Abstract
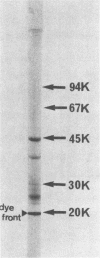
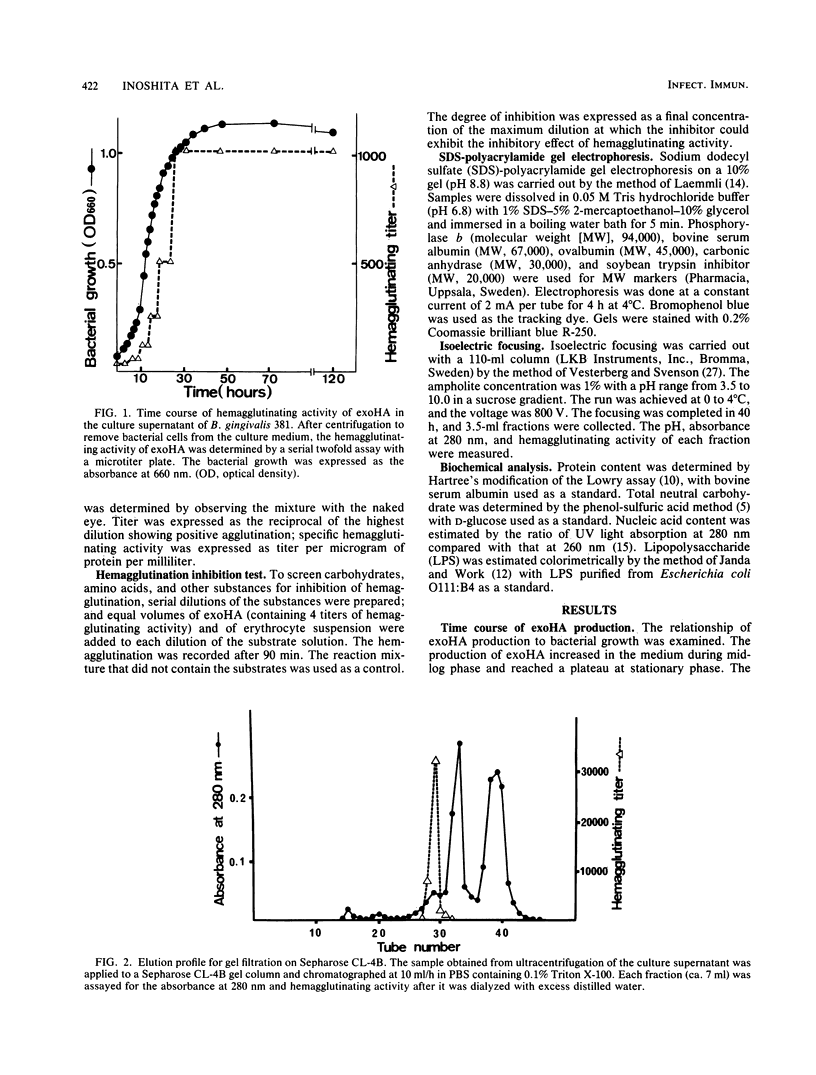
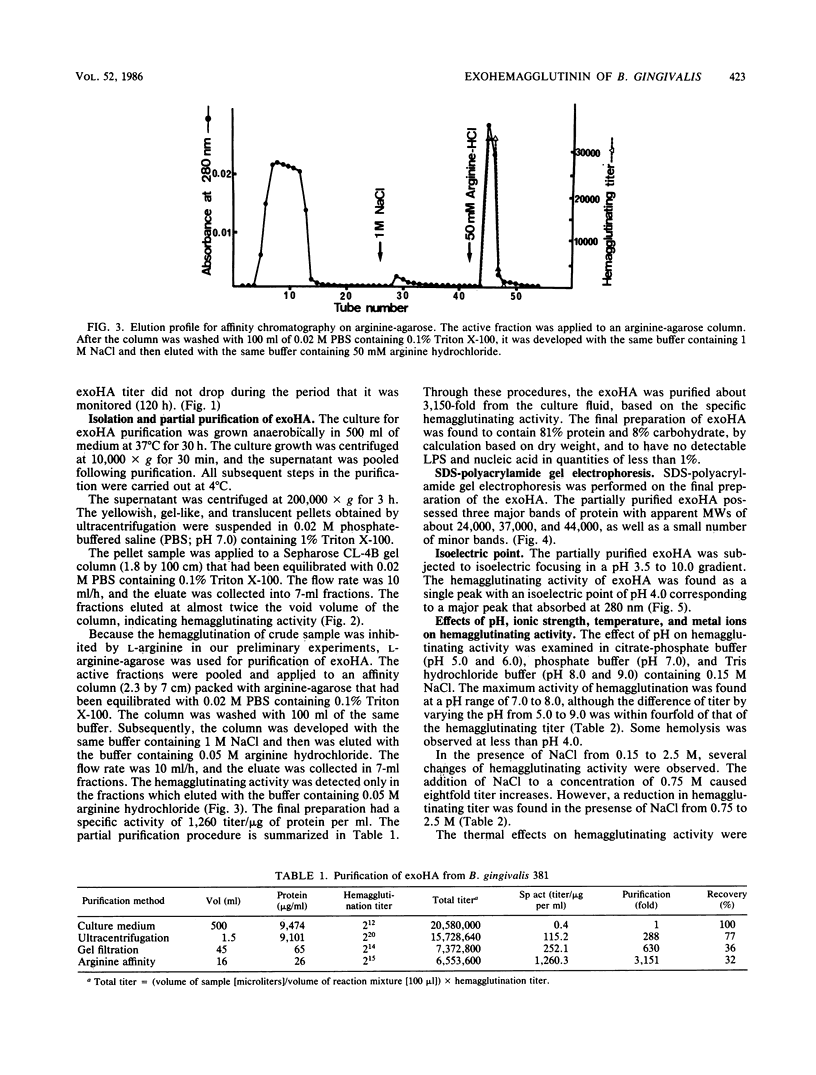
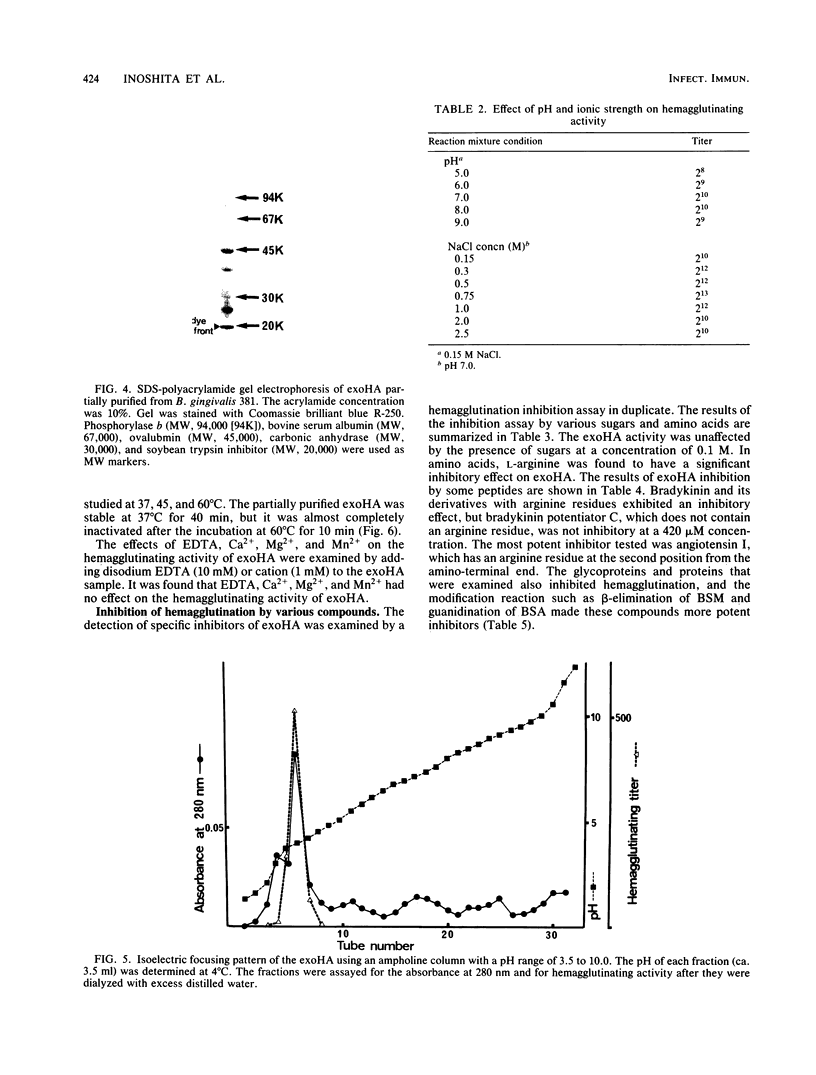
Exohemagglutinin was found in the culture medium of Bacteroides gingivalis 381. Exohemagglutinin was purified 3,150-fold from culture fluid by ultracentrifugation followed by gel filtration on Sepharose CL-4B and by affinity chromatography on arginine-agarose. Examination of the final preparation of exohemagglutinin by biochemical analysis and sodium dodecyl sulfate-polyacrylamide gel electrophoresis revealed that the isolated exohemagglutinin contained three major proteins but not a detectable lipopolysaccharide. Hemagglutination inhibition experiments showed that the activity of exohemagglutinin was inhibited by L-arginine and the arginine-containing peptides, although the activity was unaffected by the sugars tested. Some protein and glycoproteins that were examined also exhibited the inhibitory activity. When the bovine submaxillary mucin was chemically modified by beta-elimination and bovine serum albumin was modified by guanidination, the inhibitory effects on hemagglutination were significantly enhanced. These results suggest that the hemagglutination of the isolated exohemagglutinin may be involved in arginine residues as components of ligand-binding sites on erythrocytes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyd J., McBride B. C. Fractionation of hemagglutinating and bacterial binding adhesins of Bacteroides gingivalis. Infect Immun. 1984 Aug;45(2):403–409. doi: 10.1128/iai.45.2.403-409.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisar J. O., Kolenbrander P. E., McIntire F. C. Specificity of coaggregation reactions between human oral streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii. Infect Immun. 1979 Jun;24(3):742–752. doi: 10.1128/iai.24.3.742-752.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehazya P., Coles R. S., Jr Agglutination of human erythrocytes by Fusobacterium nucleatum: factors influencing hemagglutination and some characteristics of the agglutinin. J Bacteriol. 1980 Jul;143(1):205–211. doi: 10.1128/jb.143.1.205-211.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehazya P., Coles R. S., Jr Extraction and properties of hemagglutinin from cell wall fragments of Fusobacterium nucleatum. J Bacteriol. 1982 Oct;152(1):298–305. doi: 10.1128/jb.152.1.298-305.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkler W. A., Jr, Smoot C. N., Mongiello J. R. Attachment of cell fragments of Fusobacterium nucleatum to oral epithelial cells, gingival fibroblasts and white blood cells. Arch Oral Biol. 1982;27(7):553–559. doi: 10.1016/0003-9969(82)90069-3. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Etherden I. Comparative hydrophobicities of oral bacteria and their adherence to salivary pellicles. Infect Immun. 1983 Sep;41(3):1190–1196. doi: 10.1128/iai.41.3.1190-1196.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Qureshi J. V. Inhibition of adsorption of Streptococcus mutans strains to saliva-treated hydroxyapatite by galactose and certain amines. Infect Immun. 1979 Dec;26(3):1214–1217. doi: 10.1128/iai.26.3.1214-1217.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Hsieh C. C., Iwakaura K., Takagaki M., Shibata S. Exohemagglutinin derived from Streptococcus mitis ATCC 9811. J Osaka Univ Dent Sch. 1984 Dec;24:67–76. [PubMed] [Google Scholar]
- Janda J., Work E. A colorimetric estimation of lipopolysaccharides. FEBS Lett. 1971 Sep 1;16(4):343–345. doi: 10.1016/0014-5793(71)80386-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mayrand D., McBride B. C., Edwards T., Jensen S. Characterization of Bacteroides asaccharolyticus and B. melaninogenicus oral isolates. Can J Microbiol. 1980 Oct;26(10):1178–1183. doi: 10.1139/m80-197. [DOI] [PubMed] [Google Scholar]
- Nagata K., Nakao M., Shibata S., Shizukuishi S., Nakamura R., Tsunemitsu A. Purification and characterization of galactosephilic component present on the cell surfaces of Streptococcus sanguis ATCC 10557. J Periodontol. 1983 Mar;54(3):163–172. doi: 10.1902/jop.1983.54.3.163. [DOI] [PubMed] [Google Scholar]
- Okuda K., Takazoe I. Haemagglutinating activity of Bacteroides melaninogenicus. Arch Oral Biol. 1974 May;19(5):415–416. doi: 10.1016/0003-9969(74)90184-8. [DOI] [PubMed] [Google Scholar]
- Shibata S., Nagata K., Nakamura R., Tsunemitsu A., Misaki A. Interaction of parotid saliva basic glycoprotein with Streptococcus sanguis ATCC 10557. J Periodontol. 1980 Sep;51(9):499–504. doi: 10.1902/jop.1980.51.9.499. [DOI] [PubMed] [Google Scholar]
- Slots J., Gibbons R. J. Attachment of Bacteroides melaninogenicus subsp. asaccharolyticus to oral surfaces and its possible role in colonization of the mouth and of periodontal pockets. Infect Immun. 1978 Jan;19(1):254–264. doi: 10.1128/iai.19.1.254-264.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Bertolini M., Pigman W. Serine and threonine glycosidic linkages in bovine submaxillary mucin. Biochem Biophys Res Commun. 1964 Jul 27;16(5):404–409. doi: 10.1016/0006-291x(64)90366-3. [DOI] [PubMed] [Google Scholar]
- Vesterberg O., Svensson H. Isoelectric fractionation, analysis, and characterization of ampholytes in natural pH gradients. IV. Further studies on the resolving power in connection with separation of myoglobins. Acta Chem Scand. 1966;20(3):820–834. doi: 10.3891/acta.chem.scand.20-0820. [DOI] [PubMed] [Google Scholar]
- Weerkamp A. H., McBride B. C. Characterization of the adherence properties of Streptococcus salivarius. Infect Immun. 1980 Aug;29(2):459–468. doi: 10.1128/iai.29.2.459-468.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki Y. [Mechanism of Eikenella corrodens adherence to human buccal epithelial cells and partial purification of the bacterial lectin-like substance from bacterial cells which participate in adherence]. Nihon Shishubyo Gakkai Kaishi. 1984 Jun;26(2):223–242. doi: 10.2329/perio.26.223. [DOI] [PubMed] [Google Scholar]
- Yoshimura F., Takahashi K., Nodasaka Y., Suzuki T. Purification and characterization of a novel type of fimbriae from the oral anaerobe Bacteroides gingivalis. J Bacteriol. 1984 Dec;160(3):949–957. doi: 10.1128/jb.160.3.949-957.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]