ability to discriminate the chameleon from its background, we applied the model of Vorobyev & Osorio (1998), which derives the perceptual distance, ΔS, between two colours in units of ‘just noticeable differences’ (JNDs) based on differences in photoreceptor quantum catches and photoreceptor noise, ωi. We used the same model calculations as detailed in Siddiqi et al. (2004).
We first averaged each spectrum (chameleon colours, backgrounds and irradiance) over each 5 nm interval using a kernel smoothing function. Next, we derived receptor quantum catches for each cone type over the visible spectrum for birds and snakes (300–700 nm; Vorobyev & Osorio 1998; Endler & Mielke 2005), then applied the von Kries transformation to account for receptor adaptation to the light environment, which contributes to colour constancy (Vorobyev & Osorio 1998; Vorobyev et al. 1998; Siddiqi et al. 2004; Endler & Mielke 2005).
We assumed that photoreceptor noise, ωi, for the long-wavelength sensitive (LWS) photoreceptor in both birds and snakes=0.05 (see also Siddiqi et al. 2004; Stuart-Fox et al. 2004) then derived ωi for remaining photoreceptor classes. We used calculations for high illumination conditions because the habitat is open heath with no canopy cover and both predators are diurnal rather than crepuscular. We assumed a ratio of 1 : 2 : 3 : 3 for the four avian photoreceptor classes and 1 : 1.6 : 7.3 for the three snake photoreceptor classes (Vorobyev & Osorio 1998). The ratio for birds is based on the mean ratio for species with a UVS visual system from Hart (2001) and the ratio for snakes is that of the garter snake Thamnophis sirtalis from Sillman et al. (1997). To check how sensitive the results are to the choice of ωi, we also performed the calculations with a range of photoreceptor ratios, but results remained qualitatively unchanged.
Colour discrimination involves two perceptual channels: the chromatic channel, which detects variation in the spectral composition of light and the achromatic channel, which detects variation in light intensity (‘brightness’). We assumed that the three single cones in snakes and the four single cones in birds are used for chromatic discrimination, while the snake LWS single cone and the LWS photoreceptors in both the principal and accessory members of the avian double cone are used for achromatic discrimination (Siddiqi et al. 2004). For the bird double cone, we used the sum of the LWS sensitivity corrected for the transmission of the oil droplet associated with the principal member and LWS sensitivity for the accessory member, which has no oil droplet (figure 1b). For achromatic calculations, bird and snake LWS spectral sensitivities were normalized to a maximum of 1.
(d) Statistical analysis
After first testing that our data fitted model assumptions, we tested for differences in colour responses to the two predators using repeated measures ANOVA (PROC MIXED, SAS v. 9.1), with predator type as the repeated measure on the individual chameleons (subjects). Sex and body region (top, middle or bottom flank) and their respective interactions with predator type were included in the models as fixed effects and the order of predator presentation (first or second) was included as a random effect.
3. Results
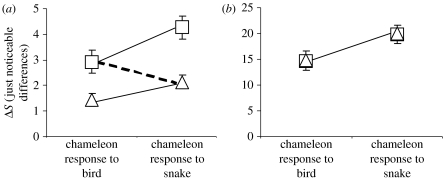
We found that chameleons consistently matched the background more closely in response to birds than snakes (table 1; electronic supplementary material, figure S3). This was consistent between the sexes and among all three focal body regions in terms of both the chromatic and achromatic (brightness) components of colour as none of the interactions between predator and sex or body region was significant (table 1). The closer background colour matching in response to birds was evident relative to both the bird visual system and the snake visual system (table 1; figure 2a,b). There were also significant differences in the degree of colour matching among body regions with the top and mid-flanks showing closer colour matching than the bottom flank.
Table 1.
Repeated measures ANOVA testing for differences in colour responses to model bird and snake predators. (Predator, sex and body region were fixed effects and order of presentation (order) was included in models as a random effect.)
| dependent variable | factor | bird vision | snake vision | ||
|---|---|---|---|---|---|
| Fd.f. | p | Fd.f. | p | ||
| achromatic | predator | 21.361,87 | <0.0001 | 21.361,87 | <0.0001 |
| sex | 1.931,87 | 0.17 | 1.931,87 | 0.17 | |
| body region | 11.462,87 | <0.0001 | 11.462,87 | <0.0001 | |
| predator×body region | 0.712,87 | 0.50 | 0.712,87 | 0.50 | |
| predator×sex | 0.571,87 | 0.45 | 0.571,87 | 0.45 | |
| order | 0.791,87 | 0.43 | 0.7987 | 0.43 | |
| chromatic | predator | 7.661,87 | 0.007 | 8.51,87 | 0.005 |
| sex | 6.781,87 | 0.01 | 3.271,87 | 0.07 | |
| body region | 12.062,87 | <0.0001 | 132,87 | <0.0001 | |
| predator×body region | 0.072,87 | 0.94 | 0.042,87 | 0.96 | |
| predator×sex | 0.011,87 | 0.92 | 0.01,87 | 0.99 | |
| order | 0.451,87 | 0.66 | 0.4587 | 0.66 | |
Figure 2.
Colour responses of chameleons to the two predators measured as their contrast against the background in units of JNDs, relative to the visual system of each predator. Lower values indicate greater camouflage. Squares, bird vision; triangles, snake vision. Bars represent standard errors around the means. Chameleon colour responses to birds are significantly more camouflaged than colour responses to snakes (thin solid line comparisons) in terms of both (a) chromatic (colour) and (b) achromatic (brightness) perceptual channels for both predators. Despite this difference, chameleon responses to snakes are less detectable to the snake visual system than responses to birds are to the bird visual system (a thick dashed line comparison; F1,87=4.61, p=0.03) because snakes have poorer colour discrimination.
There was no difference in the behaviour of chameleons towards the two predators. Chameleons consistently showed typical anti-predator behaviours (flattening themselves and flipping to the opposite side of the branch); all individuals flipped to the opposite side of the branch in response to both the predators and there was no difference in the frequency with which they flattened themselves laterally (F1,27=2.6, p=0.12).
4. Discussion
Chameleons consistently showed better background colour matching in response to birds than snakes, but even so, appear significantly more camouflaged to the snake visual system because snakes have poorer colour discrimination. There are two potential explanations for this pattern. The first is that because birds have better colour discrimination, chameleons need to match the background more closely to achieve a similar level of camouflage. While our results are consistent with this explanation, further experimental tests are required to verify that predators perceive the chameleon colour differences and respond to them differently. The second, not mutually exclusive explanation for better background matching in response to birds is that avian predators are more abundant, thereby imposing stronger natural selection for crypsis. Chameleons may therefore show a stronger background colour matching response to birds, although other behavioural responses to the two predators did not differ. Detailed data on predation rates by birds and snakes are required to test this explanation. Under both the explanations, however, birds pose a greater predation threat to chameleons, either because birds have better vision or because they are more abundant.
Although birds and snakes differ in colour discrimination, their capacity for achromatic (brightness) discrimination is similar due to conserved spectral sensitivities of long-wavelength-sensitive visual pigments (figure 1). Thus, the different achromatic responses of chameleons to birds and snakes result in corresponding differences in achromatic camouflage. Why do chameleons show close colour matching, yet are notably paler than their backgrounds in response to snakes (see also Stuart-Fox et al. 2006)? One reason may be that snakes view their prey from below against a background of high illumination, whereas birds view their prey from above against a background of low illumination. The significant difference in brightness responses to the two predators may reflect this difference in viewing angle. Overall then, chameleons show differential responses in both the chromatic and achromatic components of colour, which probably influence apparent camouflage to the two predators.
The chameleons' differential camouflage response to the two predators raises the question of why chameleons vary their coloration rather than showing maximum colour matching or the ‘bird response’ at all times. One possibility is that there is a physiological cost of colour change, although data on such costs are not, to our knowledge, available for any colour-changing organism. Maximum camouflage requires perfect background matching at all times as the animal moves, a feat that is likely to be challenging and potentially costly, even for a chameleon. Instead, chameleons may adjust their camouflage responses relative to perceived threat.
Acknowledgments
We thank the South African National Research Foundation, Stiaan Marais, George Goode, Stephanie Ritter, Bill Branch, Michael Cunningham, Kate Henderson and Ellis Loew. Eastern Cape permit WRO 11/03 WR.
Supplementary Material
Figures S1, S2, S3
References
- Endler J.A. A predator's view of animal color patterns. Evol. Biol. 1978;11:319–364. [Google Scholar]
- Endler J.A, Mielke P.W. Comparing entire colour patterns as birds see them. Biol. J. Linn. Soc. 2005;86:405–431. doi:10.1111/j.1095-8312.2005.00540.x [Google Scholar]
- Hart N.S. Variations in cone photoreceptor abundance and the visual ecology of birds. J. Comp. Physiol. A. 2001;187:685–698. doi: 10.1007/s00359-001-0240-3. doi:10.1007/s00359-001-0240-3 [DOI] [PubMed] [Google Scholar]
- Hart N.S, Hunt D.M. Avian visual pigments: characteristics, spectral tuning, and evolution. Am. Nat. 2007;169:S7–S26. doi: 10.1086/510141. doi:10.1086/510141 [DOI] [PubMed] [Google Scholar]
- Hopper K.R. Flexible antipredator behavior in a dragonfly species that coexists with different predator types. Oikos. 2001;93:470–476. doi:10.1034/j.1600-0706.2001.930312.x [Google Scholar]
- Langridge K.V, Broom M, Osorio D. Selective signalling by cuttlefish to predators. Curr. Biol. 2007;17:R1044–R1045. doi: 10.1016/j.cub.2007.10.028. doi:10.1016/j.cub.2007.10.028 [DOI] [PubMed] [Google Scholar]
- Rundus A.S, Owings D.H, Joshi S.S, Chinn E, Giannini N. Ground squirrels use an infrared signal to deter rattlesnake predation. Proc. Natl Acad. Sci. USA. 2007;104:14 372–14 376. doi: 10.1073/pnas.0702599104. doi:10.1073/pnas.0702599104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi A, Cronin T.W, Loew E.R, Vorobyev M, Summers K. Interspecific and intraspecific views of color signals in the strawberry poison frog Dendrobates pumilio. J. Exp. Biol. 2004;207:2471–2485. doi: 10.1242/jeb.01047. doi:10.1242/jeb.01047 [DOI] [PubMed] [Google Scholar]
- Sillman A.J, Govardovskii V.I, Rohlich P, Southard J.A, Loew E.R. The photoreceptors and visual pigments of the garter snake (Thamnophis sirtalis): a microspectrophotometric, scanning electron microscopic and immunocytochemical study. J. Comp. Physiol. A. 1997;181:89–101. doi: 10.1007/s003590050096. doi:10.1007/s003590050096 [DOI] [PubMed] [Google Scholar]
- Stuart-Fox D, Moussalli A. Selection for social signalling drives the evolution of chameleon colour change. PLoS Biol. 2008;6:e25. doi: 10.1371/journal.pbio.0060025. doi:10.1371/journal.pbio.0060025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart-Fox D.M, Moussalli A, Johnston G.R, Owens I.P.F. Evolution of color variation in dragon lizards: quantitative tests of the role of crypsis and local adaptation. Evolution. 2004;58:1549–1559. doi: 10.1111/j.0014-3820.2004.tb01735.x. doi:10.1111/j.0014-3820.2004.tb01735.x [DOI] [PubMed] [Google Scholar]
- Stuart-Fox D, Whiting M.J, Moussalli A. Camouflage and colour change: antipredator responses to bird and snake predators across multiple populations in a dwarf chameleon. Biol. J. Linn. Soc. 2006;88:437–446. doi:10.1111/j.1095-8312.2006.00631.x [Google Scholar]
- Templeton C.N, Shriner W.M. Multiple selection pressures influence Trinidadian guppy (Poecilia reticulata) antipredator behavior. Behav. Ecol. 2004;15:673–678. doi:10.1093/beheco/arh065 [Google Scholar]
- Vorobyev M, Osorio D. Receptor noise as a determinant of colour thresholds. Proc. R. Soc. B. 1998;265:351–358. doi: 10.1098/rspb.1998.0302. doi:10.1098/rspb.1998.0302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorobyev M, Osorio D, Bennett A.T.D, Marshall N.J, Cuthill I.C. Tetrachromacy, oil droplets and bird plumage colours. J. Comp. Physiol. A. 1998;183:621–633. doi: 10.1007/s003590050286. doi:10.1007/s003590050286 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1, S2, S3